Abstract
Leishmaniasis is one of the major parasitic diseases, caused by obligate intracellular protozoa Leishmania, having high mortality as well as morbidity rate. As there is no human licensed vaccine available against leishmaniasis, chemotherapy remains the major way of combating this disease. Many disadvantages are known to be associated with the current drug regime including severe side effects and toxicity, long duration and expensive treatment, and the emergence of resistance. An alternative approach is being utilized to search for active molecules using natural sources, rather than relying on synthetic drugs. Many plant-derived secondary metabolites like phenolic compounds, steroids, quinones, etc. are being extensively investigated for their anti-leishmanial potential. One such group of complex phenolic compounds are diarylheptanoids. These compounds have been shown to exhibit anti-inflammatory, anti-parasitic, anti-fungal, and other pharmacological activities. In the present study, a set of sixteen tetrahydropyran derivatives including three natural products were obtained in lyophilized form. These compounds with trans-2,6-disubstituted tetrahydropyrans, Diospongin A, Diospongin B (isolated from Dioscorea spongiosa) and Centrolobine (Centrolobium sclerophyllum) as parent compounds were synthesized by the reaction of 1-phenyl-1-triemthylsiloxyethylene with six-membered cyclic hemiacetals in the presence of iodine as a catalyst. All the sixteen synthesized tetrahydropyran derivatives were used for toxicity analysis against L. donovani promastigotes, amastigotes and THP-1-derived human macrophages. IC50 values and selectivity index were calculated for all the compounds. Out of these sixteen, five compounds showed the best effect in vitro in terms of both leishmanicidal activity and non-toxicity to human macrophages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is one of the many neglected tropical diseases with high rates of mortality and morbidity. It is caused by an obligate intracellular protozoan Leishmania, belonging to the family Trypanosomatidae. Transmission of this disease occurs by the bite of the infected female phlebotomine sandflies belonging to the genera Phlebotomus (old world) and Lutzomyia (new world). Different species of Leishmania are the causative agents of the four major clinical forms of the disease namely: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), visceral leishmaniasis (VL; renowned in the Indian sub-continent as kala-azar) and post kala-azar dermal leishmaniasis (PKDL), with VL being the most fatal form if left untreated (Alvar et. al. 2012).
Chemotherapeutic agents remain the major means to combat leishmaniasis because of the unavailability of any human licensed vaccines against this disease (Mohapatra 2014). The first line of drugs recommended against Leishmania are pentavalent antimonials like sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime) but these are now being used restrictively due to the emergence of resistance against them and their severe side effects (Burza et al., 2018). This has led to the administration of a second line of drugs. Amphotericin B or its lipid formulations had been recommended and were found to be effective but due to toxicity and expensive costs, its use in developing countries is restricted. Miltefosine had been registered as the first orally administered drug against leishmaniasis in India in 2002 and has been found to be highly effective with ⁓98% cure rate but was found to be teratogenic, thus preventing its administration in pregnant women (Maltezou 2010; DNDi 2016). Also, long half-life of miltefosine (150 h) along with long administration duration might result in the emergence of resistance against this drug. Paromomycin which is an aminoglycoside antibiotic has also shown promising anti-leishmanial activity, however in vitro studies have already reported emergence of resistance against this drug along with side effects (Jhingran et al. 2009; den Boer and Davidson, 2006, DNDi 2016).
The above-mentioned factors, namely, increase in the drug resistance, severe toxic side effects, high cost making their use in resource-limited countries restrictive, and the emergence of Leishmania coinfections, have led to the search for identification of new drugs and drug targets to eliminate the pathogen (Alvar et al. 2008).
An alternative approach to the search for active molecules apart from synthetic drugs is by using natural sources. Plants have been since long considered a major source of biologically active extracts, essential oils and isolated substances, active against various diseases like microbial and protozoal infections, carcinomas, diabetes, and inflammatory reactions (Harvey 2008). In many resource-limited countries traditional plant-based medicines against parasitic diseases are gaining preference over synthetic drugs due to their accessible nature (Calla-Magarinos et al. 2009).
Plant based products are currently gaining more attention because of their ability to act directly against the pathogens along with acting as immunomodulators (Cragg and Newman 2013). A few examples of the active agent obtained from medicinal plants which are being utilized as chemotheurapetic agents are artemisinin (isolated from Artemisia annua) being used for the treatment of malaria, camptothecin (isolated from Camptotheca acuminata), vinblastine and vincristine (isolated from Catharanthus roseus) and paclitaxel (isolated from Taxus brevifolia) being used against cancer.
