Abstract
In this study, we aim to evaluate the immune response of chickens to UV-treated sporulated oocysts as a means of protection against caecal coccidiosis caused by field strains of Eimeria tenella. Two groups of chicks were immunized using prepared UV-treated oocysts of E. tenella and challenged at day 20 post hatching. The first group was immunized only once at day 1 post hatching, the second group was immunized twice (day 1 and day 8 post hatching). Two non-immunized control groups were used: the first group was challenged with E. tenella, while the second group remained uninfected. The effectiveness of immunization on production and animal health was evaluated by the following criteria: body weight, feed conversion ratio, blood in faeces, mortality, lesion scores and oocyst output. The two immunized groups showed a significantly better performance in body weight, weight gain and lesion scores than the non-immunized group. However, all three groups performed significantly worse than the unchallenged group. The mortality of the non-immunized infected group was high (70%) while mortality in both immunized and unchallenged groups of chickens was significantly lower (range 2.2 to 4.4%) than the infected group (p < 0.05). The production of oocysts in faeces, post-infection, was significantly higher in the non-immunized group compared to the immunized group (p < 0.05) and both were significantly higher than the uninfected group (p < 0.05). In conclusion, immunization by prepared UV-irradiated oocysts is effective in stimulating at least a partial protective immunity in immunized chickens against caecal coccidiosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccidiosis caused by Eimeria tenella is the best known of the avian protozoan diseases. This is partly because of the serious disease it causes and partly because of its widespread economic importance in poultry farming. This parasite inhabits the caeca and causes high morbidity and mortality, hemorrhagic diarrhea, major weight loss and emaciation (McDougald and Reid 1997). Anti-coccidial drug resistance in broiler flocks is a growing global problem (Witcombe and Smith 2014) and is significant in tropical areas such as the study location for this work in Algeria (Djemai et al. 2016), moreover, vaccines are expensive and in limited supply in the tropics.
It is well known that UV radiation can be used for attenuating or inactivating micro-organisms. In particular, this is widely used in the drinking water industries. Ware et al. (2010), concluded that UV treatment of water (10-mJ/cm2 UV fluence) appears to be effective at inactivating Toxoplasma gondii oocysts under ideal conditions. Cryptosporidium oocysts are also highly resistant to chemical inactivation but several studies show that Cryptosporidium parvum oocysts are inactivated by UV radiation (Clancy et al. 1998; Bukhari et al. 1999; King et al. 2017). Kniel et al. (2007) were able to induce protective immunity in chickens immunized with sporulated oocysts of E. acervulina inactivated by UV radiation (261 mW/cm2). The idea of decreasing the pathogenicity of a strain by irradiation has been around for some time (Yvoré et al. 1993). It is an applicable approach in practice. For example, it is used in helminths in the production of a commercial vaccine against lungworm infection in cattle (Bovilis Huskvac™, MSD Animal Health) and is based on the use of viable gamma irradiated Dictyocaulus viviparus L3 larvae.
Different types of irradiation have been tried on Eimeria spp oocysts, in an attempt to reduce viability, with some success. For example: in various studies E. tenella oocysts were exposed to ultraviolet rays (El-Ashram et al. 2019), X-rays (Jenkins et al. 1991) and to gamma rays (Abu Ali et al. 1972); the advantage of irradiation is that it does not appear to affect immunogenicity at a given dose of UV or gamma radiation.
The aims of this study were to investigate the potential for UV treated sporulated oocysts of E. tenella to be used as an immunization strategy to improve animal health and production performance in chickens.
