Abstract
The leaf decoction of Sesbania sesban var. bicolor is used traditionally by Santhal tribe of Assam, India, for the treatment of intestinal helminthic infections. This study was conducted to evaluate the in vitro and in vivo anthelmintic efficacy of methanolic extract of S. sesban var. bicolor leaves using Hymenolepis diminuta-rat (cestode) and Syphacia obvelata-mice (nematode) as test parasites and models. Praziquantel (PZQ) and albendazole (ABZ) were used as reference drugs. At the highest concentration of 30 mg/ml of the plant extract, H. diminuta and S. obvelata showed mortality at 0.81 ± 0.01 h and 15.17 ± 0.05 h, respectively. The in vivo results substantiated the in vitro findings, and the extract showed a better cestocidal efficacy in a dose-dependent manner, whereby treatment of rats with 400 mg/kg of the plant extract caused 65.10% reduction in eggs per gram (EPG) of faeces and 56% reduction in worm counts. S. obvelata-infected mice treated at the same dose showed 34.32% and 47.08% reduction in EPG and worm counts at necropsy, respectively. The methanolic extract was subjected to bioassay-guided fractionation using different solvents and the ethyl acetate fraction proved to be the most active. This active fraction was subjected to column chromatography using varying concentrations of hexane:ethyl acetate. Maximum efficacy was observed in 7:3 hexane:ethyl acetate, where H. diminuta and S. obvelata showed mortality at 3.56 ± 0.12 h and 9.21 ± 0.02 h, respectively. This indicates that the isolated fraction contained the active component responsible for its anthelmintic activity, which substantiates the medicinal usage in traditional practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal helminthiasis, is an important public health problem in tropical and sub-tropical countries. It affects around 1.5 billion people globally (WHO 2015). At present the control of intestinal worms is based on mass drug treatment by two common drugs, albendazole and mebendazole. However, in some areas of the world synthetic medicines are still out of reach and therefore, alternative strategies developed from traditional knowledge to combat such diseases have emerged since times immemorial (Deori and Yadav 2016).
Sesbania sesban (L.) Merr. var. bicolor (Wight & Arn.) F. W. Andrews (Fabaceae) (Fig. 1), commonly called as “Mondormoli” in the Santhali language, is widely distributed and cultivated throughout semi-arid and sub-humid tropical regions (Göhl 1982). It is a small perennial tree and is very common throughout Africa and in Asian countries like India, Malaysia, Indonesia and the Philippines (Nigussie and Alemayehu 2013; Kumar et al. 2014). Five varieties of S. sesban have been recognised botanically (Gutteridge 1993; Mani et al. 2011) viz., S. sesban var. sesban, S. sesban var. bicolor, S. sesban var. nubica, S. sesban var. zambesiaca and S. sesban sub sp. punctata.
In traditional medicine, various parts of this plant are used as an anti-inflammatory, anthelmintic, anti-diarrheal, and anti-oxidant (Gomase et al. 2012; Goswami et al. 2016; Murugan and Muniyappan 2018). The aqueous extract of the leaves was shown to possess anti-diabetic properties in a study on normal and streptozotocin-induced diabetic rats (Pandhare et al. 2011). Kamel et al. (2011) reported a moderate in vitro effect of the methanolic extract of S. sesban against Schistosoma mansoni. Likewise, Subramanian and Kalava (2014) reported the anti-inflammatory effects of the aqueous extract of the seed in carrageenan-induced paw edema model in rats. Similarly, Ibrahim (1992) reported the in vitro efficacy of its aqueous extract against Caenorhabditis elegans. Also, Tatiya et al. (2013) isolated diosgenin and oleanolic acid which showed anti-inflammatory activity in both in vitro and in vivo studies in animal models like carrageenan- and histamine-induced rat paw edema, cotton pellet granuloma, acetic acid-induced vascular permeability and oxazolone-induced delayed-type hypersensitivity. Another study by El-Emam et al. (2015) revealed that treatment of mice infected with S. mansoni using the methanol extract along with mefloquine reduced the worm burden. Condensed tannins from S. sesban and Desmodium intortum have been shown to reduce Haemonchus contortus infection in goats (Debela et al. 2012).