Many plant-derived secondary metabolites like phenolic compounds, coumarins, alkaloids, etc. are being extensively investigated for their anti-leishmanial potential (Rodrigues et al. 2015; Goncalves de Olievera et al. 2017). Out of these, few alkaloids which have been extracted from various plant species have managed to show significant leishmanicidal activity in vitro conditions. Indole alkaloids (corinanteine, dihydrocorinanteine and corinanteidine), which were isolated from Corynanthe pachyceras, were found to be active against L. major with an IC50 of 30 μM. Coronaridine isolated from Peschirea australis, had an IC50 of 12 μg/ml against L. amazonensis, while other indole alkaloids, like harmane, pleiocarpin and buchtienin, which in turn were obtained from the bark and leaves of Kopsia griffithii, were found to be active against the promastigote form of L. donovani, with an IC50 of 6.25 μg/ml, 25.00 μg/ml and 1.56 μg/ml, respectively (Mishra et al. 2009; Polonio and Efferth 2008; Singh et al. 2014; Delorenzi et al. 2001). The steroidal saponin Racemoside A which was isolated from Asparagus racemosus was found to induce apoptosis in L. donovani promastigotes and amastigotes with IC50 values of 1.31 μg/ml and 0.61 μg/ml, respectively (Polonio and Efferth 2008).
One such group of complex phenolic compounds, known to possess a myriad of pharmacological activities, and are isolated from different parts of plants belonging to Myricaceae, Betulaceae, Zingiberaceae, Aceraceae, Leguminosae and Burseraceae are diarylheptanoids (Per et al. 2002; Kawai et al. 2008; Ibrahim et al. 2017). Their structure consists of two aromatic rings conjugated with seven carbon chains (Brand et al. 2006; Amalraj et al. 2017). Diarylheptanoids isolated from Alnus glutinosa were found to protect non-cancerous dividing cells during cancer treatment (Dinić et al. 2015).
The present study deals with the in vitro screening of a set of sixteen natural product-based compounds for leishmanicidal activity. Both L. donovani log-phase promastigotes and amastigotes were cultured in the presence of these compounds to check the effect on their viability. THP-1-derived human macrophages were also cultured with these compounds to determine the toxic effect on the survival of the macrophages. Selectivity index was also calculated for all sixteen inhibitors. Out of sixteen compounds, five compounds showed the best results on the basis of inhibitory effects on L. donovani promastigotes and intracellular amastigotes and survival of THP-1-derived macrophages.
Materials and methods
Strains and culture conditions
L. donovani Bob (LdBob; a derivative of the strain MHOM/SD/62/1SCL2D) promastigotes, originally obtained from Stephen Beverley (Washington University, St. Louis, MO) were cultured at 22 °C in M199 medium (Sigma) and were supplemented with 100 μg/ml of streptomycin, 100 units/ml of penicillin (Sigma-Aldrich, USA) and 10% heat-inactivated fetal bovine serum (FBS; Biowest, UK).
THP-1 cells (Tohuku Hospital Pediatrics-1; acute monocytic leukemia-derived human cell line; 202 TIB) are a human monocytic cell line obtained from ATCC (Rockville, MD). These cells were cultured in RPMI-1640 medium (Sigma-Aldrich, USA) which was also supplemented with 10% heat-inactivated FBS (Biowest, UK), 100 μg/ml of streptomycin and 100 units/ml of penicillin (Sigma-Aldrich, USA), and in turn maintained at 37 °C with 5% CO2.
Drugs
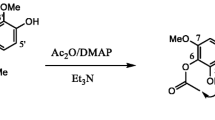
A set of sixteen compounds (tetrahydropyran derivatives) were obtained from Dr. Debendra K. Mohapatra (CSIR-Indian Institute of Chemical Technology, Hyderabad) in lyophilized form. The compounds were reconstituted by dissolving in dimethylsulfoxide (DMSO, HiMedia) to get the desired concentration (Table 1). Diospongin A, Diospongin B, Centrolobine are naturally isolated from Dioscorea spongiosa and Centrolobium sclerophyllum, respectively. These three natural compounds served as parent compounds for the synthesis of the tested tetrahydropyrans.