Materials and methods
Experimental design
Our Experimental designconforms to the guidelinesset out for evaluating the efficacy and safety of anticoccidial vaccines (Chapman et al. 2005).Based on this we used four groups of chickens for our study: (1) Unvaccinated unchallenged control birds, (2) Unvaccinated challenged control birds, and (3) Vaccinated challenged birds (two different regimes). A total of 240 one-day-old Cobb 500 broilers (non-sexed) were purchased from a commercial hatchery. Ethical approval and authorisation to conduct the animal experimentation was obtained from the University of Constantine (Veterinary Institute) and the State Veterinary Departments of both Constantine and Jijel. Experimental and rearing conditions conform to the standards of the COBB Broiler Management Guide and standards set out by other studies (Long and Millard 1977; Bedrnik et al. 1995; Chapman and Rayavarapu 2007). Broilers were reared in clean conditions, groups were maintained separately and animals exposed to the same environmental conditions.
At one-day-old, birds were randomly divided into four groups (V1I, V2I, NVI, NVNI), each containing 60 birds, divided into 3 replicates each containing 20 chicks. Group V1I (single immunization challenge group) received only one dose of the UV-treated E. tenella oocysts before challenge (summarized in Table 1). Birds from group V2I (double immunization challenge group) received two doses of the UV- irradiated oocysts and were then challenged. The control groups were, NVI and NVNI; chicks of both groups have not been immunized, however the NVI group (non-immunized challenge group) was infected and the NVNI group (non-immunizednon-challenge group) was not infected. Standard, non-medicated feed and water (without coccidiostats) were provided ad libitum for days 1–28 to all groups (Long and Millard 1977; Bedrnik et al. 1995; Chapman and Rayavarapu 2007).
Eimeria tenella oocyst collection and sporulation
E. tenella oocysts used in the inoculum and those irradiated with UV used for immunization were collected from clinical episodes of hemorrhagic caecal coccidiosis in several broiler farms located in the Wilaya of Jijel (Algeria). E. tenella oocysts used for the immunization of chickens and those used for inoculation originated from different broiler farms. Oocysts were isolated from the caeca of infected chickens and, after separation of the faecal material, were recovered using standard procedures (Ryley et al. 1976). Isolated Eimeria oocysts were suspended in 2.5% potassium dichromate and sporulated for 72 h at 29°C by aeration and continuous agitation in a shaking water bath. When sporulation had reached > 80%, the Eimeria oocysts were stored at 4°C until use (Djemai et al. 2016).
Identification of Eimeria species
Prepared inoculums and immunization isolates could potentially contain a number of species of chicken Eimeria which could potentially confound our study. To test that all samples contained only E. tenella, morphological and molecular analyses were conducted. Parasites were examined by microscopy, at 1000X magnification, and differentiated using known average lengths and widths of the 7 Eimeria species infecting chickens as described (Long and Reid 1982). Species designation was verified by PCR amplification using Eimeria species-specific ITS1 sequences following standard procedures (Jenkins et al. 2006a, b). Genomic DNA of purified oocysts was extracted using phenol chloroform extraction (Duncanson et al. 2001; Bajnok et al. 2015) with modifications for small amounts as described (Dodd et al. 2014). ITS1-PCR revealed that prepared oocyst suspensions contained only E. tenella.
Preparation of the inoculum
Sporulated E. tenella oocysts, stored in potassium dichromate solution, were washed 3–4 times in waterand resuspended in phosphate-buffered saline solution (PBS) (pH 7.2) and counted using the modified McMaster method (Taylor et al. 1995). A concentration of 2.5 × 104 oocysts/ml was prepared.
