Abstract
Human onchocerciasis is caused by the filarial worm. Onchocerca volvulus is a parasite that forms nodules under the skin. The aim of this work was to assess the nematicidal activities of Aloe vera on Onchocerca ochengi and Caenorahbditis elegans and to determine the phytochemical compounds. Nodules were collected from the umbilical region of infected cattle, dissected and male worms were cultured in RPMI-1640. Worms were incubated with different concentrations of A. vera extracts in RPMI-1640 and M9-buffer. Polyphenol, tannin and flavonoid contents of extract were determined by using gallic acid and rutin as standards. The anthelmintic effect of A. vera extract against O. ochengi was concentration dependent with LC50 of 20.71 µg/mL and 11.75 µg/mL after 48 and 72 h respectively. A. vera extract exerted concentration dependent lethal effects (LC50 = 2747 and LC50 = 31,937 µg/mL) against C. elegans (Wild Type). Methanolic-methylene chloride (MeOH–CH2Cl2) of A. vera extract exhibited high DPPH activity with an IC50 value of 15 µg/mL and 9 µg/mL for ascorbic acid. The highest activity in adult worms was observed with the MeOH (100: 0) and AcOEtMeOH fractions with LC50 values of 12.82 and 15.50 µg/mL respectively. EcOEtMeOH (8:2 v/v) was more effective (LC50 = 250 µg/mL) on WT of C. elegans. A. vera contains polyphenols (1015.05 and AcOEtMeOH = 893.60), flavonoids (25.35 and MeOH = 225.76) and tannins (401.37 and Hex = 788.89). A. vera showed in vitro nematicidal activity against O. ochengi and C. elegans. A. vera could be used as an alternative anthelmintic for onchocerciasis treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Onchocerciasis, a causative of blindness, is due to Onchocerca volvulus, a parasitic worm that forms nodules under the skin (Ayimele and Yong 2015). The disease is characterized by the presence of nodules on skin, dermatitis and the ocular syndrome (Katabarwa et al. 2013). Previous studies showed that 37 million people are infected in the world with 99% of them in intertropical Africa and 270,000 blinds (Eze and Matur 2011; Manchang et al. 2014). The control started with vector elimination by spraying insecticides and larvicides (Boatin 2008) followed by mass drug treatment using various combinations of drugs including ivermectin which is currently the recommended molecule against onchocerciasis (Dent et al. 2000a). Although this drug has been shown to significantly reduce transmission of the disease, its filaricidal effect is limited only on the juvenile form of the parasite (Cupp et al. 2011). In addition to the fact that this drug induces adverse side effects, it has a low effect on the adult form of the parasite and it was reported to be inefficient on some communities where the parasite has probably developed resistance (Borsboom et al. 2003; Osei-Tweneboana et al. 2007). Elsewhere, the vector control methods showed various limitations. For example, resistance of blackfly to insecticides and the toxicity of these insecticides against beneficial insects to ecosystem balance was noted.
In Cameroon, the prevalence of onchocerciasis is estimated at 28% and more than one million of infected people present serious lesions of skin (Tanya et al. 2013).Onchocerciasis is endemic in 61% of the Health Districts of Cameroon (Tanya et al. 2013).In Ngaoundere as part of the Adamawa Region, the prevalence of human and animal onchocerciasis is estimated at 30% and 65% respectively (Wahl et al. 1991). In animals, onchocerciasis is responsible for the loss of fertile grounds. For example in Cameroon, 110.000 km2 of cultivable grounds were abandoned because of the disease (Wahl et al. 1991). Lower agricultural output was observed, in addition to the stigmatization of the infected people. Children giving up study to guide the blind adults having river blindness have been noted by Toé et al. (2014).
However, the anti-Onchocerca and anti-Caenorahbditis activities of Aloe vera crude extract and fractions have not been evaluated on these parasites. In this work, their activities were evaluated on the adult worms of Onchocerca ochengi, the closest related species to O. volvulus and the best model for anti-O. volvulus drug screenings (Gonzalez et al. 2009). The activity of this plant was also evaluated against free-living nematode Caenorhabditis elegans, a model organism appropriate to research (Trees et al. 2000). The simple morphology, rapid development, easy cultivation and accessibility to genetics and molecular biology make C. elegans a powerful analytical tool for investigations of biological effects of toxic substances and identification of suitable pharmacological targets (Burglin et al. 1998).
Plant presents multiple beneficial properties for human health. Considering the need for a new anthelmintic drug, some plants were chosen after a screening based on their anthelmintic efficiency. The eradication of this epidemic requires effective drug intervention, good affordability, with fewer side effects. These disadvantages of conventional drugs have stimulated research for alternative control methods such as the use of traditional medicinal plants. Screening and proper evaluation of medicinal plants could reveal bioactive compounds that may be sustainable and environmentally acceptable (Kampkötter et al. 2007). Even today, plants play an important role in the health care of about 80% of the population in developing countries and it is estimated that more than half of the drugs under clinical use presently owe their origin from plants (Nisha et al. 2007). The parasitic and free-living nematodes were sensitive to the extracts of several African species (Sarin 1996) Aloe being used extensively in the cosmetic industry, has been described for centuries for its laxative, anti-inflammatory, immunostimulant, antiseptic (Bizimenyera et al. 2006), wound and burn healing (Okyar et al. 2001), antiulcer (Chithra et al. 1998), antitumor (Koo 1994), antidiabetic (Saito 1993), purgative, antibacterial, antioxidant, anesthetic, anthelmintic and antifungal properties (Bunyapraphatsara et al. 1996). Extracts of A. vera have shown activity against Aspergillus flavus, A. niger, Staphylococcus aureus, S. epidermidis, Pseudomonas aeruginosa, Trichophyton mentagraphytes, T. schoeleinii, Microsporium canis and Candida albicans, Escherichia coli, Bacillus subtilius, Salmonella typhi, Klebsiella pneumoniae (Rajesh 2015).
This study aims to evaluate the anthelmintic activities of leaves extract and fractions of A. vera on Onchocerca ochengi and free-living nematode Caenorahbditis elegans. The present work examines Aloe vera nematicidal activity against O. ochengi adult worms and C. elegans (L4). In addition, we analyse the acute toxicity in Wistar rats and the mixture phytochemical of the extracts and fractions of A. vera.
