Abstract
This study was conducted in order to compare well established used chemical anticoccidial medication (diclazuril) against natural prepared safe alternative products of garlic extract (GE), Moringa oleifera (MO) leaves extract, onion extract (OE), in order to control experimentally infected with Eimeria tenella species in chickens. Performance parameter in form of average body weight (ABW) and feed conversion rate (FCR) were studied together with biochemical parameters (malondialdehyde (MDA), superoxide dismutase (SOD) and catalase (CAT), mortality rate, oocyst count in addition to total white blood cell (WBCs), lymphocytes and heterophils counts. Histopathological examination of intestinal tract in all test groups was studied. Results revealed that the lowest mortality rate was found in group treated with MO leaves extract. All challenged herbal extract treated groups revealed ABW and FCR lower than diclazuril treated infected group. All treated groups were lower in both average lesion score and average oocyst count two weeks post challenge when compared with control positive group indicate positive impact of all studied therapies either chemical or herbal products but with variable degrees as best effect was diclazuril followed by MO group, followed by GE group and finally group treated with OE. Experimental infection of chickens with E. tenella oocysts significantly increased MDA concentration when compared with control negative non-treated group (P < 0.01). However, infected birds fed with OE, GE, MO leaves extracts and diclazuril administration for a week pre-infection had significantly declined MDA concentrations compared with infected non-treated (P < 0.01). Control positive birds showed significant decrease in SOD and CAT activities vs. the healthy birds either at week pre-infection or at two days’ post-infection (P < 0.01). However, SOD activities in birds fed with OE, MO leaves extract and diclazuril for a week pre-infection significantly higher (P < 0.01) than control positive. Histopathological finding revealed that best was group treated with diclazuril followed by group received MO, followed by group received GE and finally group received OE. It could be concluded that herbal extract may be representing a good alternative anticoccidial medications specially that the later may developed resistance for many Eimeria species in continuous use in veterinary field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Avian Coccidiosis is the most important parasitic protozoan in poultry worldwide caused by Eimeria Species. It causes poor feed utilization, weight loss resulting in huge economic loss (Tanweer et al. 2014; Chand et al. 2016). As the disease is characterized by intestinal injuries, it makes birds susceptible to other intestinal diseases (as necrotic enteritis). There are many Eimeria species infecting poultry (Gadde et al. 2020), the most clinically important strains are E. tenella and E. necatrix which both are considered the two most important Eimeria species causing severe mortalities may reach 100% in poultry (Graat et al. 1996). Chicken coccidiosis is controlled by many applications of a live vaccine or the use of chemical anticoccidials (chemoprophylaxis) (McDougald 1982; Blake and Tomley 2014).
Assessment of any anticoccidials agent based mainly on standards for performance of the bird (growth rate and feed conversion), and parasitology (oocyst shedding and intestinal lesions) (Reid and Long 1979; Chapman 1998). Effective anticoccidial agent should affect all Eimeria growth stages without alteration on host immunity or leaving residues in host tissues.
The main problem concerning control program originated from drug administration is the increasing resistance of avian coccidia against routine anticoccidial drugs in addition to the increasing public interest about drug residues in chicken meat (Innes and Vermeulen 2006; Lillehoj et al. 2007; Williams 2006). Thus, stricter regulations were applied in Europe against the use of anticoccidial drugs (Yim et al. 2011; Eckert et al. 2021).
Medicinal plants extracts were examined as an alternative approach to control coccidiosis causing promising expectations (Ogbu and Onuh 2015; Abbas et al. 2017). So, the expansion usage of herbal plants as the natural coccidiostat is important to be applied.
Garlic (Allium sativum) is one of the most effective herbs with medicinal importance (Adulugba et al. 2017). Garlic has antibacterial (Tipu et al. 2002; Safithri et al. 2011), antifungal, and anticoccidial properties in chickens (Pourali et al. 2014; El-Khtam et al. 2014). Organosulfur compounds of garlic as allicin, alliin, ajoene, diallylsulfide, dithiin, S-allylcysteine (www.wekipedia.org) have wide antimicrobial activities. Allicin prevents E. tenella sporulation in chicken as reported in vitro study (Muthamilselvan et al. 2016).
Onion (Allium cepa) possess antibacterial, antiviral, antiparasitic, antifungal properties and found in most parts of the onion plant (Lampe 1999; Abou-Elezz et al. 2011), this due to it contains various organic sulphur compounds, flavinoids, phenolic acids, sterols and a trace of volatile oil (Melvin et al. 2009). Later on, An et al. (2015) concluded that supplementation of onion extract (OE) in white mini broilers improved body weight gain and considered promising antibiotic replacer. Moreover, Aditya et al. (2017) investigated effect of OE on growth performance, blood profile and carcass characteristics in broiler chickens, results revealed that OE improved weight gain together with increasing serum IgG level and meat anti-oxidation capacity when compared with negative control group. Recently, AL-Ramamneh et al. (2017) noticed that OE containing rations improves chickens’ performance; improve small intestine absorption surface length, decrease coccidian lesion scores and decrease number of coccidian egg count in experimentally infected broiler chickens.
The Moringa tree is one of the most useful (beneficial) trees in the world. Moringa oleifera (MO) is very important for its medicinal value. Most parts of this plant have antibacterial and antifungal activities, and are used for the treatment of different diseases in the indigenous system of medicine, particularly in South Asia (Anwar et al. 2007). In poultry, it was found that use of MO leaves in ration of infected chickens with coccidiosis not only significantly improves body weight gain but also inhibit oocyst shedding (Ola-Fadunsin and Ademola 2013). Later on, different beneficial effects were confirmed including antibacterial, antifungal, anti-inflammatory, antihypercholesterolemic activities and antioxidant properties together with positive impact on hematological and biochemical values of broiler chickens (Modisaojang-Mojanaga et al. 2019).