Preparation of UV-treated sporulated oocysts
Sporulated E. tenella oocysts were collected, washed and counted as described above and a concentration of 5 × 103 oocysts/ml was prepared. A UV Lamp, TUV T8 (TUV 30 W G30T8, Philips, Holland), with an output at 253.7 nm was used to treat the oocysts. A 180 ml sample of PBS containing 5 × 103 oocysts/ml, was divided into 18 clear glass dishes with a 5–10 ml suspension of oocysts in each dish to a depth of ≤ 1 mm (Zhao et al. 2013; Abdel-Baki et al. 2009). Then each dish was placed at the centre of a closed wooden box (100 cm in length, 50 cm in width, and 50 cm in height) adjacent to a UV light meter (UV Light Meter, YK-35UV) for measuring the UV intensity and wavelength. The UV lamp was positioned above the sample and UV meter at the center of the box. The ceiling and the interior walls of the box were covered with a layer of aluminum foil (Thickness: 0.2 mm) as an efficient reflector of ultra-violet light (Abdel-Baki et al. 2009). The oocyst suspensions were exposed to UV light (160 mW/cm2) for 60 min (Abdel-Baki et al. 2009) and agitated using a magnetic agitator (Drehzahl electronic- IKA-COMBIMAG REO) to ensure homogeneous exposure of the oocysts to UV.
Immunization and challenge
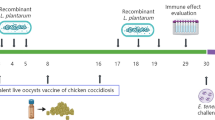
Immunization was carried out in two batches in groups V1I and V2I. One-day old chicks (18 h post-hatching) from the group V1I received only one dose of 5 × 103 UV-treated E. tenella sporulated oocysts suspended in 1 ml of PBS solution (per bird). Chicks from group V2I received two doses (also 5 × 103 oocysts per ml per bird): the first was given at one-day old (18 h post-hatching) and the second at 8 days post-hatching. UV-treated oocysts were administered by gavage into the crop using a syringe (5 ml) connected to a cat urethral catheter (Buster Cat Catheter; sterile, 1.0 × 130 mm, China). The immunization strategy is summarized in Table 1.
The E. tenella sporulated oocysts, used to challenge the immunized chickens, were non-treated wild strains of E. tenella (collected from broiler flocks during clinical events of hemorrhagic caecal coccidiosis) (Li et al. 2005). At 20 days post-hatching, each chick from groups V1I, V2I and NVI received 50 000 sporulated E. tenella oocysts (in 2 ml of PBS) by gavage as described (Table 1) (Long and Millard 1977; Jenkins et al. 1991; Li et al. 2005).
Evaluation of the efficacy and safety of immunization using UV-treated E. tenella
In order to evaluate the efficacy and safety of the immunization by UV-treated E. tenella, we collected the following parameters (Bedrnik et al. 1995):
-
(a)
Body weight and average weight gain. Birds were individually weighed at day 1; the day of inoculation (day 20 post-hatching); and days 2, 4, 6 and 8 post-challenge (at day 22, 24, 26, 28). Average weights gains were calculated for all groups of chicks (including dead birds).
-
(b)
Feed conversion ratio (FCR). The feed conversion ratio (FCR) was calculated by dividing the average amount of feed consumed by the average weight gain in each replicate of each group.
-
(c)
Oocyst output in faeces. Oocysts were counted as described (Taylor et al. 1995) for determination of oocyst output per gram of faeces (OPG). Fecal samples for OPG counting were taken from four random spots from the floor of each pen. They were samples at the frequency of 4 morning samples/repetition/day between the following time intervals: 5th and 9th day of post-hatching (after the first immunization); 12th and 16th day post-hatching (after the second immunization); 5th and 8th day post-challenge (25th and 28th day post-hatching). The average oocyst output was calculated per gram of faeces (OPG) at each interval and for each group.
-
(d)
Faecal score. A scoring system was used to record the daily occurrence of bloody faeces seen on the litter for each repetition of all groups. The evaluation was applied according to the method suggested by Williams (1997): (− ) = no bloody droppings seen; ( +) = < 1 bloody dropping per chick; ( +) = > 1 < 2 bloody droppings per chick; (+ +) = > 2 bloody droppings per chick. Measurements were taken at the following time intervals: 5th and 16th day post-hatching (after immunization); 5th and 8th days post-challenge.