The phytochemical analysis of S. sesban has shown the presence of alkaloids, proteins, flavonoids and phytosterols in the chloroform, methanol, ethanol and aqueous extracts. Its methanol and ethanol extracts were found to contain phenolic compounds and the aqueous extract showed the presence of saponins. Methanol, ethanol and aqueous extract showed the presence of carbohydrates (Kumar et al. 2014). Also, Kohli (1988) reported a saponin, stigmasta-5, 24(28)-diene-3β-O-β-D-galactopyranoside from the seeds from S. sesban. A saponin isolated from the plant identified as 3-O-[α-L-rhamnopyranosyl-(1 → 3)-β-D-glucuronopyranosyl]-oleanolic acid showed molluscicidal activity (Dorsaz et al. 1988).
In many studies to investigate the bioefficacy of plant extracts, most workers have used in vitro methods. However, in vitro studies alone do not validate the anthelmintic efficacy of the plant (Athanasiadou et al. 2007). For example, in some studies the in vitro and in vivo bioefficacy effects of a plant did not supplement each other (Bøgh et al. 1996; El-Bahy and Bazh 2015). In vitro activity of a plant does not guarantee comparable in vivo effect. Hence, in vitro studies need to be supplemented or validated with in vivo studies (Deori and Yadav 2016). Several active components have been isolated from S. sesban by several workers. Anthocyanins isolated were found to have anti-oxidant and anti-microbial activity (Kathiresh et al. 2012). Kumar et al. (2014) showed that the extract of the whole plant possessed campesterol, β-sitosterol, cyanidine, delphinidin glycosides, α-keto glutaric acid, oxaloacetic acid, pyruvic acid, oleanolic acid, saponins, palmitic acid, stearic acid, oleic acid, linoleic acid, cyanidin and delphinidin glycosides. Also, Singh et al. (2017) isolated β-sitosterol and kaempferol from roots and stem of S. sesban.
To the best of our knowledge, in vitro and in vivo anthelmintic activity of this plant has not been recorded using intestinal helminth parasite models. Based on these facts, this study was undertaken to investigate and validate the anthelmintic efficacy of this plant using in vitro and in vivo assays and also to find its active column fraction.
Materials and methods
Chemicals and drugs
All chemicals and drugs used were of analytical grade. Albendazole (Ambalal Sarabhai Enterprises Ltd., Vadodara) and praziquantel (Distocide, Chandrabhagat Pharma Pvt. Ltd., Mumbai) were used as reference drugs.
Plant material
A field survey in the Santhal-inhabited districts of Assam, viz, Kokrajhar, Udalguri, Baksa and Chirang, revealed that the local traditional healers prescribe the leaves of S. sesban var. bicolor to treat intestinal helminth infections. The leaves are ground, made into pellets and taken orally by patients having intestinal helminth infections. The plant material was collected from Kokrajhar district of Assam, India, during August and September, 2015. It was identified by a plant taxonomist in the Department of Botany, North-Eastern Hill University (NEHU), Shillong and a voucher specimen (NEHU-12084) was deposited in the herbarium museum of the same department. The leaves were washed with distilled water to remove impurities, dried in shade and then powdered. The powdered material was extracted with methanol in a Soxhlet extractor. The extract (yield 20.21%, w/w) was stored in glass vials at 4 °C.
Phytochemical testing
The extract was subjected to phytochemical testing to confirm the presence of various secondary metabolites. The qualitative analysis was undertaken using the methods of Harborne (1973), Evans (1989) and Sofowora (1993).
Bio-assay guided fractionation
Different fractions of the methanolic crude extract of S. sesban var. bicolor were separated out using solvents such as hexane, diethyl ether, chloroform, ethyl acetate and n-butanol using a fractionating funnel to obtain different solvent phases. The most active solvent phase was obtained by subjecting each extracted solvent phase to in vitro testings (Gueye et al. 2011). The most active solvent phase was then subjected to silica gel column chromatography to obtain different column fractions using different ratios of hexane:ethyl acetate. The column length was 35 × 3 cm filled with silica gel and mesh size was 60–120. Each column fraction was then subjected to in vitro testings to find the most efficacious fraction (Devi and Muthu 2015).