Parasitic infection generally led to oxidative stress (Pourali et al. 2014). This type of stress lead to production of free radicals and decrease the antioxidant enzymes activities as CAT and SOD (Abo-Aziza et al. 2019). Oxidative stress causes damage to unsaturated fatty acids, DNA, and proteins when the free radicals’ concentrations are above normal levels (Prisacaru 2016). Natural antioxidants can control the production of free radicals and inhibit their actions in the biological system (Maarman 2017) Many plant extracts contain antioxidant agents (Simioni et al. 2018) Garlic (Pourali et al. 2014), Onion (AL-Ramamneh et al. 2017) and Mo (Abdel-Tawab et al. 2020). Lipid peroxidation is another symptom of oxidative stress. The measurement of MDA can be used as indicator for lipid antioxidation statues (Swiatkiewicz et al. 2015). Leukogram could be used as indicator of the degree of the treatment and the relieve of stress (Okorie-Kanu et al. 2018). So, the aim of this study was to investigate promising safe alternatives natural product against extensively used chemical anticoccidial drugs on broiler chicken performance, MDA level, antioxidant enzymes activities, leukogram, intestinal lesion scores, in addition to oocyte shedding.
Material and methods
Moringa oleifera (MO) leaves preparation leaves of MO were collected from the farm of Egyptian Scientific Society of Moringa; the plant was kindly identified by Professor Aboelfetoh Mohammed Abdelalla. National Research Centre; Giza. Egypt. Collected leaves was air-dried, powdered and kept for extraction.
Moringa oleifera (MO) leaves extraction dried MO leaves powder were extracted with 1 L of 70% ethanol and shacked each 8 h by 24 h for preparation of hydro-alcoholic extract. Prepared hydro-alcoholic extract was filtered using cotton funneling four times, this followed by concentration of filtered extract using a rotator evaporator under reduced pressure. Concentrated extracts were lyophilized and kept at -20 ̊C till reconstituted and used in a dose of 250 mg/kg live body weight according to Khalil et al. (2014).
Garlic extract (GE) GE was prepared by homogenized of cloves of garlic in sterile saline, centrifuged at 5000 revolution per minute and sterilized by filtration using bacteriological filter with 0.45 µm diameter to prepare final concentration 40 mg/mL that used orally at the rate 0.5 mL/bird according to Dar et al. (2014).
Dicoxal Commercially available product contains Diclazuril 1%, Reg. No. 574, Batch No. 0200539.
Onion extract (OE) OE was prepared according to Aditya et al. (2017). Briefly, the bulbs of onion were cut into small pieces and mixed with salt (3 g/kg) and then heated at 60 °C for 30 min. The onions minced using electric blender then extracted through coffee filter to get liquid portion as the OE, subsequently the OE stored at 4 °C. Prior to experiment, OE mixed to the diet for 10 min using feed mixer in inclusion rate of 7.5 g of OE / kg poultry ration, then stored to the feedbag until the experiment started.
E. tenella sporulated oocyst E. tenella oocyst was kindly supplied by department of parasitology, Veterinary Research Division, National Research Centre, Egypt. Oocyst were primary isolated from caecum of natural infected pullet according to Chapman and Shirley (2003). The obtained oocysts were allowed to sporulate at 26–28 °C in 2.5% potassium dichromate solution, cleared and counted per 1 mL of the solution using McMaster technique as described by Long and Rowell (1958), then propagated in two weeks old Eimeria free Cobb chicks with challenge dose of 4 × 104 sporulated oocyst/ bird by oral infection according to El-Shazly et al. (2020). Infected birds showed typical signs and postmortem lesions of E. tenella infection. Oocyst were recovered after 8 days from dropping of experimentally infected birds using flotation technique according to Soulsby (1986), allowed to sporulate in solution of 2.5% potassium dichromate at room temperature for 6 days then kept in refrigerator at 4 °C till used for experimental infection with the previously mentioned challenge dose.
Experimental design
A total number of 240 broiler chickens were divided into six equal groups as shown in Table 1, 40 chicks per each. Dropping of all groups were examined for freedom from Eimeria species oocyst twice, first time at ten days of age and second time at 20 days of age by flotation technique (using saturated NaCl solution, specific gravity 1.28). All groups were fed broiler rations free from anticoccidial drug. Group (1) was treated with diclazuril at a dose rate of 2.5 ppm in drinking water according to El-Shazly et al. (2020) 5 days post challenge with E. tenella. Groups (2), (3) and (4) were treated with GE, naturally prepared MO and OE in ration respectively starting from ten days of age till the end of the experiment. Group (5) was kept as control positive challenged non-treated group while group (6) was kept as control negative non challenged non-treated group. At 21 day of age, groups (1–5) were experimentally infected by administration of 4 × 104 sporulated oocyst of E. tenella directly to the crop using syringes and cannula according to El-Shazly et al. (2020). Ration was prevented for 12 h before challenge application for all groups. Experiment was terminated two weeks post challenge, dead birds during experiment together with all birds at the end of the experiment were directed to postmortem examination for the presence of gross lesions consistent with coccidiosis. Lesions observed in infected birds starts from 4th day of infection in control non treated infected group, while other treated infected group was delayed from 6th days post infection, lesions was varied according treatment used. The observed lesions were assigned scores from 1 to 4 using the criteria described by Conway and McKenzie (2007). Blood sampling were collected from each group at 14th and 23 days of age, and the oocyst per gram of fecal material (OPG) was counted using the McMaster counting technique as described by Long et al. (1976). Performance parameter including average body weight (ABW) and feed conversion rate (FCR) were studied. Biochemical parameters as malondialdehyde (MDA), superoxide dismutase (SOD) and catalase (CAT), hematological parameters as White blood cell (WBCs), lymphocytes and heterophils counts) in addition to histopathological examination of intestinal tract in all test groups were also studied.
Blood samples
Blood samples were collected from birds in each group at day 8 post-infection with E. tenella via the wing vein-puncture using sterile syringes and needles. Each blood sample was transferred immediately into a set of sterile tubes containing ethylene diamine tetra acetic acid (EDTA) as anticoagulant for hematological and biochemical investigations.