-
(e)
Caecal lesion score. On 5th, 12th and 25th (after-challenge) days post-hatching, 15 chicks/group/day (5 chicks/repetition/day) were killed. The caeca were removed and opened. The infected caeca of each bird were examined and scored on a scale of 0–4 according to the method described by Johnson and Reid (1970). To confirm the attribution of the lesions observed during the autopsy to the coccidiosis, scrapings of the caecal mucosa were inspected by microscopy (100 × and 400 × magnification), for unsporulated oocysts, schizonts and gametocytes. Unsporulated oocysts present in the pool of the products of the scrapings were purified and suspended in 2.5% potassium dichromate solution. The oocysts in the suspension were sporulated by incubation in a stirring water bath at 29 °C for 3 days (Coudert et al. 1995). DNA extraction and ITS1 PCR (Jenkins et al. 2006a, b) were carried out, as described above, to confirm the presence of E. tenella. The only species identified in the products of these scrapings, from the inoculated and immunized chicks, was E. tenella.
-
(f)
Mortality. Between 20 and 28th day (day 1 to day 8 post-challenge), mortality (%) was recorded for each of the 4 batches. Additionally, any dead subjects were also weighed and autopsied to determine possible coccidiosis lesions.
Statistical analysis
Data were analyzed by one-way ANOVA using SPSS 10.0 software (Statistical Package for the Social Sciences). Treatment groups were compared by ANOVA using the following parameters, weight gain (the first day post-hatching; 20th day post-hatching; 2nd, 4th, 6th, 8th days post-challenge), feed conversion ratio (2nd, 4th, 6th and 8th days post-challenge), and lesion scores (5, 12 and 25th days post-hatching), oocyst output in the faeces (between 5 and 9th day post-hatching; between 12 and 16th day post-hatching; between 5 and 8th days post-challenge), and cumulative mortality (20th and 28th day post-hatching). Immunized groups were compared to the non-immunized challenge control group (NVI) and non-immunized non-challenge control group (NVNI) and a statistically significant difference of p < 0.05 was used. Tukey’s post-test was used to identify significantly different groups.
Results
The average body weight and average weight gains recorded in the immunized challenge groups (V1I, V2I) were significantly lower (p < 0.05) than those of the non-immunized non-challenge group (NVNI), but significantly higher (p < 0.05) than those of the non-immunized challenge group (NVI) (Table 2). Significantly poor growth performance was observed in NVI compared to NVNI (p < 0.05). The administration of the UV-treated oocysts of E. tenella using in V1I and V2I caused a significant reduction in the average weight gain after the challenge compared to the uninfected groups (V1I = 24.24%; V2I = 20%). However, this compared positively to NVI which showed a very low weight gain (NVI = 50%) compared to the uninfected controls. Interestingly the repeated immunization of chickens with the UV-treated E. tenella produced no significant increase in growth performance when compared to the group immunized only once.
After immunization, V1I and V2I developed light caecal lesions, scoring from 0 to 0.6 (5th, 12th days post-hatching), low oocyst output in faeces (from 0 to 600 OPG) with light output scores between 5 and 9 days of age and between 12 and 16 days of age (Table 3). These were not significantly different from the uninfected groups (p > 0.05).
After challenge, the caecal lesion scores (day 5 post-challenge), the oocyst excretion (day 5–8 post-challenge) and mortality (day 1–8 post-challenge) in the 2 immunized groups were significantly lower (p < 0.05) than those observed in NVI. There was no significant difference between the singly and doubly immunized groups. Mortality of NVI is significantly higher at 70% than either the immunized or uninfected control group (p < 0.05) (Table 3). Post-mortem testing, as described (Materials and Methods), indicated that this mortality was due to infection with Eimeria. There is no significant effect of immunization on mortality. Faecal scores (day 5 to 8 post inoculation) observed in the 2 immunized groups show some damage caused by the immunization but this later attenuates (Table 4). The faecal score observed in NVI showed that the birds were all very seriously affected compared to the immunized or uninfected groups (Table 5).