Experimental animals
Albino rats of both sexes (Wistar strain), infected with H. diminuta, weighing about 180–220 g and Swiss albino mice of both sexes, infected with S. obvelata, weighing about 25–30 g were used to perform in vivo experiments. The animals were maintained in separate acrylic cages and were given food and water ad libitum. S. obvelata infection was identified in mice using the perianal cellophane tape test, as described by Meade and Watson (2014), with slight modifications. H. diminuta infection was maintained in rats as described by Tangpu et al. (2006). All experiments on rats and mice were done after due approval from the Institutional Animal Ethics Committee (Animal Models) of North-Eastern Hill University, Shillong. Experimental animals were provided adequate living conditions and procedures were performed with adequate anaesthesia to ensure painless death as per ethical guidelines.
Anthelmintic assay
In vitro tests
Adult live worms of H. diminuta and S. obvelata were collected from freshly necropsied rats and mice. After washing in phosphate-buffered saline (PBS), the specimens were maintained in small petridishes containing PBS at 37 ± 1 °C inside the incubator. Extract was tested at 10, 20 and 30 mg/ml concentrations. The reference drugs, PZQ and ABZ were tested at 1 mg/ml and 5 mg/ml concentrations, respectively. An additional set of worms placed in PBS, served as control. All experiments were undertaken in triplicates, with five worms of each parasite in each petridish. The efficacy of extract was judged on the basis of physical motility of worms, as evident by their paralysis and mortality (Vijaya and Yadav 2016).
In vivo tests
The in vivo testing protocols in H. diminuta-rat model have been described previously (Yadav and Tangpu 2012). For S. obvelata, mice were kept in infected bed for 2 weeks. Later, establishment of infection was confirmed by cellophane tape test. Animals were divided into five groups, with 5 animals in each. Group 1 served as negative control. Group 2, 3 and 4 of animals were treated with 100 mg/kg, 200 mg/kg and 400 mg/kg doses of extract. Group 5 of mice served as positive control and received 20 mg/kg of ABZ. Eggs per gram (EPG) count of animals was done for 3 days, prior to and after dosing of extract. Treatment with extract was done for 5 days. On day 12, all the mice were sacrificed and the percentage reductions in EPG and worm counts were undertaken as described by Kozan et al. (2006), with minor modifications.
Statistical analysis
All data are presented as mean ± standard error of the mean (S.E.M). Origin Pro version 8.0 SR6 was used for graphical representation. Data was analysed using unpaired Student’s t test and one-way analysis of variance (ANOVA) followed by Tukey test with p < 0.001 being considered statistically significant.
Results
Phytochemical tests
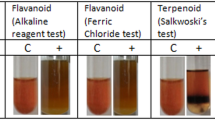
The extract showed the presence of several plant secondary metabolites such as alkaloids, flavonoids, terpenoids, tannins, phlobatannins, saponins and glycosides (Table 1).
In vitro tests
The efficacy of the extract was observed to be dose-dependent against the test parasites. At its highest dose (30 mg/ml), the extract showed mortality of H. diminuta at 0.81 ± 0.01 h, as compared to PZQ with a mortality time at 5.89 ± 0.02 h. Control worms survived till 41.92 ± 0.02 h. Likewise, at the highest dose, extract revealed mortality of S. obveleta at 15.17 ± 0.05, as compared to 7.15 ± 0.02 h by reference drug, ABZ (Fig. 2). Control worms survived till 30.07 ± 0.09 h. The plant showed a better in vitro efficacy against H. diminuta compared to S. obvelata indicating that the plant possesses a better cestocidal activity.