Material and methods
Location and place of study
The study was conducted at the University of Ngaoundere (Cameroon), Faculty of Science, in the Department of Biological Sciences Applied Zoology’s Laboratory. All experimental procedures performed in animals were approved by the regional delegation of Livestock, Fisheries and Animal industries at Ngaoundere (N°075/16/L/RA/DREPIA). Aloe vera leaves were harvested in the Adamawa Region, Department of Vina (Lat 7° 33′ N and Long 13°56’E).
Experimental materials used
Chemicals and reagents
All chemicals used in the laboratory have been purchased from Sigma Aldrich (Deisenhofen, Germany).
Plant collection and identification of Aloe vera
The plant identification was done by the lecturers of the Department of Biological Sciences of the Faculty of Sciences (University of Ngaoundere, Cameroon). The specimen was registered in the national herbarium of Cameroon under the reference number 87406/HNC.
Preparation of crude extract for tests
The plant was harvested and dried in the laboratory, protected from light, for a few weeks at room temperature. Afterwards, 50 g extract of leaves of A. vera were ground, weighed and macerated for 48 h at room temperature for each of the following solvents: 500 mL of distilled water and 70% methanol (70:30, v/v) and in the mixture of methanol/methylene chloride (1:1, v/v). The mixture was then centrifuged (3,500 × g, 10 min). After decantation, the organic phase of these different extracts was recovered and filtered using Wattman No. 413 filter paper (VWR International, Darmstadt, Germany) The filtrate was then put in rotary evaporator (Buchi Rotavapor R-210, Germany) at 40 °C under reduced pressure, lyophilized. The obtained powder was weighed and kept at 4 °C for further investigation (Ndjonka et al. 2011).
Fractionation of crude extract for tests
One of half (1.5) kg of A. vera leaves powder was macerated with 4 L of MeOH–CH2Cl2 (1: 1 v/v) for 48 h. Filtration was carried out using Wattman No. 1 paper (Irshad et al. 2011). The solvents were concentrated at 40 °C by, using a rotary evaporator under reduced pressure to give a crude leaves extract (5.45%) (Emran et al. 2015). The crude extract (81.88 g) of leaves was resuspended in MeOH–CH2Cl2 and then partitioned with hexane (Hex) (1:0 v/v), hexane: acetate (HexAcOEt) (8:2 v/v), hexane: acetate (HexAcOEt) (6:4 v/v), acetate (AcOEt) (1:0 v/v), acetate: methanol (AcOEtMeOH) (8:2 v/v), acetate: methanol (AcOEtMeOH) (7:3 v/v) and methanol (MeOH) (1:0 v/v) respectively (Abdullahi et al. 2014). The resulting solutions were concentrated under reduced pressure to dryness and stored at 4 °C. A small amount were used for bioassay and phytochemical analysis. The obtained crude extract and fractions were diluted with 0.2% dimethyl sulfoxide (DMSO) in M9 buffer (1.5 g KH2PO4, 3 g Na2HPO4, 2.5 g NaCl, 0.5 mL 1 M MgSO4) for C. elegans or RPMI-1640 for O. ochengi at a final concentration of 100 and 50 mg/mL respectively. Solutions were carefully mixed and stored for anthelmintic activity determination against O. ochengi and C. elegans.
Obtaining and culture of Onchocerca ochengi adult worms
The adult worms of O. ochengi were obtaining according to the method described by Ndjonka et al. (2011). Portions of the infected umbilical skin were bought from the slaughterhouse in Ngaoundere and then transported to the laboratory (at the University of Ngaoundere) where the obtaining and the dissection of the nodules took place. The obtaining of worms was carried out under a dissecting microscope (maximum magnification × 50). The obtaining adult worms were washed following the standard procedure. Viable worms were then collected and numbered for anthelmintic assays according to the method described by Borsboom et al. (2003).
Obtaining and culture of Caenorhabditis elegans
The different strains used in this study were obtained from the Caenorhabditis Genetic Center (CGC, Minneapolis, MN, USA). The are: N2 Bristol, called wild type (WT); levamisole-resistant strain CB211 (lev-1(e211) IV), albendazole-resistant strain CB3474 (ben-1 (e1880) III) and ivermectin-resistant strain VC722 (glc-2 (ok1047) I). The culture of C. elegans was carried out on a solid medium NGM (Nematode Growth Medium)—agar as well as in the liquid medium M9. The solid culture medium NGM-Agar was obtained by dissolving in 1000 mL of distilled water 17 g agar, 3 g of NaCl and 2.5 g of casein peptone, then autoclaved. 25 mL of 1 M KH2PO4/K2HPO4; 1 mL of 1 M MgSO4; 1 mL of 1 M CaCl2; 1 mL cholesterol were added before use. This culture was carried out in petri dishes. Petri dishes with solidified Nematode Growth Medium (NGM-Agar) were partially seeded by 600 µL of Escherichia coli OP50 and dried for 1 h under the ventilated hood. A block of NGM containing worms from the old stock was collected with a previously flaming scalpel and the piece was transferred and spilled onto the new growing medium (Brenner 1974). Each petri dish was observed under a microscope to check the viability of the worms and was then surrounded by the parafilm. The petri dishes have been incubated at 20 °C for 48–72 h, to allow the multiplication of worms. The presence of eggs attests when the plate was ready for synchronization (Ndjonka et al. 2011).
Anthelmintic screening assay of extract and fraction on Onchocerca ochengi
Based on Borsboom et al. (2003) protocol, six adult worms of O. ochengi were incubated with increasing concentrations (0–40 µg/mL) of Aloe vera extracts in the RPMI supplemented by 100 IU/mL/100 mg/mL of penicillin/streptomycin. Positive tests were carried out with ivermectin, albendazole and levamisole. The tubes were incubated at 37 °C in a 5% CO2 incubator and mortality was checked using the MTT/formazan test after 48 and 72 h (Ndjonka et al. 2011).