Hematological analysis
White blood cells (WBCs) were counted within 2 h using of blood sampling the Neubauer hemocytometer method (Esievo and Saror 1992). For lymphocytes and heterophils counts, blood smears were immediately prepared using 5 μL of blood and stained with Giemsa stain for cell counts (Irizaary-Rovira 2004).
Biochemical investigations
Blood samples were centrifuged at 3000 rpm for 15 min for separation of blood plasma that was used for measurement of malondialdehyde (MDA), superoxide dismutase (SOD) and catalase (CAT) using commercial SPECTRUM kits (BioMerieux, SA). Absorbencies were measured at 560 nm and 520 nm respectively by spectrophotometer according to the manufacturer’s instruction.
Histopathological studies
Cecal samples from experimental birds’ post challenge and treatment from each group were fixed in 10% formalin, dehydrated in absolute alcohol, cleared in xylene, and embedded in paraffin for preparation of fine blocks in paraffin wax. Sections of 5-μm thicknesses were cut and subjected to routine hematoxylin and eosin staining according to Bancroft and Gamble (2008).
Statistical analysis of the data
All available data were summarized as mean ± SD and subjected to statistical analysis using Student’s t-test. Values of P < 0.05 were considered statistically significant.
Results and discussion
Intensive poultry production gets rise of huge amount of white meat to cover human need unfortunately many disease conditions appear with either in clinical or subclinical forms causing severe economic losses such as coccidiosis. Our study investigates experimental infection with coccidiosis in presence of either dietary natural feed additives or treatment with chemical anticoccidial commercially available product.
Results of mortality rate, ABW and FCR are showed in Table 2. Mortality rate for all groups revealed that no mortalities in control negative group. On the other hand, among all treated groups the lowest mortality rate was in group treated with MO leaves extract which was 5%, followed by group treated with diclazuril which was 7.5%, followed by both groups treated with GE and OE which have the same findings 12.5% mortality rate, finally the highest mortality rate was showed in positive control group which was 40%. Both chemical anticoccidial diclazuril and natural extract reduce mortality rate when compared to control positive group indicate their beneficial value. Results of mortality rate of infected non treated group was the highest (40%), this due to that E. tenella one of diseases of poultry that causing mortalities varies according infestation dose, this results was parallel with Bachaya et al. (2012), on the other hand mortality rate decreased dramatically in groups treated with MO (5%) followed by group treated with Diclazuril (7.5%), this may be due to beneficial effect of MO extract including anti-inflammatory effect (Modisaojang-Mojanaga et al. 2019), anticoccidial effect (El Banna et al. 2016) and inhibit oocyst shedding Ola-Fadunsin and Ademola (2013) this may the cause of decreased mortalities in group treated with MO. While group treated with Diclazuril mortality rate was improved when compared with infected non-treated group this may be due to that Diclazuril is a chemical anticoccidial medication which results in high cure rate from Eimeria protozon, this result was parallel with Elkomy et al. (2015) who reported that Diclazuril treatment in E. tenella infected chickens resulted in reduction in mortalities, lowered number of oocysts and the lesion score. For the other two extracts (GE and OE) mortality rate was decreased (12.5%) when compared to control infected no-treated group, this may be due to the antiparasitic activities of GE (Muthamilselvan et al. 2016) and OE (AL-Ramamneh et al. 2017).
Results of final ABW and FCR revealed that control negative (group 6) was the highest ABW (2320.5 g) and best FCR (1.58) followed by group (1) treated with diclazuril which was 2140 g ABW and 1.61 FCR. However, the lowest was control positive group (group 5) which was 1870 ABW and 1.90 FCR. This may be due to that control negative has healthy intestinal tract surface with normal villi with no challenge affecting intestinal health for any period of time within our experiments that results in maximum utilization of feed ingredients. In addition, group (1) diclazuril help in treat causative agent with high cure rate due to chemical anticoccidial characteristics results in rapid cure rate complete clearance of coccidiosis results in regeneration of intestinal mucosa this was parallel with results found by Amer et al. (2007), Amer et al. (Amer et al. 2010) and El-Shazly et al. (2020). On the other hand, control positive group (group 5) there is no control of E. tenella results in high destruction of intestinal mucosa with no treatment which lead to decrease ABW and increase FCR in this group. This result was matched with Du and Hu (2004), Arczewska-Wlosek and Swiatkiewicz (2012) and Györke et al. (2016) who reported that E. tenella challenge results in reduction in body weight and affecting FCR due to destruction of intestinal mucosa which affects absorption and consequently weight gain.
All herbal extract groups have higher ABW and improved FCR when compared with control positive group, the highest was group (3) challenged treated with MO leaves extract which was 2100 g final ABW and 1.62 FCR, followed by group (2) treated with GE which was 1990 g final ABW and 1.63 FCR, followed by group (4) treated with OE which was 1975.5 g final ABW and 1.65 FCR, this positive impact could be attributed to each herbal extract mode of action. MO leaves extract was highest ABW and best FCR within all used herbal extract; this may be due two points, first is antioxidant activity, as it was found that antioxidant compounds reduce severity of E. tenella infection of enterocytes through enhancement of intestinal lipid peroxidation, this was documented by Siddhuraju and Becker (2003) and Anwar et al. (2005), the second points is that its growth—promoting effects which is documented by Ghazalah and Ali (2008) and Liaqat et al. (2016) who attributed this effect due to its direct nutritional and immune-stimulating actions of its phytochemical components. The present finding in performance parameter (final ABW and FCR) in GE treated group maybe explained due to GE has anticoccidial effects which decrease negative impact of E. tenella. This was supported by Dkhil et al. (2011) who reported that GE exerts anticoccidial activity. Similar results were found by Arczewska-Wlosek and Swiatkiewicz (2012) and Dar et al. (2014) who reported that GE not only has anticoccidial, but also anti-inflammatory properties. Group received OE showed improved final ABW and FCR when compared to control positive group this due to onion contains numerous organic sulphur compounds in OE which proved to have anticoccidial activities (Lee et al. 2003; AL-Ramamneh et al. 2017). Moreover, Aji et al. (2011) reported that diets contain fresh onion bulbs has higher body weight and feed conversion rate when compared to control negative group which was parallel with our results.