Discussion
In this study, the efficacy of the UV-treated E. tenella immunization was determined primarily on comparative growth rates of immunized and non-immunized chickens following virulent challenge as described previously (Williams and Catchpole 2000), with feed conversion ratios and lesion scores used as secondary parameters (Crouch et al. 2003). The pathogenic potential of an E. tenella challenge was confirmed by the ability to individually cause significant reductions in weight gain. We demonstrate that immunization with UV-treated oocysts significantly improves weight, weight gain, compared with non-immunized birds. However, the effect is not complete since the uninfected birds gained significantly higher weights which was achieved more rapidly than those immunized. Furthermore, feed conversion was unaffected by either infection or immunization. Broadly, this shows that this immunization has the potential to improve productivity in part. Where UV-treated oocyst immunization showed a clear effect was in reducing mortality from 70% to between 2.2 to 4.4%. This would have a major influence on productivity. Taken together these data suggest that this approach shows promise as an approach to improving productivity compared to the non-immunized state.
In addition to investigating productivity, this study considered parameters that influenced the health of the birds. Immunization was shown to have a beneficial effect on the development of caecal lesions as judged by lesion scores and blood in the droppings–both were significantly reduced. Furthermore, oocyst production was significantly reduced in immunized birds compared to their non-immunized counterparts thus potentially reducing the degree of transmission from bird to bird. Overall, while immunization using UV-treated oocysts was not absolute, there may be benefits to tropical countries or communities where other anti-coccidial approaches are restricted. We were unable to demonstrate any value in giving a second immunization–there was no significant difference between the two immunized groups of chickens.
In other studies, Crouch et al. (2003) indicated that protection against the reduced weight gain induced by virulent challenge could be demonstrated in chicks vaccinated at 1-day old for E. acervulina, E. maxima, E. mitis, E. tenella using the commercial PARACOX™-5 vaccine. Maes et al. (1991), observed very good growth performance in a group of chickens challenged by 200,000 oocysts of E. tenella/bird and immunized by repeated doses of 2000 virulent oocysts of E. tenella/bird/day (at day 4, 6, 8, 11 and 13). Nakai et al. (1992) obtained an improvement in the growth performance in chickens immunized daily throughout 13 successive days (50 virulent oocysts of E. tenella/bird/day). These studies all demonstrate the value of immunization and the measurement of growth and growth rate as an indicator of success.
On the other hand, it has been shown that the use of the severity of coccidial lesions, as a major criterion in the evaluation of the protective immunity induced by a anticoccidial vaccines, can lead to incorrect conclusions due to a lack of correlation between body weight and the coccidial lesions (Williams 1997). According to Norton et al. (1989), Weber (1989) and Williams (1997), the absence of coccidial lesions following a virulent challenge of an immunized bird, is a sign of immune protection, while the presence of the lesions does not necessarily indicate a weak protection and a significant depression of growth rate. According to the above-mentioned studies, we can conclude that immunization by the prepared UV-treated sporulated oocysts of E. tenella, carried out in this study, generates a good protection against caecal coccidiosis. However, we observe that the above-mentioned parameters are slight in spite of the large immunization dose administered (5 × 103 oocysts/bird).
There are a number of hypotheses that could explain what we are observing with the UV-treated oocyst immunization. Firstly, we may be observing attenuation which preserves the capacity for sexual and asexual reproduction but has resulted in the attenuation of pathogenicity. Secondly, there could be a proportion of the UV-treated E. tenella sporulated oocysts which remain viable and provide low level infection but which stimulates an immune response. Thirdly, inactivation of a part of the population of UV-treated E. tenella oocysts (dead oocysts) which results in attenuation of viable oocysts by stimulation of the immune response against them. Unfortunately, we are unable to confirm the proportion of oocysts that the UV-treatment attenuates or kills and, at present, we are unable to distinguish between these possibilities or determine the full extent of the immunity to E. tenella. We also think that vaccination with a large number of UV-treated sporulated oocysts of E. tenella can cause clinical signs, hence the importance of studying immunizations with lower doses of oocysts.