Anthelmintic screening assay of extract and fraction on Caenorhabditis elegans
The eggs obtaining from the same strain after treatment for clorox were poured into new plates containing NGM-agar. After eggs hatching, the synchronized L4 were transferred from the solid medium into 24-well sterile plates containing M9 buffer (each well contains 10 L4 larvae). Increasing concentrations (0–8 × 103 µg/mL) of A. vera leaves were added in each well. The mortality rate of the L4 worms was determined after 48 h or 72 h at 20 °C. Ivermectin, levamisole and albendazole (0–20 µg/mL) were used as positive control and 0.2% DMSO was used as a negative control. Each experiment was repeated three times (Ndjonka et al. 2011).
Worm mortality and LC50 determination by MTT reduction assay
The death of O. ochengi and C. elegans was revealed by the methyl-test thiazolyl-tetrazolium (MTT) after 72 h of incubation with the extracts and fractions. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-dipphenyltetrazolium bromide) was reduced to a dark blue product, formazan, by living worm cells. After 48 and 72 h incubation at 37 °C, the treated worms were removed and washed in fresh PBS (Phosphate Buffered Saline) and M9 Buffer for O. ochengi and C. elegans respectively. O. ochengi were incubated in 0.5 mg/mL MTT solution for 30 min and C. elegans in 5 mg/mL concentrated MTT solution for 3 h incubation (James and Davey 2007). All MTT assays were performed in the dark as the MTT reagent is light sensitive (Loveland et al. 1992).
Phytochemical tests for determination of secondary metabolites
Qualitative analysis
The extract of A. vera was tested for the presence of tannins, flavonoids, polyphenols and saponins according to the standard protocols for detection of the different phytochemicals of plant extracts as described by Priyanka and Alok (2013); and Dharajiya et al. (2017).
Quantitative analysis
The dosage of polyphenols was carried out according to the method described by Wolfe et al. (2003). The Folin-Ciocalteu (FC) method was used to quantify polyphenols present in the different extracts. This method consists of carrying out dilutions with distilled water and gallic acid: 50 µL of the sample at 0.2 mg/mL are mixed with 200 µL of Na2CO3 at 35% (w/w) and 250 µL of the CF reagent diluted to 1/10 (v/v). The mixture is stirred and incubated in the dark at 40 °C for 30 min and the absorption is measured at 765 nm using a spectrophotometer (UV-biowave Cambridge, England). The results were expressed in mg equivalent of gallic acid per gram of dry materials (mg of GAE/g). Polyphenols quantity was determined by calculation from the standard curve of gallic acid titration.
The quantification of the tannins in the leaves extract of Aloe vera was carried out by the method described by Wolfe et al. (2003). 200 µL of the sample were mixed with 35% (w/v) Na2CO3 and 100 µL of FC. The solution was vortexed for one minute, incubated for five minutes and absorption at 640 nm was then measured. The results were expressed in gallic acid equivalent mg per gram of dry materials (GAE/g) (Kumaran and Karunakaran 2006).
The determination of total flavonoids was carried out using the method described by Boizot and Charpentier (2006). 2 mL of the extraction solvent which consists of 1400 µL of methanol 100%, 500 µL of distilled water and 100 µL of acetic acid was added to 0.1 g of each extract to be analyzed. The mixture was filtered using whatman paper in a 25 mL beaker and completed at 10 mL with the extraction solvent. 0.25 mL of this solution was transferred to a 14-tube mL and completed up to 5 mL with the extraction solvent. The analysed solution (Y) is obtained. At 1 mL solution of (Y), 0.2 mL of distilled water and 0.5 mL of AlCl3 were added. The whole mixture was well mixed and incubated at room temperature for 1 min. Blank is made by 1 mL (Y) and 0.5 mL distilled water and rutin at 0.1 mg/mL. Absorbance was then measured at 430 nm. The results were expressed in mg equivalent rutin/gram dry materials by referring to the rutin calibration curve.
DPPH radical-scavenging activity assay
The antiradical free test used was carried out according to the method of Talla et al. (2016) with little modification. Thus, a methanolic solution of 3 mL of DPPH (0.037 mg/mL) was placed in a dry and sterile test tube. Subsequently 100 μL of the extract’s solution were added and the mixture was vigorously stirred for 30 s using the vortex. After incubation for 30 min in the dark and at room temperature, the absorbance was measured at 517 nm against a blank (Athamena et al. 2010). The results were expressed in mg equivalent of ascorbic acid per gram of dry materials (mg of GAE/g). DPPH quantity was determined by calculation from the standard curve of ascorbic acid titration.
Acute toxicity studies of crude extract of Aloe vera in Wistar rats
Wistar rats were maintained at room temperature (22 ± 3 °C) with a relative humidity of 30% and artificial lighting. The five (05) female animals were 8–12 weeks old and had an average weight ranging from 125.3 ± 0.2 to 241.6 ± 0.5 g. The animals were randomly selected, marked and kept in their cages for at least five days prior to oral administration of the single 2000 mg/kg dose of the extract. Administration was by gavage through a gastric tube. The animals are fasted before administration, i.e. they are deprived of food for 1–2 h, but not water, and suspended overnight. After the fasting period, the animals must be weighed before administration of the substance. The fasting body weight of each animal is determined according to the body weight of the substance to be administered. We administered our first dose to the 1st animal followed by observations before administering the dose to the other four animals. The control group received an equal volume of distilled water. The animals were observed individually every 30 min and then 24 h with particular attention during the first 4 h and every day thereafter for 14 days. Symptoms of toxicity were observed and recorded systematically after administration (Nghonjuyi et al. 2016). Acute oral toxicity was performed in accordance with the Organisation for Economic Co-operation and Development (OECD 2001) recommendations and guidelines for chemical testing. The toxicity data used for Wistar rats is presented in Table 4.
Statistical analysis
Data comparison was done using one-way ANOVA followed by multiple tests of comparison of Turkey. The curves and graphs were plotted using Graph Pad prism 5.10. Values of P < 0.05 were considered statistically significant.