Results of final ABW and FCR in groups received herbal extract was superior to challenged groups which indicate their positive impacts in neutralizing E. tenella infection negative effect on ABW and FCR. On the other hand, all challenged herbal extract treated groups revealed ABW and FCR lower than diclazuril treated infected group, this was parallel with results found by Habibi et al. (2016). This may be due to effect herbal extracts results in reduction with no complete eradication of Eimeria species population results in delayed regeneration of intestinal tract, this was matched with Muthamilselvan et al. (2016). Moreover, Brander et al. (1991) explained the highest efficacy of diclazuril as chemical anticoccidial in that diclazuril breaks down all intracellular developmental stages of both asexual and sexual cycles of E. tenella result of rapid and high cure rate and consequently fast enterocytes regeneration, while any other extract is a mixture of several chemical compounds.
Results of average lesion score and average oocyst count post challenge by the end of week two were showed in Table 3, results of average lesion score revealed that control negative showed no lesion score (0.0 ± 0.0), the highest lesion score was control positive group (5) which was 3.61 ± 0.12, followed by group (4) received OE which was 2.95 ± 0.19, followed by group (2) received GE which was 2.89 ± 0.21, followed by group (1) treated with Diclazuril chemical anticoccidial which was 1.29 ± 0.18, followed by group (3) received MO leaves extract which was 1.11 ± 0.17.
Results of average oocyst count two weeks post challenge revealed that no oocyst shedding in control negative group (6) indicate no infection, while the highest oocyst shedding was positive control group (5) which was 20,100 ± 1165, followed by group (4) received OE which was 12,800 ± 1135, followed by group (2) received GE which was 11,700 ± 1065, followed by group (3) received MO leaves extract which was 11,700 ± 1065, and the lowest was group (1) treated with Diclazuril chemical anticoccidial which was 910 ± 80.
All treated groups were lower in both average lesion score and average oocyst count two weeks post challenge when compared with control positive group indicate positive impact of all studied therapies either chemical or herbal products but with variable degrees as best effect was diclazuril followed by MO groups showing the lowest lesion score and average oocyst count two weeks post challenge. This maybe due to that diclazuril as chemical anticoccidial has strong effect on E. tenella all developmental stages, this finding was parallel with results found by Zhou et al. (2010), Shen et al. (2012) and Zyan et al. (2017), while MO positive impact in decrease both lesion score and oocyst shedding was parallel to results found by Ola-Fadunsin and Ademola (2013) who reported that using of MO leaves extract in various doses results in not only decease in lesion score but also variable inhibitory effect on oocyst shed in the broiler dropping which was 96.4%, 97.4%, 99.1 and 99.8% according dosing program per groups (1.0, 2.0, 3.0, 4.0 and 5.0 g/kg body weight) respectively. This may be due to MO leaves extract anti-inflammatory, antioxidant properties and immune-stimulating actions (Olugbemi et al. 2010; Abou-Elezz et al. 2011; Liaqat et al. 2016), also this results may be attributed to the presence of phytochemicals and essential oils contained in MO leaves (Chuang et al. 2007), moreover results found by Alabi et al. (2017) and Sharma and Paliwal (2013) revealed that MO may serve in production of new therapeutic compound as anticoccidial drug due to it is high in saponins content. Other both groups treated with GE and OE showed improvement in average lesion score and average oocyst count post challenge when compared with control positive group. Our GE group results was matched with Dar et al., (2014) and Włosek and Światkiewicz (2012), this may be due to phenolic compounds in GE that affect cytoplasmic membranes cationic permeability of coccidian cells results in death of it (Sikkema et al. 1995) and anti-inflammatory effect of GE (Dkhil et al. 2011). Finally Results of groups received OE in aspect of improvement of average lesion score and average oocyst count than control positive group, this may be due to its anti-inflammatory effect, antioxidant and antiparasitic effects (Lampe 1999; Kumar et al. 2010) this due to bioactive compounds including organic Sulphur compounds that present in OE (Lee et al. 2003) together with several flavonoids including flavonols, flavones, quercetin and catechin that explains onion beneficial activities (Nemeth and Piskula 2007).
Blood MDA concentrations and the activities of antioxidant enzymes SOD and CAT was investigated (Table 4). Experimental infection of chickens with E. tenella oocysts significantly increased MDA concentration when compared with control negative non-treated group (P < 0.01). However, infected birds fed with OE, GE, MO leaves extracts and diclazuril administration for a week pre-infection had significantly declined MDA concentrations compared with infected non-treated birds (P < 0.01). MDA level is an indicator of lipid peroxidation and oxidative damage in chickens (Moustafa et al. 2020). In the current study, serum MDA level results suggested that OE, GE, MO leaves extracts and diclazuril could improve lipid antioxidation status. However, it was found that diclazuril administered birds at two days’ post-infection significantly declined MDA concentration compared with infected group (P < 0.05). Eventually, E. tenella oocysts triggered the lipid peroxidation at the specified period and quantity. The occurrence of oxidative damage may be contributed directly to the mechanism of negative effects of the disease. Diclazuril itself as well led to the oxidative damage at the specified period and dosage. The intensity of this damage was not as strong as the damage induced by the coccidium strain itself. On the other hand, the drug administrations were effective against this oocyst, regarding the obtained results.