According to Rose (1970), the term immunity, as used in most studies, refers to the acquisition of resistance to an infection with an Eimeria species. Resistance may be measured by a reduction in the pathogenic effects of infection, a reduction in the extent of macroscopically visible lesions, or a decrease in the numbers of parasites, as measured by the production of oocysts in the feces.
In this study, immunization of chickens with sporulated oocysts of E. tenella treated with UV radiation does not prove effective at completely preventing the development of caecal lesions, oocyst excretion and bloody droppings irrespective of the single or double immunization regime. However, it clearly influences survivability and growth rate and shows potential to be a useful tool in countries or regions where other approaches are restricted or too costly.
The cost of commercial anticoccidial vaccines is high and this may be prohibitive in some parts of the tropical world. Currently, the cost of the PARACOX™-8 vaccine in Algeria, as an example location, is 0.35–0.45 € per laying pullet–which scales up to large sums of money in an animal production system that relies on large numbers of animals. Furthermore, in many parts of the tropical world, little is known of the identity of Eimeria strains and species present in local chicken farming systems. Development of methods that enable the use of local strains or species may offer opportunities to vaccinate in areas where traditional vaccines may not have been tried or might not be effective. While, we recognise the value of using established vaccines there may be a role for the use of UV-treated oocyst vaccines in local settings to make improvements in animal health and production.
References
Abdel-Baki A, Allam G, Sakran T, El-Malah E (2009) Attenuated Sarcocystis ovicanis sporocysts induced protective immunity to lambs. Korean J Parasitol 47:131–138. https://doi.org/10.3347/kjp.2009.47.2.131
Abu Ali N, Binnerts WT, Klimes B (1972) Immunization by irradiated Eimeria acervulina. J Protozool 19:177–180. https://doi.org/10.1111/j.1550-7408.1972.tb03432.x
Bajnok J, Boyce K, Rogan MT, Craig PS, Lun ZR, Hide G (2015) Prevalence of Toxoplasma gondii in localized populations of Apodemus sylvaticus is linked to population genotype not to population location. Parasitology 142:680–690. https://doi.org/10.1017/S0031182014001760
Bedrnik P, Yvoré P, Hiepe Th, Mielke D, Drossigk U (1995) Guidelines for evaluation of the efficacy and safety in chickens of live vaccines against coccidiosis and recommendations for registration. In: Eckert J, Braun R, Shirley MW, Coudert P (eds) Biotechnology Guidelines on Techniques in Coccidiosis Research. European Commission, UK, pp 190–201
Bukhari Z, Hargy TM, Bolton JR, Dussert B, Clancy JL (1999) Medium-pressure UV for oocyst inactivation. J Am Water Works Assoc 91:86–94. https://doi.org/10.1002/j.1551-8833.1999.tb08602.x
Chapman HD, Rayavarapu S (2007) Acquisition of immunity to Eimeria maxima in newly hatched chickens on new or reused litter. Avian Pathol 36:319–323. https://doi.org/10.1080/03079450701460773
Chapman HD, Roberts B, Shirley MW, Williams RB (2005) Guidelines for evaluating the efficacy and safety of live anticoccidial vaccines, and obtaining approval for their use in chickens and turkeys. Avian Pathol 34:279–290. https://doi.org/10.1080/03079450500178378
Clancy JL, Hargy TM, Marshall MM, Dyksen JE (1998) UV light inactivation of Cryptosporidium oocysts. J Am Water Works Assoc. 9:92–102. https://doi.org/10.1002/j.1551-8833.1998.tb08501.x