Results
Anthelmintic activity of Aloe vera and fractions against Onchocerca ochengi
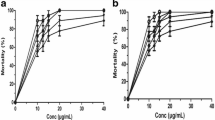
The anthelmintic activity of leaves of A. vera on O. ochengi adult males was evaluated in terms of mortality after 48 and 72 h of incubation. The plant extract induced mortality of O. ochengi adult male in a concentration-dependent manner (Fig. 1). The leaves’ extract killed worms, with LC50 = 20.71 ± 3.14 and 11.75 ± 0.73 µg/mL after 48 and 72 h respectively (Table 1). Positive controls were active against O. ochengi with LC50 of 2.23 ± 1.96 µg/mL, 3.62 ± 1.88 µg/mL, and 4.34 ± 0.71 µg/mL for ivermectin, levamisole and albendazole respectively after 72 h (Table 1).
The HexAcOEt (6: 4 v/v) and AcOEt (1, v/v) fractions were less active on adult worms with LC50 of 50.52 μg/mL for each. As shown in Fig. 2, the highest activity in adult worms was observed with the AcOEtMeOH (8: 2 v/v) and MeOH (100: 0) fractions with LC50 of 15.50 and 12.82 µg/mL respectively.
Anthelmintic activity of Aloe vera and its fractions on Caenorhabditis elegans wild-type and mutants
The anthelmintic activity of A. vera leaves’ extract was assessed in vitro against C. elegans wild-type (WT) and exhibited moderate activity. Figure 3 shows that worm motility decreased with increasing extract concentrations. The lowest concentration of the extract required to cause 50% of mortality (LC50) was 1937 µg/mL after 72 h (Table 2). The anthelmintic activity of ivermectin, levamisole and albendazole on the WT strain displayed LC50 of 2.17, 4.12 and 4.26 µg/mL respectively after 72 h incubation (Table 2). Statistical higher LC50 was observed between the MeOH–CH2Cl2 activity on C. elegans WT strain compared to ivermectin, levamisole and albendazole after 72 h incubation (Table 2) (P < 0.001).
The anthelmintic activity was assessed in vitro against three resistant strains of the free-living nematode namely CB211 resistant to levamisole, CB3474 resistant to albendazole and VC722 resistant to ivermectin. A tenfold increase of the LC50 value of C. elegans albendazole-resistant mutant CB3474, VC722 and CB211, was observed at 1950, 3920 and 4360 µg/mL for 72 h respectively for the extract studied (Table 2).
Table 2 shows that the methanolic-methylene chloride extract of A. vera had activity with LC50 values of (1950 ± 0.45 µg/mL) to albendazole-resistant strain (CB3474), (4360 ± 0.53 µg/mL) to levamisole-resistant strain (CB211) and (3920 ± 0.84 µg/mL) to ivermectin-resistant strain: (VC722) at 5% threshold after 72 h incubation with highly significant difference between the means (P < 0.001). From the comparison between the WT strain and the mutants, the methanolic-methylene chloride extract of A. vera had more effect on the WT strain displaying the lowest lethal concentrations, followed by the albendazole mutant strain.
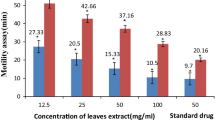
The anthelmintic activity of plant fractions was assessed in vitro against the free-living nematode C. elegans WT and mutant strains. The LC50 value of fraction obtained after 48 h on WT are recorded in Fig. 4. The WT strain was very sensitive to the fractions with LC50 ranging between 250 ± 0.1 and 790 ± 0.1 µg/mL except for Hex and HexAcOEt fraction, for which the WT strain was not very sensitive (LC50 higher than 6180 ± 0.12 µg/mL). It appears from Fig. 4 that the WT strain has been very sensitive to different factions compared to the mutant strains. A tenfold increase of the LC50 value on the three resistant mutants (CB3474, VC722 and CB211) was observed compared to that of the WT (Fig. 4, 5, 6, 7).
The sensitivity to fractions varies with mutant strains. Some mutants were highly sensitive to some fractions and not to others. LC50 values on CB4374 varying between 4590 and 6548 µg/mL, VC722 (4960 and 6565 µg/mL) and CB211 (4590 and 6565 µg/mL) for all fractions (Fig. 5, 6, 7). Figure 5 shows that except for the fraction AcOEtMeOH (LC50 = 6565 µg/mL), all the other fractions have an effect on the mutant and resistant strains of ivermectin VC722 (LC50 = 4960, LC50 = 6180 µg/mL). While for CB3474 strain (Fig. 6), with the exception of the fraction HexAcOEt with LC50 = 6 548 µg/mL, all other fractions were active with LC50 varying between 4960 and 7700 µg/mL. For the levamisole-resistant strain CB211 (Fig. 7), all fractions were active (LC50 = 4590 and 1364 µg/mL) with the exception of the fraction AcOEtMeOH (LC50 = 6565 mg/mL). After comparing the activity of the fractions on the different mutant strains, it came out that fractions which acted on the different mutant strains had a LC50 that varied between 4590 and 6100 µg/mL.
Secondary metabolites of methanolic-methylene chloride extracts and fractions of Aloe vera
The phytochemical composition of methanolic/methylene chloride extract was assessed to evaluate the chemical families present in this plant extract and which might be involved in its anthelmintic activity. Table 3 shows result of the quantification for tannins, polyphenols, flavonoids and saponins contents. It appears that polyphenol and tannins contents are the highest compared to flavonoids and saponins.
It appears that the MeOH–CH2Cl2 extract of A. vera content higher amount/proportion of polyphenols (1015.01 ± 0.05 mg/g), tannins (401.37 ± 0.05 mg/g), flavonoids (25.35 ± 0.01 mg/g) and saponins (1.20 ± 0.05 mg/g).
The phytochemical study of MeOH–CH2Cl2 fractions revealed the presence of unevenly distributed bioactive elements (Table 3). The polyphenol and tannin content of the Hex, AcOEtMeOH and MeOH leaves’ extracts reflects its higher anthelmintic activity (Table 3). Other fractions, although containing these chemical families, appeared to have no anthelmintic activity.
DPPH radical-scavenging activity
The investigation of the antioxidant activity of MeOH–CH2Cl2 leaf extract of A. vera was carried out by the free radical scavenging method DPPH. The more the value of IC50 is low, the more the extract has strong antioxidant activity. The MeOH–CH2Cl2 extract had an IC50 = 15 ± 0.12 µg/mL against standard which was not too far from that of the ascorbic acid used as standard (IC50 = 9 ± 0.22 µg/mL).