The activities of the antioxidant enzymes SOD and CAT, which protect tissues against oxidation, increased with dietary supplementation of OE, GE and MO (Table 4). Concerning SOD activities, the control positive birds showed significant decrease in SOD activities vs. the healthy birds either at week pre-infection or at two days’ post-infection (P < 0.01). However, SOD activities in birds fed with OE, MO leaves extract and diclazuril for a week pre-infection significantly higher (P < 0.01) than control positive birds. Moreover, starting the administration of diclazuril at two days before infection significantly increase SOD activities comparing with infected non-treated birds (P < 0.01). No significant difference in SOD activities was observed when starting the feeding of OE, GE and MO leaves extract at two days’ post-infection. CAT activity was significantly lower in infected birds than healthy control birds (P < 0.01). Feeding with basal diet supplemented with OE and MO leaves extractor after administration of diclazuril for a week pre-infection resulted in a significant increase in CAT activity (P < 0.01) comparing to control positive birds.
The imbalance between reactive oxygen species (ROS) creation and antioxidant efficiency is known as oxidative stress. Recently, huge work was done on antioxidant substance to relieve the oxidative stress (Hassan et al. 2018). Pourali et al. (2014) concluded that addition of 0.5% garlic powder in food od Eimeria infected broiler chickens was resulted in a decrease in MDA levels while the activities of total antioxidant status (TAS), total oxidant status (TOS), SOD and GPX were not affected. On the other hand, Aydogan et al. (2020) reported that, the supplementation of garlic powder was significantly improved antioxidant status of broiler chickens infected with Eimeria oocysts. Coccidiosis usually primes to the induction of oxidant substances to be effective against oocytes but it directed to the body animals (Muthamilselvan et al. 2016; Ognik and Krauze 2016) indicated that the impairment of the anti-oxidant/pro-oxidant equilibrium in favor of pro-oxidants, indicating oxidative stress as a result of this poultry parasitosis. In the study of Lu et al. (2016) and Cui et al. (2018), it showed that dietary MOL supplementation quadratically increased SOD and GSH-PX, and decreased MDA contents in plasma of growing and laying hens respectively. In addition, El Banna et al. (2016) reported that MO possessed a marked anticoccidial activity and could be useful as alternative product for the control of avian coccidiosis in poultry production. Tijani et al. (2015) concluded that MO can be incorporated into broiler diets at 15% level without adverse effects on the haematological and serum biochemical indices of the birds.
Leukogram in the studied birds is presented in Table 5. Coccidiosis caused by E. tenella induced a significant reduction in WBCs (P < 0.05). Data revealed a significant decrease in lymphocytes while a significant increase in heterophils was observed in infected birds vs. healthy control. The data showed increase in WBCs count in birds starts feeding on MO leaves extract or diclazuril at two days’ post-infection compared to control positive birds (P < 0.05). Moreover, a marked increase in lymphocytes in E. tenella infected birds after feeding of GE or MO leaves extract one week pre-infection (P < 0.05). However, feeding of GE at two days’ post-infection resulted in a significant elevation in lymphocytes count compared to infected non-treated birds (P < 0.01). Furthermore, a significant decrease in heterophils count was observed in birds fed with OE, GE, MO leaves extract and diclazuril administration for one week pre-infection compared to infected non-treated birds (P < 0.05). However, starting feeding with OE, GE, MO leaves extractor diclazuril administration at two days’ post-infection did not show any significant differences in neutrophils count.
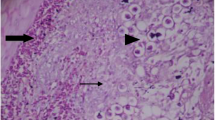
Histopathological examination of two ceci in challenged non-treated group (5) showed thickened intestinal mucosa, large numbers of enterocytes (approximately 90%) were swollen and contain intra and extracellular various developmental stages or oocysts of E. tenella (Fig. 1E, F), later on this inflammation persist with various intracellular and extracellular developmental stages of E. tenella (Fig. 1G), on the other hand control negative group (Fig. 1H) showed no histopathological changes in all examined fields of two ceci during whole experiment as enterocytes and mucosal crypt epithelium appeared normal and free from developmental stages and oocysts of Eimeria tenella.
Histopathological examination of two ceci in treated and non-treated groups. A Chicken ceci showing thicken of the mucosa due to mild inflammatory cells infiltration, (Mild infestation) H&E × 200. B Chicken intestine suffering from mild inflammatory cells infiltration mainly lymphocytes and heterophils H&E × 200. C Chicken ceci suffering from moderate inflammatory cells infiltration mainly lymphocytes and heterophils H&E × 200. D Chicken ceci showing moderate thicken of the mucosa due to moderate inflammatory cells infiltration, various intracellular and extracellular developmental stages of Eimeria tenella (arrows) H&E × 200. E Chicken ceci suffering from severe inflammatory cells infiltration mainly lymphocytes and heterophils (arrow) H&E × 200. F Chicken ceci suffering from severe inflammatory cells infiltration mainly lymphocytes and heterophils (arrow) H&E × 200. G Chicken ceci showing thicken of the mucosa due to severe inflammatory cells infiltration, various intracellular and extracellular developmental stages of Eimeria tenella H&E × 200. H Normal H&E × 200. Scale bars = 50 μm
Among all challenged treated groups, it was found that group 1 (challenged with E. tenella and treated with diclazuril) showed mild inflammatory cells infiltration mainly lymphocytes and heterophils (Fig. 1B) indicated mild infestation, group 3 (challenged treated with Moringa oleifera leaves extract) showed thicken of the mucosa due to mild inflammatory cells infiltration, various intracellular and extracellular developmental stages of E. tenella indicated positive impact on intestinal mucosa inspit of challenge (Fig. 1A), on the other hand group 2 (challenged treated with Garlic extract) and group 4 (challenged treated with Onion extract) showed moderate thicken of the mucosa due to moderate inflammatory cells infiltration mainly lymphocytes and heterophils (Fig. 1C), some field showed moderate thicken of the mucosa with moderate inflammatory cell infiltration various intracellular and extracellular developmental stages of E. tenella (Fig. 1D).