An effective synthetic protocol for the synthesis of trans-2,6-disubstituted tetrahydropyrans was developed by following Mukaiyama type aldol reaction through C–C bond formation of a cyclic hemiacetal with trimethyl(1-phenylvinyloxy)silane as a nucleophile via the formation of an oxocarbenium ion intermediate using molecular iodine (Table 2). The protocol offers an efficient, protective group tolerance and practical approach to 2,6-trans-disubstituted tetrahydropyrans with good diastereoselectivity. The effectiveness and practicality of the method used was successfully exhibited by the production of diospongin A and B in good yields (Bharath et al. 2019). Centrolobine was also synthesized by following the above protocol.
All sixteen synthesized tetrahydropyrans were tested for toxicity analysis against L. donovani promastigotes, intracellular amastigotes and THP-1-derived human macrophages.
In vitro inhibitor susceptibility assay using L. donovani promastigotes
In order to establish the susceptibility profile of wild-type promastigotes of L. donovani to the above-mentioned inhibitors, MTT assay[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma] was performed as described previously (Mosmann1983). Log-phase promastigotes were seeded in a 96-well flat-bottom plate (Nunc) at the cell density of 5 × 104 cells/well, and were in turn, incubated with increasing inhibitor concentrations (5, 10, 20, 40, 80, 160 μg/ml) at 22 °C. Amphotericin B was added as a positive control at the concentration of 0.5 μg/ml, to the promastigotes. After 48 h of incubation, 20 μl of MTT solution (Stock solution—5 mg/ml) was added to each well, at a final concentration of 0.5 mg/ml. This was followed by incubation of plates at 37 °C for 4 h. The reaction was finally stopped by adding 50 μl of stop solution, which consisted of 50% isopropanol and 20% SDS. This was followed by incubation at 37 °C with gentle shaking for 30 min to 1 h. The absorbance at 570 nm was then, measured in a SpectraMax M2 microplate reader (Molecular Devices). All the experiments were performed in triplicates and the results were expressed as mean ± standard deviation (SD). IC50 values were calculated using GraphPad Prism Version 5 software.
In vitro inhibitor susceptibility assay using THP-1 derived human macrophages
In order to establish the susceptibility profile of THP-1 derived human macrophages to the above-mentioned inhibitors, MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma] was performed as described previously (Mosmann,1983). THP-1 cells (plated at cell density of 6 × 103 cells/well) were first treated with phorbol-12-myristate-13-acetate (PMA) (Sigma-Aldrich, USA) at the concentration of 50 ng/ml for 48 h to induce their differentiation into macrophage-like cells prior to initiation of drug treatment. These cells were seeded in RPMI-1640 medium containing 10% FBS in a 96-well flat-bottom plate (Nunc). PMA treated cells were then incubated with different inhibitor concentrations (5, 10, 20, 40, 80, 160 μg/ml) at 37 °C in a humidified CO2 incubator for 48 h. MTT solution was prepared in RPMI-1640 medium (5 mg/ml MTT stock solution diluted appropriately in RPMI-1640 medium to achieve the final dilution of 0.5 mg/ml). After incubation of 48 h, 50 μl of MTT solution was further added to each well. This was followed by incubation of the plates at 37 °C for 2 h. The reaction was finally terminated by adding 100 μl of stop solution (5% formic acid in isopropanol). This was followed by incubation at 37 °C with gentle shaking for 30 min. The absorbance at 570 nm was then measured in a SpectraMax M2 microplate reader (Molecular Devices). All the experiments were performed in triplicates and the results have been expressed as mean ± standard deviation (SD). IC50 values were calculated using GraphPad Prism Version 5 software.
In vitro inhibitor susceptibility assay using L. donovani amastigotes in infected macrophage model
The susceptibility of wild-type L. donovani amastigotes to the compounds was ascertained by microscopic visualization of infected THP-1 derived human macrophages at 48 h after treating these cells with increasing concentrations of the inhibitors and subsequent calculation of the intracellular parasite load by Giemsa staining. THP-1 cells (plated at cell density of 106 cells/ml) were first treated with phorbol-12-myristate-13-acetate (PMA) (Sigma-Aldrich, USA) at the concentration of 50 ng/ml for 48 h to induce their differentiation into macrophage-like cells prior to initiation of drug treatment. These cells were grown on cover-slips in 6-well plates in RPMI-1640 medium containing 10% FBS. The matured adherent cells were then infected with late log-phase L. donovani promastigotes at a MOI (multiplicity of infection) of 20:1 for 4 h. All the excess non-adherent promastigotes were removed by washing the cells with phosphate-buffered saline (PBS)for 30 s. These cells were subsequently maintained in RPMI-1640 medium containing 10% FBS with 5% CO2 at the temperature of 37 °C and then incubated with different concentrations of the inhibitors. After 48 h, the cells were first washed with PBS and then fixed with 100% methanol (Merck) for 15 min. To visualize the intracellular parasite load Geimsa staining was performed. The experiments were performed in triplicates and the results have been expressed as mean ± standard deviation (SD). IC50 values were calculated using GraphPad Prism Version 5 software.