Coudert P, Licois D, Drouet-Viard F (1995) Eimeria species and strains of rabbits. Guidelines for evaluation the efficacy and safety in chickens of live vaccines against coccidiosis and recommendations for registration. In: Eckert J, Braun R, Shirley MW, Coudert P (eds) Biotechnology Guidelines on Techniques in Coccidiosis Research. European Commission, UK, pp 190–201
Crouch CF, Andrews SJ, Ward MJ, Francis MJ (2003) Protective efficacy of a live attenuated anticoccidial vaccine administered to 1-day-old chickens. Avian Pathol 32:297–304. https://doi.org/10.1080/10307945031000097912
Djemai S, Mekroud A, Jenkins MC (2016) Evaluation of ionophore sensitivity of Eimeria acervulina and Eimeria maxima isolated from the Algerian to Jijel province poultry farms. Vet Parasitol 224:77–81. https://doi.org/10.1016/j.vetpar.2016.04.040
Dodd NS, Lord JS, Jehle R, Parker S, Parker F, Brooks DR, Hide G (2014) Toxoplasma gondii: prevalence in species and genotypes of British bats (Pipistrellus pipistrellus and P. pygmaeus). Exp Parasitol 139:6–11. https://doi.org/10.1016/j.exppara.2014.02.007
Duncanson P, Terry RS, Smith JE, Hide G (2001) High levels of congenital transmission of Toxoplasma gondii in a commercial sheep flock. Int J Parasitol 31:1699–1703. https://doi.org/10.1016/S0020-7519(01)00282-X
El-Ashram SA, Aboelhadid SM, Gadelhaq SM (2019) Oral inoculation of ultraviolet-irradiated Eimeria species oocysts protects chickens against coccidiosis. Parasitol Res 118:3173–3183. https://doi.org/10.1007/s00436-019-06455-y
Jenkins MC, Augustine PC, Danforth HD, Barta JR (1991) X-irradiation of Eimeria tenella oocysts provides direct evidence that sporozoite invasion and early schizont development induce a protective immune response. Infect Immun 59:4042–4048. https://doi.org/10.1128/IAI.59.11.4042-4048.1991
Jenkins MC, Miska K, Klopp S (2006a) Improved polymerase chain reaction technique for determining the species composition of Eimeria in poultry litter. Avian Dis 50:632–635. https://doi.org/10.1637/7615-042106R.1
Jenkins MC, Miska K, Klopp S (2006b) Application of polymerase chain reaction based on ITS1 rDNA to speciate Eimeria. Avian Dis 50:110–114. https://doi.org/10.1637/7439-091305R.1
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30–36. https://doi.org/10.1016/0014-4894(70)90063-9
King B, Fanok S, Phillips R, Lau M, Akker BV, Monis P (2017) Cryptosporidium attenuation across the waste water treatment train: recycled water fit for purpose. Appl Environ Microbiol 83:e03068-e3116. https://doi.org/10.1128/AEM.03068-16
Kniel KE, Shearer AEH, Cascarino JL, Wilkins GC, Jenkins MC (2007) High hydrostatic pressure and UV light treatment of produce contaminated with Eimeria acervulina as a Cyclospora cayetanensis surrogate. J Food Prot 70:2837–2842. https://doi.org/10.4315/0362-028X-70.12.2837
Li GQ, Kanu S, Xiao SM, Xiang FY (2005) Responses of chickens vaccinated with a live attenuated multi-valent ionophore-tolerant Eimeria vaccine. Vet Parasitol 129:179–186. https://doi.org/10.1016/j.vetpar.2004.09.034
Long PL, Reid WM (1982) A guide for the diagnosis of coccidiosis in chickens. Research report 404. University of Georgia, Athens, Georgia
Long PL, Millard BJ (1977) Eimeria: immunisation of young chickens kept in litters pens. Avian Pathol 6:77–92. https://doi.org/10.1080/03079457708418214
Maes L, Vanparijs O, Marsboom R (1991) Effect of diclazuril (Clinacox) on the development of protective immunity against Eimeria tenella: laboratory trial in broiler chickens. Poult Sci 70:504–508. https://doi.org/10.3382/ps.0700504