Best histopathological finding was group treated with diclazuril rather all herbal extract This may be due to effect herbal extracts results in reduction with no complete eradication of Eimeria species population results in delayed regeneration of intestinal tract Muthamilselvan et al. (2016). Moreover the highest efficacy of diclazuril as chemical anticoccidial maybe due to that diclazuril breaks down all intracellular developmental stages of both asexual and sexual cycles of E. tenella result of rapid and high cure rate Elkomy et al. (2015) and Noack et al. (2019).
It could be concluded that herbal extract showing promising alternative for chemical anticoccidial medication in spite of superior efficacy of chemical anticoccidial on Eimeria tenella infestation, as chemical anticoccidial my results in resistant Eimeria species strain in long run. The use of combinations of more than one herbal extract specially if prepared with nano technology may result in superior effect, this needs further investigation in term of safety and efficacy.
References
Abbas A, Iqbal Z, Abbas RZ, Khan MK, Khan JA (2017) Immunomodulatory activity of Pinusradiata extract against coccidiosis in broiler chicken. Pak Vet J 37:145–149
Abdel-Tawab H, Abdel-Haleem HM, Abdel-Baki AAS, Al-Quraishy S, El-Mallah AM (2020) Anticoccidial and antioxidant activities of Moringa oleifera leaf extract on murine intestinal eimeriosis. Acta Parasit 65:823–830
Abo-Aziza FAM, Oda SS, Aboelsoued D, Farag TK, Almuzaini AM (2019) Variabilities of hydatidosis in domestic animals slaughtered at Cairo and Giza abattoirs, Egypt. Vet World 12:998–1007
Abou-Elezz FMK, Sarmiento-Franco L, Santos-Ricalde R, Solorio-Sanchez F (2011) Nutritional effects of dietary inclusion of Leucaenaleucocephala and Moringa oleifera leaf meal on Rhode Island Red hens’ performance. Cuban J Agric Sci 45:163–169
Aditya S, Ahammed M, Jang SH, Ohh SJ (2017) Effects of dietary onion (Allium cepa) extract supplementation on performance, apparent total tract retention of nutrients, blood profile and meat quality of broiler chicks. Asian-Australas J Anim Sci 30(2):229–235
Adulugba IA, Goselle ON, Ajayi OO, Tanko JT (2017) Development of a potent anti-coccidial drug: a phyto-synthetic approach. Am J Phytomed Clin Ther 1:1–7
Aji SB, Ignatius K, Ado AAY, Nuhu JB, Abdulkarim A, Aliyu U, Gambo MB, Ibrahim MA, Abubakar H, Bukar MM, Imam HAM, Numan PT (2011) Effects of feeding onion (Allium cepa) and garlic (Allium sativum) on some performance characteristics of broiler chickens. Res J Poult Sci 4:22–27
AL-Ramamneh D, Almassad M, Hussein N (2017) Effect of using onion as Anticoccidial agent on broiler physiology and production. Bull Environ Pharmacol Life Sci 6(7):60–64
Alabi OJ, Malik AD, Ng’ambi JW, Obaje P, Ojo BK (2017) Effect of aqueous Moringaoleifera (Lam) leaf extracts on growth performance and carcass characteristics of Hubbard broiler chicken. Braz J Poult Sci 19:273–280
Amer MM, Amer AM, Abdel-Ghany WA (2007) The efficacy of diclazuril liquid formulation in the prevention and control of coccidiosis in broiler chickens. In: The 5th scientific conference of the Faculty of Veterinary Medicine, Beni-Suef University, vol November, 6th–9th, pp 96–101
Amer MM, Awad NMN, Abo-Elezz NMN, El-Khateeb RM, Sherein-Said A, Ghetas MM, Kutkat MA (2010) Experimental study on the efficacy of some commonly used anticoccidial drugs in controlling of Coccidiosis with mixed field isolates in broiler chickens. World App Sci 9:359–366
An BK, Kim JY, Oh ST, Kang CW, Cho S, Kim SK (2015) Effects of onion extracts on growth performance, carcass characteristics and blood profiles of white mini broilers. Asian Australas J Anim Sci 28:247–251
Anwar F, Ashraf M, Bhanger MI (2005) Interprovenance variation in the composition of Moringa oleifera oil seeds from Pakistan. J Am Oil Chem Soc 82:45–51
Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res 21:17–25
Arczewska-Wlosek A, Swiatkiewicz S (2012) The effect of a dietary herbal extract blend on the performance of broilers challenged with Eimeria oocysts. J Anim Feed Sci 21:133–142
Aydogan I, Yildirim E, Kurum A, Bolat D, Cinar M, Basalan M, Yigit A (2020) The effect of dietary garlic (Allium Sativum), black cumin (Nigella Sativa) and their combination on performance, intestine morphometry, serum biochemistry and antioxidant status of broiler chickens. Braz J Poult Sci. https://doi.org/10.1590/1806-9061-2020-1317
Bachaya H, Raza M, Khan M, Iqbal Z, Abbas R, Murtaza S, Badar N (2012) Predominance and detection of different Eimeria species causing coccidiosis in layer chickens. J Anim Pl Sci 22:597–600
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, London
Blake DP, Tomley FM (2014) Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol 30:12–19
Brander GC, Pugh DM, Bywater RJ, Jenkins WL (1991) Veterinary applied pharmacology and therapeutic, 5th edn. Bailliere Tindall, London, p 552
Chand N, Faheem H, Khan RU, Qureshi MS, Abudabos AM (2016) Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ Sci Pollut Res 23:23930–23935
Chapman HD (1998) Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int J Parasitol 28:1141–1144
Chapman HD, Shirley MW (2003) The Houghton strain of Eimeria tenella: a review of the type strain selected for genome sequencing. Avian Pathol 32:115–127