Assessment of acute toxicity of methanolic-chloride methylene extract of Aloe vera
In the assessment of acute toxicity, the Wistar rats showed no mortality to the dose up to 2000 mg/kg and no signs of toxicity to oral administration of the methanol- chloride methylene leaf extract of A. vera. The acute toxicity results suggest that this methanol- chloride methylene extract from A. vera leaves is non-toxic to this dose (2000 mg/kg) (Table 4).
Discussion
This study was carried out to assess anthelmintic activities of Aloe vera leaf extract and its fractions on O. ochengi and C. elegans, then to quantitatively assess its phytochemical content and ultimately assay it antioxidant reduction power and its acute toxicity.
The methanolic-methylene chloride extracts of A. vera have shown an anthelmintic activity similar to ivermectin, levamisole and albendazole after 48 h post incubation (P < 0.05). The LC50 values obtained are similar to the finding of Dikti et al. (2017) and Kalmobé et al. (2017) who evaluated the effect of Acacia nilotica on O. ochengi males (11.5 ± 0.1 µg/mL), and leave of Lophira lanceolata on O. ochengi males (11.6 ± 0.4 µg/mL).
These values are higher than that found by Ndjonka et al. (2011) testing the activity of Anogeissus leiocarpus on O. ochengi (90 μg/mL). These LC50 values are also lower than those observed by Cho-Ngwa et al. (2010) during their study that evaluated Margaritaria discoidea and Homalium africanum activities on O. ochengi (LC50 of 31.25 μg/mL). The difference between the LC50 values obtained by certain authors can be explained by the nature of the plants, the places of harvest and without forgetting the solvents used for the extraction. Daoudi et al. (2015) have shown in their work that the solubility of phenolic compounds is affected by the polarity of the solvent used. Ali et al. (2011) who demonstrated in their work that the leaves of L. lanceolata contain tannins, saponins, terpenoids, alkaloids and flavonoids that vary with solvent.
Results of the fractions on O. ochengi are in the same range as those observed with the fractions of Craterispermum laurinum and Morinda lucida on O. ochengi (LC50 ranked from 7.80 to 46.80 μg/mL) (Samje et al. 2014). The study showed a lower range of values of 15.7–55.7 µg/mL as compared to the results of Metuge et al. (2014). These authors tested secondary metabolites from Cyperus articulates on O. ochengi (LC50 of 15.7 μg/mL on males and 55.7 μg/mL on females). Some fractions are more active as compared to the crude extract while others are less active. This may explain the synergistic effect of the crude extract. These results are similar to those observed by Rios and Recio (2005) and Sarker et al. (2005). These authors concluded that the activity of an extract is probably due to the presence of synergy between a number of components, which when separated would become less active in some fractions. The efficacy of our extract is also probably due to a combination/synergy of effects of various compounds present in the extract with potentially different mechanisms of action. Some of these active components in the extract and fractions may act by the same mechanism as synthetic anthelmintic drugs warranting the use of the 3 drug resistant strains of C. elegans.
Our results can then be explained by the partition of the bioactive molecules present in the different fractions. The variability of their results from one strain to another could be due to the modification of the genes of this nematode into several resistant mutants to the conventional drugs. The leaves of A. vera may act on the same receptors as ivermectin. The ivermectin-resistant mutant (VC722) is a single mutant in which the Glucl glc-2 subunit has been mutated. Glc-2 represents the acceptor of ivermectin in pharyngeal muscle cells (Gendrel et al. 2009). The paralysis and death of the nematode are due to the massive entry of chloride ions (ivermectin effects) into the cells (Gendrel et al. 2009). Levamisole has been proven to be a nicotinic receptor agonist responsible for muscle hyper-contraction in nematodes. Death of the worm would result from the prolonged excitation of nicotinic muscle receptors (Dent et al. 2000). The expression of the lev-1 allele allows the regulation of locomotion and egg laying. The lev-9 allele codes for the synthesis of new extracellular proteins. CB211 (lev-1) resistance to levamisole is associated with specific mutations in the lev-1 gene (Culetto et al. 2004).
The activity of certain fractions would be due to the presence of the bioactive molecules quantified (Table 3). Some authors have demonstrated the activity of these compounds on parasites, listed above. Our results are similar to the finding of Mahmoudi et al. (2013), concluding that the solubility of phenolic compounds depends on their chemical nature in the plant, which varies from single to strongly polymerized compounds.
The anthelmintic activity of the extract of the plant studies could be due to their bioactive compounds which could be responsible for the death of the parasite. The compounds in the extract may act in synergy with the other molecules to kill the parasite. Our results are similar to those observed by Arunkumar and Muthuselvam (2009), indicating that A. vera contains flavonoids, tannins and saponins. These results are also in accordance with the findings of several authors like Arunkumar and Muthuselvam (2009), demonstrating that tannins, flavonoids and polyphenols are responsible of antimicrobial, antidiarrhoeal, anthelmintic and anticancer activities. Massamha et al. (2010) have also shown that Aloe species contain tannins, saponins, flavonoids, phenols, alkaloids and anthraquinones. The tannins according to Massamha et al. (2010) would react directly with surface proteins of the worm. They cause physiological dysfunctions with regard to the mobility and the absorption of nutrients, leading to the death of worms. Tannins also interfere with energy production in parasitic helminths by decoupling oxidative phosphorylation (Mali et al. 2007).
Nevertheless, it has been shown that plants with anthelmintic activities contain phytochemicals such as polyphenols, tannins, flavonoids, and saponins (Ndjonka et al. 2013) which may act synergistically to kill worms. The present work confirms that polyphenols and tannins were the major anthelmintic secondary metabolites present in the A. vera leaf extract studied. In our study, the MeOH–CH2Cl2 extract showed the highest anthelmintic activity on both parasite and free-living nematodes. This extract also had the highest polyphenol and tannin content. The anthelmintic activity could therefore be assumed to be related to its richness in polyphenols and tannins. Prashant et al (2011) also supported, by their result that polyphenols and tannins have anthelmintic activities. Prashant et al (2011) have highlighted in their work that the mechanisms of action of these bioactive molecules, for example polyphenols and tannins act on influenza enzyme inhibitors by adhering, depriving them of substrate and complexing with cells resulting in membrane disruption and consequently antimicrobial activity; increasing the supply of digestible proteins by animals allows the formation of protein complexes in the rumen, interfering with energy regeneration by uncoupling oxidative phosphorylation, thus causing a decrease in metabolism; while saponins prevent the release of histamine in vitro, possessing membrane permeable properties, leading to vacuole and integument disintegration. According to Massamha et al (2010), tannins react directly with the surface proteins of the parasite (O. ochengi), leading to physiological dysfunction in the nematodes, such as mobility and nutrient absorption, which causes the worm to die. Tannins also interfere with energy production in parasitic helminths by uncoupling oxidative phosphorylation (Mali 2007).