Results
Cytotoxicity analysis of inhibitors against L. donovani promastigotes
L. donovani log phase promastigotes were incubated in the presence of increasing concentrations of these sixteen compounds to test their efficacy. Their growth was assessed by means of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Most of the compounds exhibited inhibition of the growth of promastigotes in a dose-dependent manner (Figs. 1, 2). The effective concentration, which resulted in 50% inhibition of growth (IC50) after 48 h of drug treatment, was found to be as follows,: DKM-B-BRAT—3.689 μg/ml; DKM-B-VIAT—9.887 μg/ml; DKM-B-DIOB—40.16 μg/ml; DKM-B-INACT—147.8 μg/ml; DKM-B-ANAT—6.603 μg/ml; DKM-B-CYL—11.18 μg/ml; DKM-B-DIOA—9.141 μg/ml; DKM-B-EPI—1.558 μg/ml; DKM-B-CENT—1.348 μg/ml; DKM-B-LCAT—78.07 μg/ml; DKM-B-RBAT—12.10 μg/ml; DKM-B-PHAT—14.93 μg/ml; DKM-B-IVAT—13.13 μg/ml; DKM-B-PHOBN—6.388 μg/ml; DKM-B-SBAT—15.21 μg/ml; DKM-B-TOBN—296 μg/ml.
Cytotoxicity analysis of inhibitors against L. donovani amastigotes in infected macrophage model
The sensitivity of amastigotes against the inhibitors was also tested by the means of Giemsa staining in the intracellular amastigote-macrophage model. All the compounds were found to affect the growth of L. donovani amastigotes in a dose-dependent manner (Figs. 3, 4). The effective concentration which resulted in 50% inhibition of growth (IC50) after 48 h of drug treatment was found to be as follows: DKM-B-BRAT—7.739 μg/ml; DKM-B-VIAT—50.01 μg/ml; DKM-B-DIOB—35.06 μg/ml; DKM-B-INACT—191.7 μg/ml; DKM-B-ANAT—14.37 μg/ml; DKM-B-CYL—36.45 μg/ml; DKM-B-DIOA—21.37 μg/ml; DKM-B-EPI—2.250 μg/ml; DKM-B-CENT—1.466 μg/ml; DKM-B-LCAT—148.6 μg/ml; DKM-B-RBAT—10.64 μg/ml; DKM-B-PHAT—40.53 μg/ml; DKM-B-IVAT—28.64 μg/ml; DKM-B-PHOBN—26.49 μg/ml; DKM-B-SBAT—38.3 μg/ml; DKM-B-TOBN—130.4 μg/ml.
Cytotoxicity analysis of inhibitors against THP-1 derived human macrophages
To test the toxicity of these sixteen compounds on human macrophages, THP-1 derived macrophages were incubated with increasing concentrations of inhibitors. Their survival was estimated by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. All the compounds were found to affect the growth of THP-1-derived human macrophages at high concentrations (Figs. 5, 6). The effective concentration which resulted in 50% inhibition of growth (IC50) after 48 h of drug treatment was found to be as follows: DKM-B-BRAT—34 μg/ml; DKM-B-VIAT—61.10 μg/ml; DKM-B-DIOB—59.47 μg/ml; DKM-B-INACT—149.3 μg/ml; DKM-B-ANAT—42.17 μg/ml; DKM-B-CYL—269.9 μg/ml; DKM-B-DIOA—34.71 μg/ml; DKM-B-EPI—5.865 μg/ml; DKM-B-CENT—1.965 μg/ml; DKM-B-LCAT—166.78 μg/ml; DKM-B-RBAT—42.52 μg/ml; DKM-B-PHAT—41.15 μg/ml; DKM-B-IVAT—48.01 μg/ml; DKM-B-PHOBN—111.9 μg/ml; DKM-B-SBAT—91.40 μg/ml; DKM-B-TOBN—135.6 μg/ml.