Chuang PH, Lee CW, Chou JY, Murugan M, Shieh BJ, Chen HM (2007) Anti-fungal activity of crude extracts and essential oil of Moringa oleifera Lam. Bioresour Technol 98:232–236
Conway DP, McKenzie ME (2007) Poultry coccidiosis diagnostic and testing procedures, 3rd edn. Blackwell Publishing, Ames, pp 7–9
Cui Y, Wang J, Lu W, Zhang H, Wu SG, Qi GH (2018) Effect of dietary supplementation with Moringa oleifera leaf on performance, meat quality, and oxidative stability of meat in broilers. Poult Sci 97:2836–3284
Dar A, Verma P, Ashfaque M, Ahmad A, Mir IA (2014) Effect of garlic extract on haematobiochemical changes in Eimeria tenella infected broiler chicken. Nat Acad Sci Lett 37:311–316
Dkhil MA, Abdel-Bakia AS, Wunderlicha F, Siesa H, Al-Quraishy S (2011) Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol 175:66–72
Du A, Hu S (2004) Effects of a herbal complex against Eimeria tenella infection in chickens. J Vet Med B Inf Dis Vet Public Health 51:194–197
Eckert J, Carrisosa M, Hauck R (2021) Network meta-analysis comparing the effectiveness of anticoccidial drugs and anticoccidial vaccination in broiler chickens. Vet Parasitol 291:109387
El Banna HA, Atef M, Nabil G (2016) Anti-coccidial activity of Moringa oleifera plant. Anim Vet Sci 4(2):19–25
El-Khtam AO, El-Latif AA, El-Hewaity MH (2014) Efficiency of turmeric (Curcuma longa) and garlic (Allium sativum) on Eimeria species in broilers. Int J Basic Appl Sci 3:349–356
Elkomy A, Aboubakr M, Medhat Y (2015) Anticoccidial efficacy of diclazuril on experimentally Eimeria tenella infected broiler chickens. Benha Vet Med J 29:23–28
El-Shazly KA, El-Latif AA, Abdo W (2020) The anticoccidial activity of the fluoroquinolonelomefloxacin against experimental Eimeria tenella infection in broiler chickens. Parasitol Res 119:1955–1968
Esievo KAN, Saror DI (1992) Haematological assays. A laboratory manual in veterinary clinical pathology, 1st edn. Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria
Gadde UD, Rathinam T, Finklin MN, Chapman HD (2020) Pathology caused by three species of Eimeria that infect the turkey with a description of a scoring system for intestinal lesions. Avian Pathol 49(1):80–86
Ghazalah AA, Ali AM (2008) Rosemary leaves as a dietary supplement for growth in broiler chickens. Int J Poult Sci 7:234–239
Graat EA, Ploeger HW, Henken AM, De VriesReilingh G, Noordhuisen JP, Van Beek PN (1996) Effects of initial litter contamination level with Eimeria acervulina on population dynamics and production characterstics in broilers. Vet Parasitolol 65:223–232
Györke A, Kalmár Z, Pop LM, Şuteu OL (2016) The economic impact of infection with Eimeria spp. in broiler farms from Romania. Rev Bras Zootec 45:273–280
Habibi H, Firouzi S, Nili H, Razavi M, Asadi SL, Daneshi S (2016) Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: in vitro and in vivo study. J Parasit Dis Off Organ Indian Soc Parasitol 40(2):401–407
Hassan F, Roushdy E, Kishawy A, Zaglool A, Tukur H, Saadeldin I (2018) Growth performance, antioxidant capacity, lipid-related transcript expression and the economics of broiler chickens fed different levels of rutin. Anim Open Access J MDPI 9(1):7
Innes EA, Vermeulen AN (2006) Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora. Parasitology 133:S145-168
Irizaary-Rovira AR (2004) Avian and reptilian clinical pathology (Avian hematology & biochemical analysis), Section XI. In: Cowell RL (ed) Veterinary clinical pathology secrets. Elsevier, St. Louis, pp 282–313
Khalil WKB, Ghaly IS, Diab KAE, ELmakawy AI (2014) Antitumor activity of Moringa oleifera leaf extract against Ehrlich solid tumor. Int J Pharm 4:68–82
Kumar S, Sharadamma KC, Radhakrishna PM (2010) Effects of a garlic active based growth promoter on growth performance and specific pathogenic intestinal microbial counts of broiler chicks. Int J Poult Sci 9:244–246
Lampe JW (1999) Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr 70:475s–490s
Lee EJ, Love J, Ahn DU (2003) Effect of antioxidants on consumer acceptance of irradiated turkey. Meat J Food Sci 68:1659–1663
Liaqat S, Mahmood S, Ahmad S, Kamran Z, Koutoulis KC (2016) Replacement of canola meal with Moringa oleifera leaf powder affects performance and immune response in broilers. J Appl Poult Res 25:352–358
Lillehoj HS, Kim CH, Keeler CL Jr, Zhang S (2007) Immunogenomic approaches to study host immunity to enteric pathogens. Poult Sci 86:1491–1500
Long PL, Rowell JG (1958) Counting oocysts of chicken coccidia. Lab Pract 7:515–518
Long PL, Millard BJ, Joyner LP, Norton CC (1976) A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat 6:201–217
Lu W, Wang J, Zhang HJ, Wu SG, Qi GH (2016) Evaluation of Moringa oleifera leaf in laying hens: effects on laying performance, egg quality, plasma biochemistry and organ histopathological indices. Ital J Anim Sci 15:658
Maarman GJ (2017) Natural antioxidants as potential therapy, and a promising role for melatonin against pulmonary hypertension. In: Wang Y-X (ed) Pulmonary vasculature redox signaling in health and disease. Springer, Cham, pp 161–178
McDougald L (1982) Chemotherapy of coccidiosis (chapter 9). In: Long P (ed) The biology of the coccidiosis. University Park Press, Baltimore, pp 373–427
Melvin JM, Jayachitra J, Vijayapriya M (2009) Antimicrobial activity of some common spices against certain human pathogens. J Med Plants Res 3:1134–1136
Modisaojang-Mojanaga MM, Ogbuewu IP, Oguttu JW, Mbajiorgu CA (2019) Moringa leaf meal improves haemato-biochemical and production indices in broiler chickens: a review. Comp Clin Pathol 28:621–632