This may be the result of their lack of solubility in RPMI and M9-Buffer. These results are similar to those of Mahmoudi et al. (2013) who concluded that the solubility of phenolic compounds depends on their chemical nature in the plant, which varies from single to strongly polymerized compounds. The activity of A. vera MeOH–CH2Cl2 extracts and fractions demonstrated on the filarial nematode O. ochengi and C. elegans might be due to the presence of these phytochemical products which might act synergistically. Another possible anthelmintic mechanism of action of tannins is that they can link to glycoproteins on the cuticle of the parasite and can indirectly cause death (Iqbal et al. 2007). Mortality observed may also be the consequence of the presence of polyphenols. Polyphenols such as ellagic acid, gentisic acid and gallic acid have been shown to kill O. ochengi (Ndjonka et al. 2012). It has long been known and demonstrated in various studies that tannins and other polyphenolic compounds are protein coagulants which could result in a broad spectrum worm killing activity (Yin 2010). Iqbal et al. (2007) suggested that condensed tannins may also bind to the cuticle (which is rich in glycoprotein) of larvae and cause death.
It is thus likely that the active compounds in A. vera are predominantly polar. This corroborates previous studies, which showed that polar components are nematocidal (Prashant et al. 2011). This activity will be due to the presence of the bioactive compounds identified from methanol extract of the stem of A. barbadensis by Gas Chromatography–Mass Spectrometry (GC–MS) analysis. GC–MS is the best technique to identify the bioactive constituents of long chain hydrocarbons, alcohols, acids, esters, alkaloids, steroids, amino acids and nitro compounds (Muthulakshmi et al. 2012). But it is demonstrated that these biological activities should be assigned to a synergetic action of the compounds contained therein rather than a single chemical substance (Scalbert and Williamson 2000).
MeOH–CH2Cl2 extract of Aloe vera with good anthelmintic activity has also been shown to have good antioxidant capacity. The high antioxidant capacity has been demonstrated shown to positively affect anthelmintic activity (Pradyuthaand and Maheswara 2022). The antioxidant capacity of plant extracts is influenced by various factors, including their composition (polyphenol content) and the conditions (DPPH essay) under which they are tested (Parejo et al. 2002).
Our toxicity results are similar to those observed by Michayewicz (2013), testing the ethanolic activity of A. vera leaves and obtaining no mortality up to 2000 mg/kg. This result is observed by Saritha and Anilakumar (2010), testing the methanolic leaves extract of A. vera in wistar rats. A multiple oral administration of the extract at a single dose of 2000 mg/kg body weights for 14 days did not produce signs of toxicity, behavioral appearances, or changes in gross appearance. A similar result was observed by Nghonjuyi et al. (2016) when using the leaves of A. vera, the acute toxicity test, none of the four studied hydroalcoholic extracts induced mortality or significant behavioural changes.
Conclusion
The present study assessed the MeOH–CH2Cl2 extracts and fractions of leaves of A. vera for in vitro nematicidal activity by using the cattle parasite nematode O. ochengi and free-living nematode C. elegans as models. The results showed the toxicity of A. vera against O. ochengi and C. elegans. Therefore, these results could be used in the traditional treatment of onchocerciasis and other worm infections. Moreover, A. vera possesses significant anthelmintic potency without noticeable adverse effects in animal experiments. AcOEtMeOH and MeOH extract showed high activities on O. ochengi with LC50 values of 12.82 µg/mL, proof for further studies is required for HPLC or LC–MS analysis, to isolate and characterize the bioactive constituents responsible of its anthelmintic activity.
Data availability
Data of the study are available upon request.
References
Abdullahi MI, Musa AM, Haruna AK et al (2014) Isolation and characterization of an anti-microbial biflavonoid from the chloroform-soluble fraction of methanolic root extract of Ochna schweinfurthiana (Ochnaceae). Afr J Pharm Pharmacol 8:93–99
Ali SA, Sule IM, Ilyas M et al (2011) Antimicrobial studies of aqueous extract of the leaves of Lophira Lanceolata. Res J Pharm, Biol Chem Sci 2:637–643
Arunkumar S, Muthuselvam M (2009) Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci 5:572–576
Athamena SI, Chalghem A, Kassah-Laouar S et al (2010) Activité anti-oxydante et antimicrobienne d’extraits de Cuminum cyminum L. Leban Sci J 11:69–81
Ayimele GA, Yong JN (2015) Natural anti-onchocercals from cameroon medicinal plants. Afr J of Integ Health 5:41–46
Bizimenyera ES, Githiori JB, Eloff JN et al (2006) In vitro activity of Peltophorum africanum Sond. (Fabaceae) extracts on the egg hatching and larval development of the parasitic nematode Trichostrongylus colubriformis. Vet Parasitol 142:336–343
Boatin BA (2008) The onchocerciasis control programme in West Africa (OCP). Ann Trop Med Parasitol 102:13–17. https://doi.org/10.1179/136485908X337427
Boizot N, Charpentier JP (2006) Méthode rapide d’évaluation du contenu en composés phénoliques des organes d’un arbre forestier. Le Cahier des Techniques de l’INRA, numéro spécial, p 79–82
Borsboom GJJJM, Boatin BA, Nagelkerke NJD et al (2003) Impact of ivermectin on onchocerciasis transmission: assessing the empirical evidence that repeated ivermectin mass treatments may lead to elimination/eradication in West-Africa. Filaria J 2:1–25