Discussion
Recently, there has been a renewed interest in traditional plant-based medicines. Current studies are being undertaken for the development of safe and cheaper therapies using medicinal plants for the treatment of infectious and non-infectious diseases. As discussed before, since chemotherapeutics still remain the first choice to treat leishmaniasis, the search for finding novel drugs against this disease remains continuously on. The development of natural products-based chemotherapeutics against leishmaniasis will provide us with a new class of safer, less expensive molecules for the already rapidly exhausting arsenal of anti-leishmanial drugs. WHO is also in support of the development and use of plant extracts as potential leishmanicidal agents in view of increasing cases of resistance against current chemotherapeutic drugs (WHO technical reports 2010). Many plant-based compounds are already being studied for their anti-leishmanial activity. Diospyrin, isolated from Euclea natalensis, is a specific inhibitor of the topoisomerase of parasites, and was found to inhibit L. donovani promastigotes at an IC50 of 0.1 μg/ml (Ray et al. 1998; Lall et al. 2001). Essential oils obtained from Chenopodium ambroisoides showed IC50 values of 3.7 μg/ml and 4.6 μg/ml against promastigotes and amastigotes of L. amazonensis respectively (Monzote et al. 2021). 6,7-dihydroneridienone, a sterol derivative, isolated from a Mexican plant, Pentalinon andrieuxii, displayed high leishmanicidal activity with an IC50 values of 0.03 μM against L. mexicana, with negligible cytotoxicity on healthy murine bone marrow macrophages (Cheuka et al. 2017).
The present study shows the results of the in vitro screening of a set of sixteen natural product-based compounds for leishmanicidal activity. The sixteen tetrahydropyrans were synthesized using centrolobine and diaspongin A and B as parent compounds. Both centrolobine and diaspongin A and B belong to the class of diarylheptanoids. Diarylheptanoids are a class of plant secondary metabolites isolated from various plant sources. These phenolic compounds consist of two phenolic aromatic rings which are linked by a chain of seven carbons. Several studies have shown various health benefits of diarylheptanoids. Curcumin is a known diarylheptanoid compound used as a nutraceutical, and has been studied extensively for its role in protection against many diseases (Kunnumakkara et al. 2017). The compound des-O-methylcentrolobine, a phenolic diarylheptanoid derived from Centrolobium sclerophyllum, was found to be highly effective against L. amazonesis promastigotes (Araujo et al. 1998, 1999). Other diarylheptanoids isolated from Diascorea spongiosa, Diospongin B and C have been shown to have anti-osteoporotic activity (Yin et al. 2004). These compounds are able to exhibit a wide spectrum of pharmacological activities as described above and hence, can be used as an alternative source for the development of therapeutics (Ganapathy et al. 2019; Jeong et al. 2010; Beniddir et al. 2012; Lee et al. 2009; Tezuka et al. 2000).
All the sixteen tetrahydropyran inhibitors were incubated with both L. donovani promastigotes and amastigotes (infected macrophage model) and the effect of the extracts on the survival of the parasite was studied. Moreover, the toxicity of the extracts on THP-1-derived human macrophages was also studied. For each case, IC50 values and selectivity index were also calculated for all the sixteen inhibitors (Table 3).
Cytotoxicity analysis of inhibitors against L. donovani promastigotes revealed that at higher concentrations all the compounds except three, DKM-B-LCAT, DKM-B-INACT and DKM-B-TOBN (IC50–78.07 μg/ml,147.8 μg/ml and 296 μg/ml respectively), were lethal for the promastigotes. All the other compounds showed toxicity even at lower concentrations. DKM-B-BRAT, DKM-B-ANAT, DKM-B-CYL, DKM-B-RBAT and DKM-B-PHOBN (IC50—3.689 μg/ml, 6.603 μg/ml, 11.18 μg/ml, 12.10 μg/ml and 6.388 μg/ml respectively) showed 80% inhibition of the promastigotes at lower concentrations between 10 and 20 μg/ml.
Similarly, cytotoxicity analysis of these inhibitors against L. donovani amastigotes in infected macrophage model revealed that, all the compounds at higher concentrations were lethal for the amastigotes in the infected macrophage model. DKM-B-BRAT, DKM-B-ANAT, DKM-B-RBAT and DKM-B-PHOBN (IC50—7.739 μg/ml, 14.37 μg/ml, 10.64 μg/ml and 26.49 μg/ml respectively) showed 80% inhibition of the amastigotes at concentrations between 20 and 60 μg/ml.