Moustafa N, Aziza A, Orma O, Mohamed T (2020) Effect of supplementation of broiler diets with essential oils on growth performance, antioxidant status, and general health. Mansoura Vet Med J 21:14–20
Muthamilselvan T, Kuo T, Yang W, Yang W (2016) Herbal remedies for coccidiosis control: a review of plants, compouns, anticoccidial actions. Evid Based Complement Alternat Med 2016:1–19
Nemeth K, Piskula MK (2007) Food content, processing, absorption and metabolism of onion flavonoids. Crit Rev Food SciNutr 47:397–409
Noack SH, Chapman D, Selzer PM (2019) Anticoccidial drugs of the livestock industry. Parasitol Res 118:2009–2026
Ogbu CC, Onuh SS (2015) Oocyst output, performance and haematological indices of broiler chickens infected with coccidian oocysts and fed Ocimumgratissimum leaf extract. Glob J Poult Farm Vaccin 3:146–153
Ognik K, Krauze M (2016) The potential for using enzymatic assays to assess the health of turkeys. Worlds Poult Sci J 72:535–550
Okorie-Kanu CO, Okorie-Kanu OJ, Okoye JA (2018) Effects of vaccination on the haematological parameters of cockerels and ducks infected with a Velogenic Newcastle disease virus. Anim Res Int 15(1):2926–2936
Ola-Fadunsin SD, Ademola IO (2013) Direct effects of Moringa oleifera Lam (Moringaceae) acetone leaf extract on broiler chickens naturally infected with Eimeria species. Trop Anim Health Prod 45(6):1423–1428
Olugbemi TS, Mutayoba SK, Lekule FP (2010) Evaluation of Moringa oleifera leaf meal inclusion in cassava chip based diets fed to laying birds. Livest Res Rural Dev 22(6):363–367
Pourali M, Kermanshahi H, Golia A, Razmi G, Soukhtanloo M (2014) Antioxidant and anticoccidial effects of garlic powder and sulfur amino acids on Eimeria-infected and uninfected broiler chickens. Iran J Vet Res 15(3):227–232
Prisacaru AE (2016) Effect of antioxidants on polyunsaturated fatty acids: review. Acta Sci Pol Technol Aliment 15:121–129
Reid WM, Long PL (1979) A diagnostic chart for nine species of fowl coccidia. University of Georgia. Research report, p 335
Safithri M, Bintang M, Poeloengan M (2011) Antibacterial activity of garlic extract against some pathogenic animal bacteria. Med Pet 34:155–158
Sharma V, Paliwal R (2013) Isolation and characterization of saponins from Moringaoleifera (Moringaceae) pods. Int J Pharm 5:179–183
Shen X, Wang C, Zhu Q, Li T, Yu L, Zheng W, Fei C, Qiu M, Xue F (2012) Effect of the diclazuril on Hsp90 in the second-generation merozoites of Eimeria tenella. Vet Parasitol 185:290–295
Siddhuraju P, Becker K (2003) Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro-climatic origins of drumstick tree (Moringa oleifera Lam.). J Agric Food Chem 15:2144–2155
Sikkema J, Bont JAM, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, Neri LM (2018) Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9:17181
Soulsby EJL (1986) Helminths, arthropods and protozoa of domestic animals, 7th edn. Bailliere Tindall, London, p 231
Swiatkiewicz S, Swiatkiewicz M, Arczewska-Wlosek A, Jozefiak D (2015) Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J Anim Physiol Anim Nutr 99:1–12
Tanweer AJ, Chand N, Saddique U, Bailey CA, Khan RU (2014) Antiparasitic effect of wild rue (Peganumharmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol Res 113:2951–2960
Tijani LA, Akanji AM, Agbalaya K, Onigemo M (2015) Haematological and serum biochemical profiles of broiler chickens fed diets containing moringa leaf meals. J Trop Agric Food Environ Ext 14:7–11
Tipu MA, Pasha TN, Ali Z (2002) Comparative efficacy of salinomycin sodium and Neem fruit (Azadirachta indica) as feed additive anticoccidials in broilers. Int J Poult Sci 1:91–93
Williams RB (2006) Tracing the emergence of drugresistance in coccidia (Eimeria spp.) of commercial broiler flocks medicated with decoquinate for the first time in the United Kingdom. Vet Parasitol 135:1–14
Włosek AA, Światkiewicz S (2012) The effect of a dietary herbal extract blend on the performance of broilers challenged with Eimeria oocysts. J Anim Feed Sci 2:133–142
Yim D, Kang SS, Kim DW, Kim SH, Lillehoj HS, Min W (2011) Protective effects of Aloe vera-based diets in Eimeria maxima-infected broiler chickens. Exp Parasitol 127:322–325
Zhou BH, Wang HW, Wang XY, Zhang LF, Zhang KY, Xue FQ (2010) Eimeria tenella: effects of diclazuril treatment on microneme genes expression in second-generation merozoites and pathological changes of caeca in parasitized chickens. Exp Parasitol 125:264–270
Zyan KA, Elshorbagy MA, Aggour MG, Abdelfatah MA (2017) Molecular identification of Eimeria tenella in broiler chickens in Kalyoubia governorate and evaluation of different strategies for control cecal coccidiosis. BVMJ 33:175–182
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FAMA-A, TME-M, ERH, ZMSAG, MAB. The first draft of the manuscript was written by NSR, KME, HMM, ZMSAG and MAB. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University B (Date 2-12-2021/No. 44712012022).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abo-Aziza, F.A.M., El-Metenawy, T.M., Rabie, N.S. et al. Comparative study between chemical anticoccidial medication and natural prepared products on experimentally infected broiler chickens. J Parasit Dis 47, 101–112 (2023). https://doi.org/10.1007/s12639-022-01545-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-022-01545-8