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Bunyapraphatsara N, Yongchaiyudha S, Chokechaijaroenporn O (1996) Antidiabetic activity of Aloe vera L. juice. II. Clinical trials in diabetes mellitus patients in combination with glibenclamide. Phytomedicine 3:245–248
Burglin TR, Lobos E, Blaxter ML (1998) Caenorhabditis elegans as a model for parasitic nematodes. Int J Parasitol 28:395–411
Chithra P, Sajithlal GB, Chandrakasan G (1998) Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol 59:195–201
Cho-Ngwa F, Abongwa M, Ngemenya MN et al (2010) Selective activity of extracts of Margaritaria discoidea and Homalium africanum on Onchocerca ochengi. BMC Complement Altern Med 10(62):1–7. https://doi.org/10.1186/1472-6882-10-62
Culetto E, Baylis HA, Richmond JE et al (2004) The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J Biol Chem 279:42476–42483
Cupp EW, Sauerbrey M, Richards F (2011) Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop 120:100–108
Daoudi A, Sabiri M, Bammou M et al (2015) Valorisation des extraits de trois espèces du genre Urtica : Urtica urens L., Urtica membranacea Poiret et Urtica pilulifera L. J Appl Biosci 87:8094–8104
Dent JA, Smith MM, Vassilatis DK (2000) The genetics of ivermectin resistance in Caenorhabditis elegans. PrOc Natl Acad Sci 97:2674–2679
Dharajiya D, Pagi N, Jasani H et al (2017) Antimicrobial activity and phytochemical screening of Aloe vera (Aloe barbadensis Miller). Int J Curr Microbiol App Sci 6:2152–2162
Dikti VJ, Kalmobé J, Djafsia B et al (2017) Anti-Onchocerca and anti-Caenorhabditis activity of a hydro-alcoholic extract from the fruits of Acacia nilotica and some proanthocyanidin derivatives. Molecules 22:1–19
Emran TB, Rahman M, Uddin MMN et al (2015) Effects of organic extracts and their different fraction of five Bangladeshi plants on in vitro thrombolysis. BMC Complem Altern M 15:128
Eze J, Matur BM (2011) Assessment of the epidemiology of Onchocerca volvulus after treatment with ivermectin in the federal capital territory, Abuja, Nigeria. Int J Recent Res Appl Stud 7:319–332
Gendrel M, Rapti G, Richmond JE et al (2009) A secreted complement-control-related protein ensures acetylcholine receptor clustering. Nature 461:92–996
Gonzalez RJ, Cruz-Ortiz N, Rizo N et al (2009) Successful interruption of transmission of Onchocerca volvulus in the Escuintla-guatemala focus. Guatemala PLOS Negl Trop Dis 3:1–7
Iqbal Z, Sarwar M, Jabbar A et al (2007) Direct and indirect anthelmintic effects of condensed tannins in sheep. Vet Parasitol 144:125–131
Irshad S, Butt M, Younus H (2011) In-vitro antibacterial activity of Aloe Barbadensis Miller (Aloe Vera). Intl r J of Pharmaceuticals 01:59–64
James CE, Davey MW (2007) A rapid colorimetric assay for the quantitation of the viability of free-living larvae of nematodes in vitro. Parasitol Res 101:975–980
Kalmobé J, Ndjonka D, Boursou D et al (2017) Phytochemical analysis and in vitro anthelmintic activity of Lophira lanceolate (Onchnaceae) on the bovine parasite Onchocerca ochengi on drug resistant strains of the free living nematode Caenorhabditis elegans. BioMed Cent Complement Altern Med 17:1–12. https://doi.org/10.1186/s12906-017-1904-z
Kampkötter A, Nkwonkam CG, Zurawski RF et al (2007) Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology 5:113–123
Katabarwa MN, Eyamba A, Nwane P et al (2013) Seventeen years of annual distribution of ivermectin has not interrupted onchocerciasis transmission in the north region, Cameroon. Am J Trop Med Hyg 58:1041–1049. https://doi.org/10.1155/2013/420928
Koo MWL (1994) Aloe vera, antiulcer and antidiabetic effects. Phytother Res 8:461–464
Kumaran A, Karunakaran RJ (2006) Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem 97:109–114
Loveland BE, Johns TG, Mackay IR et al (1992) Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem Int 27:501–510
Mahmoudi S, Khali M, Mahmoudi N (2013) Etude de l’extraction des composés phénoliques de différentes parties de la fleur d’artichaut (Cynara scolymus L.). Nature Technol 09:35–40
Mali RG, Mahajan SG, Mehta AA (2007) In vivo anthelmintic activity of stem bark of Mimusops elengi Linn. Phcog Mag 3:73–76
Manchang TK, Ajonina-Ekoti I, Ndjonka D (2014) Immune recognition of Onchocerca volvulus proteins in the human host and animal models of onchocerciasis. J Helminthol 89:1–12. https://doi.org/10.1017/S0022149X14000224
Massamha B, Gadzirayi CT, Mukutirwa I (2010) Efficacy of Allium sativum (Garlic) controlling nematode parasites in sheep. Intern J Appel Res Vet Med 8:161–169
Metuge JA, Babiaka SB, Mbah JA et al (2014) Anti-Onchocerca metabolites from Cyperus articulatus: isolation, in vitro activity and in silico ‘drug-likeness.’ Nat. Prod. Bioprospect 4:243–249. https://doi.org/10.1007/s13659-014-0023-5
Michayewicz N (2013) L’Aloe vera, plante médicinale traditionnellement et largement utilisée depuis des millénaires, aux nombreuses propriétés thérapeutiques. Plante miracle?,” Thèse présentée et soutenue publiquement le 29 octobre pour obtenir le diplôme d'état de docteur en pharmacie, p151
Muthulakshmi A, Margret JR, Mohan VR (2012) GC-MS analysis of bioactive components of Feronia elephantum correa (Rutaceae). J App Pharm Sci 2:69–74
Ndjonka D, Agyare C, Lüersen K et al (2011) In vitro activity of Cameroonian and Ghanian medicinal plants on parasitic (Onchocerca ochengi) and living free-living (Caenorhabditis elegans) nematodes. J Helminthol 10:6–9
Ndjonka D, Jonina-Ekoti I, Djafsia B et al (2012) Anogeissus leiocarpus extract on the parasite nematode Onchocerca ochengi and on drug resistant mutant strains of the free-living nematode Caenorhabditis elegans. Vet Parasitol 190:136–142. https://doi.org/10.1017/S0022149X1300045X
Ndjonka D, Rapado LN, Silber AM et al (2013) Natural Products as a source for treating neglected parasitic diseases. Int J Mol Sci 14:3395–3439. https://doi.org/10.3390/ijms14023395