McDougald LR, Reid WM (1997) Coccidiosis. In: Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM (eds) Diseases of Poultry, 10th edn. Iowa State University Press, USA, pp 865–883
Nakai Y, Uchida T, Kanazawa K (1992) Immunization of young chicks by trickle infection with Eimeria tenella. Avian Dis 36:1034–1036
Norton CC, Catchpole J, Evans NA (1989) Performance of an attenuated coccidiosis vaccine in floor-pen challenge studies. In: Yvoré P (ed) Coccidia and Intestinal Coccidiomorphs. INRA Editions, France, pp 677–682
Rose ME (1970) Immunity to coccidiosis: effect of betamethasone treatment of fowls on Eimeria mivati infection. Parasitology 60:137–146. https://doi.org/10.1017/S0031182000077301
Ryley JF, Meade R, Hazelhurst J, Robinson TE (1976) Methods in coccidiosis research: separation of oocysts from faeces. Parasitology 73:311–326. https://doi.org/10.1017/S0031182000046990
Taylor M, Catchpole J, Marshall C, Norton C, Green J (1995) Eimeria species of sheep. In: Eckert J, Braun R, Shirley MW, Coudert P (eds) Biotechnology Guidelines on Techniques in Coccidiosis Research. European Commission, UK, pp 25–39
Ware MW, Augustine SAJ, Erisman DO, See MJ, Wymer L, Hayes SL, Dubey JP, Villegas EN (2010) Determining UV inactivation of Toxoplasma gondii oocysts by using cell culture and a mouse bioassay. Appl Environ Microbiol 76:5140–5147
Weber GM (1989) Immunisation of chicks by trickle infection with Eimeria tenella under medication with Lasalocide. In: Yvoré P (ed) Coccidia and Intestinal Coccidiomorphs. INRA Editions, France, pp 693–696
Williams RB (1997) The mode of action of Anticoccidial Quinolones (6 Decyloxy-4-hydroxyquinoline-3-carboxylates) in Chickens. Int J Parasitol 27:101–111. https://doi.org/10.1016/S0020-7519(96)00156-7
Williams RB, Catchpole J (2000) A new protocol for challenge test to assess the efficacy of live anticoccidial vaccines for chickens. Vaccine 18:1178–1185. https://doi.org/10.1016/S0264-410X(99)00387-4
Witcombe DM, Smith NC (2014) Strategies for anti-coccidial prophylaxis. Parasitology 141:1379–1389. https://doi.org/10.1017/S0031182014000195
Yvoré P, Pery P, Laurent F, Bessay M (1993) Vaccins anticoccidiens Bilan et perspectives. Vet Res 24:229–250
Zhao Y, Huang B, Huang S, Zheng H, Li Y, Lun Z, Shen J, Wang Y, Kasper LH, Lu F (2013) Evaluation of the adjuvant effect of pidotimod on the immune protection induced by UV-attenuated Toxoplasma gondii in mouse models. Parasitol Res 112:3151–3160. https://doi.org/10.1007/s00436-013-3491-3
Funding
Authors declare to receive no funding.
Author information
Authors and Affiliations
Contributions
The study was designed, guided and carried out by SD. AM and GH analyzed the data. DK and IB performed the extraction and purification of parasitic DNA. The manuscript was written by SD and GH.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
Our Experimental design conforms to the guidelines set out for evaluating the efficacy and safety of anticoccidial vaccines stated by Chapman et al. (2005). Experimental and rearing conditions conform to the standards of the COBB Broiler Management Guide and standards set out by other studies (Chapman and Rayavarapu 2007; Bedrnik et al. 1995; Long and Millard 1977).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Djemai, S., Mekroud, A., Hide, G. et al. Investigation into the potential of using UV-treated sporulated oocysts of Eimeria tenella as a local solution to immunization of chickens against caecal coccidiosis. J Parasit Dis 47, 238–245 (2023). https://doi.org/10.1007/s12639-022-01562-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-022-01562-7