Cytotoxicity analysis of these inhibitors against THP-1 derived human macrophages revealed that most of the compounds were in fact lethal for human macrophages at higher concentrations (80–160 μg/ml). Two compounds, DKM-B-EPI and DKM-B-CENT, had lethal effect on THP-1 derived human macrophages at very low concentrations (2–5 μg/ml) deeming them unfit for consideration even though they had lethal effect on L. donovani promastigotes at very low concentrations (Table 3). Out of the sixteen, only six, DKM-B-INACT, DKM-B-CYL, DKM-B-LCAT, DKM-B-PHOBN, DKM-B-SBAT and DKM-B-TOBN showed an IC50 of ≥ 100 μg/mL (Table 3) for THP-1 derived human macrophages, indicating low toxicity towards human macrophages.
The selectivity index can be defined as the ratio of 50% cytotoxic concentration of the drug against macrophages to the 50% inhibitory concentration of the drug against Leishmania spp. amastigotes (CC50/IC50). A compound with an SI value greater than 1 is considered to be more selective against Leishmania spp. parasites and is regarded as a promising potential agent in the treatment of leishmaniasis (Koutsoni et al. 2019). Out of sixteen inhibitors, only one, DKM-B-INACT, displayed a selectivity index less than one (0.77) rest all had a selectivity index greater or equal to one (Table 3). DKM-B-CYL had the highest selectivity index of 7.40, with a high IC50 value for THP-1 macrophages (269.9 μg/ml) and low IC50 values for both promastigotes and amastigotes (11.18 μg/ml and 36.45 μg/ml respectively), indicating comparatively higher selectivity of the drug towards the parasite than the host cells. DKM-B-BRAT, also showed a high selectivity index of 4.39, with low IC50 values for both promastigotes and amastigotes (3.689 μg/ml and 7.739 μg/ml respectively) but a comparatively higher IC50 value for THP-1 macrophages (34 μg/ml). A similar pattern was also observed in the case of DKM-B-PHOBN, DKM-B-RBAT, and DKM-B-ANAT, while the rest of the compounds had comparatively lower values of selectivity index (Table 3).
Conclusion
From the present study, it can be concluded that out of the given set of sixteen compounds, five compounds, DKM-B-CYL, DKM-B-PHOBN, DKM-B-BRAT, DKM-B-RBAT and DKM-B-ANAT showed the best effect in vitro in terms of both leishmanicidal activity and human macrophage survival (Table 3). All these compounds also had a high selectivity index (Table 3), indicating higher selectivity of the compounds towards Leishmania amastigotes than the human macrophage cells. Further studies will be required to decipher the exact modus operandi of these inhibitors in Leishmania, followed by checking their efficacy in vivo in a murine model. If required, further development of better compounds, in terms of leishmanicidal activity and human macrophage survival, using these compounds as scaffolds can be done, as there is a desperate need for the discovery of new lead compounds, which can be used to combat leishmaniasis, amidst the fear of emerging resistance against the existing drug arsenal.
Abbreviations
- CL:
-
Cutaneous leishmaniasis
- VL:
-
Visceral leishmaniasis
- MCL:
-
Mucocutaneous leishmaniasis
- PKDL:
-
Post kala-azar dermal leishmaniasis
- L. donovani :
-
Leishmania donovani
- L. major :
-
Leishmania major
- L. amazonensis :
-
Leishmania amazonensis
- THP-1:
-
Tohuku Hospital Pediatrics-1
- DMSO:
-
Dimethylsulfoxide
- FBS:
-
Fetal Bovine Serum
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- SDS:
-
Sodium Dodecyl Sulphate
- PMA:
-
Phorbol-12-myristate-13-acetate
- IC50 :
-
Inhibitory concentration 50%
- WHO:
-
World Health Organization
References
Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, Gradoni L, Ter Horst R, Lopez-Velez R, Moreno J (2008) The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21:334–359
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, Boer MD (2012) Leishmaniasis worldwide and global estimates of its incidence. PloS one 7(5):e35671. https://doi.org/10.1371/journal.pone.0035671
Amalraj A, Pius A, Gopi S, Gopi S (2017) Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—a review. J Tradit Complement Med 7(2):205–233