Nghonjuyi NW, Tiambo CK, Taïwe GS et al (2016) Acute and sub-chronic toxicity studies of three plants used in Cameroonian ethnoveterinary medicine: Aloe vera (L.) Burm. f. (Xanthorrhoeaceae) leaves, Carica papaya L. (Caricaceae) seeds or leaves, and Mimosa pudica L. (Fabaceae) leaves in Kabir chicks. J Ethnopharmacol 178:40–49
Nisha M, Kalyanasundaram M, Paily KP et al (2007) In vitro screening of medicinal plant extracts for macrofilaricidal activity. Parasitol Res 100:575–579. https://doi.org/10.1007/s00436-006-0294-9
Okyar A, Can A, Akev N et al (2001) Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother Res 15:157–161
Organisation for Economic Co-operation and Development (OECD) (2001) Guidelines for the testing of chemicals, no. 423, OECD
Osei-Tweneboana MY, Eng JKL, Boakye DA et al (2007) Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet 369:2021–2029
Parejo I, Viladomat F, Bastida J et al (2002) Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. J Agric Food Chem 50:6882–6890
Pradyutha AC, Maheswara VUR (2022) Antioxidant potential, anthelmintic activity and quantification of total phenols, tannins, flavonoids of E. milii leaf and flower extracts. Asian J Biol Life Sci 11(3):705
Prashant T, Bimlesh K, Mandeep K et al (2011) Phytochemical screening and extraction: a review. Int Pharm Sci 1:1–9
Priyanka D, Alok KS (2013) Phytochemical extraction and characterization of the leaves of Aloe vera barbadensis for its anti-bacterial and anti-oxidant activity. Int J Sci Res 6:658–661
Rajesh KS (2015) The effect of Aloe Vera juice to control the physical, chemical and biological parameters of soils and protect the life of earthworm. Int J Environ Sci Technol 3:60–66
Rios JL, Recio MC (2005) Medicinal plants and antimicrobial activity. J Ethnopharmacol 100:80–84
Saito H (1993) Purification of active substances of Aloe arborescens Miller and their biological and pharmacological activity. Phytother Res 7:14–19
Samje M, Metuge J, Mbah J et al (2014) In vitro anti-Onchocerca ochengi activities of extracts and chromatographic fractions of Craterispermum laurinum and Morinda lucida. BMC Complement Altern Med 14:1–12. https://doi.org/10.1186/1472-6882-14-325
Sarin YK (1996) Illustrated manual of herbal drug used in ayurveda, New Delhi, joint of publication of C.S.I.R. and I.C.M.R
Saritha V, Anilakumar KR (2010) Toxicological evaluation of methanol extract of Aloe vera in rats. Int J Pharm Biomed Res 1:142–149
Sarker SD, Latif Z, Gray AI (2005) Natural product isolation. In: Sarker SD, Latif Z, Gray AI (eds) Natural products isolation. Humana Press, Totowa, p 1–23
Scalbert A, Williamson D (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073–2085
Talla E, Nyemb JN, Tiabou TA et al (2016) Antioxidant activity and a new ursane-type triterpene from Vitellaria paradoxa (Sapotaceae) stem barks. Eur J Med Plant 16:16–20
Tanya VN, Wandji S, Kamgno J et al (2013) Recent advances in onchocerciasis research and implication for control. The Cameroon Academy of Sciences. Yaounde/Cameroon. Numéro 9956-26-38-x, p 91
Toé LD, Koala L, Burkett-Cadena ND et al (2014) Optimization of the Esperanza window trap for the collection of the African onchocerciasis vector Simulium damnosum sensu lato. Acta Trop 137:9–43. https://doi.org/10.1016/j.actatropica.2014.04.029
Trees AJ, Graham SP, Renz A et al (2000) Onchocerca ochengi infections in cattle as a model for human onchocerciasis: recent developments. Parasitology 120:133–142
Wahl G, Ekale D, Enyong P, Renz A (1991) The development of Onchocerca dukei and O. ochengi microfilariae to infective-stage larvae in Simulium damnosum s.l. and in members of the S. medusaeforme group, following intra-thoracic injection. Ann Trop Med Parasit 85:329–337
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51:609–614
Yin CY (2010) Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem 45:1437–1444
Acknowledgements
All equipments used in this study were kindly donated to Prof. Dr. D. Ndjonka by the Alexander von Humboldt Foundation (AvH). This donation is gratefully acknowledged. The authors would like to thank the laboratory of the Institute for Research in Agriculture for Development (IRAD, WAKWA) of Ngaoundere and the Laboratory of Applied Zoology of the University of Ngaoundere-Cameroon where this study was carried out.
Funding
The authors declare that they have not received funding.
Author information
Authors and Affiliations
Contributions
Justin Kalmobe and Dieudonne Ndjonka designed and conducted the study. Justin Kalmobe, Dieudonne Ndjonka and Simeon Fogue Kouam conducted the fractionation of the extracts. Justin Kalmobe, Jacqueline Dikti Vildina, Boursou Djafsia, Honore Ndouwe Tissebe Menga and Dieudonne Ndjonka performed the statistical analysis and drafted the manuscript. All authors contributed substantially to the manuscript and approved its final version. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests in this study.
Ethics approval and consent to participate
This work was carried out in accordance with the Animal Ethical Committee of the Regional Delegation of Livestock; Fisheries and animal Industries Authority, Cameroon (number 075/16/ L/RA/DREPIA).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalmobe, J., Vildina, J.D., Boursou, D. et al. Anthelmintic activity of crude and separated extract of Aloe vera (Xanthorrhoeaceae) against bovine adults parasites of Onchocerca ochengi and infected larvae of drug resistant strains of the free-living nematode Caenorhabditis elegans. J Parasit Dis (2024). https://doi.org/10.1007/s12639-024-01701-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12639-024-01701-2