Araujo CAC, Alegrio LV, Leon LL (1998) Antileishmanial activity of compounds extracted and characterized from Centrolobium sclerophyllum. Phytochemistry 49(3):751–754
Araujo CAC, Alegrio LV, Gomes DCF, Lima MEF, Gomes-Cardoso L, Leon LL (1999) Studies on the effectiveness of diarylheptanoids erivatives against Leishmania amazonensis. Mem Inst Oswaldo Cruz 94(6):791–94
Beniddir MA, Grellier P, Rasoanaivo P, Loiseau PM, Bories C, Dumontet V, Gueritte F, Litaudon M (2012) Diarylheptanoid glucosides from Pyrostria major and their antiprotozoal activities. Eur J Org Chem 5:1039–1046
Bharath Y, Choudhary UM, Sadhana N, Mohapatra DK (2019) The Mukaiyama type aldol reaction for the synthesis of trans-2,6-disubstituted tetrahydropyrans: synthesis of diaspongin A and B. Org Biomol Chem 17:9169
Brand S, Holscher D, Schierhorn A, Svatoš A, Schroder J, Schneider B (2006) A type III polyketide synthase from Wachendorfia thyrsiflora and its role in diarylheptanoid and phenylphenalenone biosynthesis. Planta 224:413–428
Burza S, Croft SL, Boelaert M (2018) Leishmaniasis. Lancet 392(10151):951–970. https://doi.org/10.1016/S0140-6736(18)31204-2
Calla-Magarinos J, Gimenez A, Troye-Blomberg M, Fernandez C (2009) An alkaloid extract of Evanta, traditionally used as anti-leishmania agent in Bolivia, inhibits cellular proliferation and interferon-gamma production in polyclonally activated cells. Scand J Immunol 69:251–258
Cheuka PM, Mayoka G, Mutai P, Chibale K (2017) The role of natural products in drug discovery and development against neglected tropical diseases. Molecules 22(1):58. https://doi.org/10.3390/molecules22010058
Cragg GM, Newman DJ (2013) Natural products: A continuing source of novel drug leads. Biochim Biophys Acta 1830(6):3670–3695. https://doi.org/10.1016/j.bbagen.2013.02.008
Delorenzi JC, Attias M, Gattass CR, Andrade M, Rezende C, Pinto AC, Henriques AT, Bou-Habib DC, Saraiva EMB (2001) Anti-leishmanial activity of an indole alkaloid from Peschiera australis. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.45.5.1349-1354
den Boer M, Davidson RN (2006) Treatment options for visceral leishmaniasis. Expert Rev Anti Infect Ther 4(2):187–197. https://doi.org/10.1586/14787210.4.2.187
Dinić J, Randelovic T, Stankovic T, Dragoj M, Isakovic A, Novakovic M, Pesic M (2015) Chemoprotective and regenerative effect of diarylheptanoids from the bark of black alder (Alnus glutinosa) in human normal keratinocytes. Fitoterapia. 105:169–76. https://doi.org/10.1016/j.fitote.2015.07.003
DNDi; https://dndi.org/diseases/visceral-leishmaniasis/facts/
Ganapathy G, Preethi R, Moses JA, Anandharamakrishnan C (2019) Diarylheptanoids as nutraceutical: a review. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2019.101109
Goncalves de Olievera LF, Pereira BAS, Gilbert B, Correa AL, Rocha L, Alves CR (2017) Natural products and phytotherapy: an innovative perspective in leishmaniasis treatment. Available from: https://www.arca.fiocruz.br/handle/icict/20075
Harvey AL (2008) Natural products in drug discovery. Drug Discov Today 13(19–20):894–901. https://doi.org/10.1016/j.drudis.2008.07.004
Ibrahim SR, Mohamed GA, Khedr AI, Aljaeid BM (2017) Anti-oxidant and anti-inflammatory cyclic diarylheptanoids from Alnus japonica stem bark. Int J Pharmacol Res 16:83–91
Jeong MS, Choi SE, Kim JY, Kim JS, Kim EJ, Park KH, Lee DI, Joo SS, Lee CS, Bang H, Lee MK (2010) Atopic dermatitis-like skin lesions reduced by topical application and intraperitoneal injection of Hirsutenone in NC/Nga mice. Clin Dev Immunol. https://doi.org/10.1155/2010/618517
Jhingran A, Chawla B, Saxena S, Barrett MP, Madhubala R (2009) Paromomycin: uptake and resistance in Leishmania donovani. Mol BiochemParasitol 164(2):111–117
Kawai S, Nakata K, Ohashi M, Nishida T (2008) Myricanol and myricanone biosynthesis in Myrica rubra: incorporation of two molecules of 4-coumaric acid. J Wood Sci 54(3):256–260
Koutsoni OS, Karampetsou K, Dotsika E (2019) In vitro screening of antileishmanial activity of natural product compounds: determination of IC50, CC50 and SI values. Bio Protoc 9(21):e3410. https://doi.org/10.21769/BioProtoc.3410
Kunnumakkara AB, Bordoloi D, Harsha C, Banik K, Gupta SC, Aggarwal BB (2017) Curcumin mediates anticancer effects by modulating multiple cell signalling pathways. Clin Sci (Lond) 131(15):1781–1799. https://doi.org/10.1042/CS20160935
Lall N, Meyer JJ (2001) Inhibition of drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis by diospyrin, isolated from Euclea natalensis. J Ethnopharmacol 78(2–3):213–216. https://doi.org/10.1016/s0378-8741(01)00356-7
Lee HB, Lee HK, Kim JR, Ahn YJ (2009) Anti-Helicobacter pylori diarylheptanoid identified in the rhizome of Alpinia officinarum. J Korean Soc Appl Biol Chem 52(4):367–370
Maltezou HC (2010) Drug resistance in visceral leishmaniasis. J Biomed Biotechnol; 617521.https://doi.org/10.1155/2010/617521 PMID: 19888437
Mishra BB, Singh RK, Srivastava A, Tripathi VJ, Tiwari VK (2009) Fighting against leishmaniasis: search of alkaloids as future true potential anti-leishmanial agents. Mini Rev Med Chem 9:107–123
Mohapatra S (2014) Drug resistance in leishmaniasis: newer developments. Trop Parasitol 4:4–9. https://doi.org/10.4103/2229-5070.129142
Monzote L, García J, González R, Scotti MT, Setzer WN (2021) Bioactive essential oils from cuban plants: an inspiration to drug development. Plants 10:2515. https://doi.org/10.3390/plants10112515
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferative and cytotoxicity assays. J Immunol Methods 65(1–2):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Organization WH (2010) Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, Switzerland, 22–26 March. WHO technical report series. 2010; (949).
Per C, Claeson UP, Tuchinda P, Reutrakul V (2002) Occurrence, structure and bioactivity of 1, 7-diarylheptanoids. Nat Prod Chem 26:881–908
Polonio T, Efferth T (2008) Leishmaniasis: drug resistance and natural products (review). Int J Mol Med. https://doi.org/10.3892/ijmm_00000020
Ray S, Hazra B, Mittra B, Das A, Majumder HK (1998) Diospyrin, a bisnaphthoquinone a novel inhibitor of type I DNA topoisomerase of Leishmania donovani. Mol Pharmacol 54(6):994–999
Rodrigues IA, Mazotto AM, Cardoso V, Alves RL, Amaral ACF, Silva JRA, Pinheiro AS, Vermelho AB (2015) Natural products: Insights into leishmaniasis inflammatory response. Mediat Inflamm. https://doi.org/10.1155/2015/835910
Singh N, Mishra BB, Bajpai S, Singh RK, Tiwari VK (2014) Natural product-based leads to fight against leishmaniasis. Bioorg Med Chem 22:18–45
Tezuka Y, Ali MS, Banskota AH, Kadota S (2000) Blepharocalyxins C-E: three novel antiproliferative diarylheptanoids from the seeds of Alpinia blepharocalyx. Tetrahedron Lett 41(31):5903–5907
Yin J, Kouda K, Tezuka Y, Le Tran Q, Miyahara T, Chen Y, Kadota S (2004) New diarylheptanoids from the rhizomes of Dioscorea spongiosa and their antiosteoporotic activity. Planta Med 70(1):54–58
Acknowledgements
We thank the Central Instrumentation Facility at the School of Life Sciences, Jawaharlal Nehru University, for providing the imaging facility.
Funding
Rentala Madhubala is an AS Paintal Distinguished Scientist Chair of ICMR. Smriti Tandon is a recipient of funding from the Indian Council of Medical Research, India. Madhu Puri is a recipient of the University Grants Commission- D.S. Kothari Post-Doctoral Fellowship.
Author information
Authors and Affiliations
Contributions
ST: Methodology (Screening of inhibitors for antileishmanial activity), Writing—original draft, writing—review and editing. MP: Methodology (Screening of inhibitors for antileishmanial activity), writing—review and editing. YB: Methodology (synthesis of the sixteen compounds), writing—review and editing. UMC: Methodology (synthesis of the sixteen compounds), writing—review and editing. DKM: Supervision, methodology (synthesis of the sixteen compounds) writing—review and editing, RM: Supervision, writing—review and editing. RM: Conceptualization, supervision, funding acquisition, writing- original draft, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
None to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tandon, S., Puri, M., Bharath, Y. et al. In vitro screening of natural product-based compounds for leishmanicidal activity. J Parasit Dis 47, 644–658 (2023). https://doi.org/10.1007/s12639-023-01605-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-023-01605-7