In vivo tests
Administration of S. sesban var. bicolor leaf extract to rats infected with H. diminuta worms showed a significant reduction (p < 0.001) in EPG counts and worm recovery rate in a dose-dependent manner. The animals treated with 400 mg/kg dose of extract, for 5 days, showed 65.10% reduction in EPG counts, compared to the EPG counts of pre-treatment period. The reduction in worm count was found to be 56%. The animals treated with reference drug PZQ at a concentration of 5 mg/kg b.w. showed 86.62% and 76% reduction in EPG and worm counts, respectively. The EPG count in control group did not show much variation during pre-and-post treatment periods (Table 2).
The plant extract showed comparatively less activity in S. obvelata-mice model. During the first 3 days (pre-treatment EPG), eggs were detected in the cellophane test of all the animals. However, after 5 days treatment, EPG and worm counts reduced by 34.32% and 47.08%, respectively. ABZ (20 mg/kg), however, showed 84.72% and 93.04% reduction in EPG and worm counts, respectively (Table 3). The in vivo results also confirm that the plant possess a greater cestocidal activity than nematicidal activity.
In vitro testing of solvent phases and column fractions
Maximum in vitro efficacy was observed in the ethyl acetate phase against H. diminuta, which showed worm mortality at 10.17 ± 0.06 h, followed closely by chloroform phase which showed mortality at 10.76 ± 0.02, n-butanol phase at 21.59 ± 0.09 h, methanol phase at 21.84 ± 0.06 h and hexane phase at 27.46 ± 0.25 h in decreasing order of efficacy. Control worms showed physical activity till 39.37 ± 0.28 h (Fig. 3a).
Maximum in vitro efficacy was observed in the ethyl acetate phase against S. obvelata, which caused worm mortality at 20.70 ± 0.17 h, followed closely by chloroform phase which caused mortality at 21.12 ± 0.16 h, n-butanol phase at 21.59 ± 0.09 h, methanol phase at 21.84 ± 0.06 h and finally hexane phase at 27.46 ± 0.25 h in decreasing order of efficacy. Control worms showed physical activity till 29.63 ± 0.16 h (Fig. 3b).
The ethyl acetate phase being the most active solvent phase, was subjected to column chromatography using varying concentrations of hexane:ethyl acetate. The fractions were subjected to in vitro tests at 100 μg/ml concentration to find the active fraction. Maximum in vitro efficacy was observed in 7:3 hexane:ethyl acetate, where H. diminuta and S. obvelata showed mortality at 3.56 ± 0.12 h and 9.21 ± 0.02 h respectively (Fig. 4), indicating that this fraction possesses the active principle.
Discussion
Sesbania sesban var. bicolor has not yet undergone proper validation to support its traditional anthelmintic claims. In vitro results of the present study revealed that at the highest concentration, maximum efficacy was observed against H. diminuta as compared to S. obvelata in a dose-dependent manner, indicating that the plant possesses a better cestocidal activity. These findings are in agreement with the reports of other workers who studied the anthelmintic potentials of medicinal plants. In vitro studies of S. sesban have shown its hydroethanolic and aqueous leaf extract to be effective against cestode, Moneizia expansa and Paramphistomum flukes (Limsay et al. 2014). Tandon et al. (1996) reported that the root tuber peel extract of Flemingia vestita, when tested against commonly available helminth parasites such as, Ascaris suum, A. lumbricoides, Heterakis gallinarum, Raillietina echinobothrida, Paramphistomum sp., showed in vitro anthelmintic efficacy. An in vitro anthelmintic assay of Alpinia nigra showed that the ethanolic extract possessed effective anthelmintic efficacy against Fasciolopsis buski (Swargiary and Roy 2015). In a related study by Bøgh et al. (1996), on anthelmintic efficacy of the dried fruits of Embelia schimperi, the plant extract showed significant in vitro as well as in vivo effects against H. diminuta. However, there was no in vivo effect against Hymenolepis microstoma, Echinostoma caproni and Heligmosomoides polygyrus, although there was in vitro efficacy. Thus, the in vitro study indicates that S. sesban var. bicolor possess anthelmintic property and to substantiate the present findings, further study was undertaken to test the extract in two in vivo models.
In vivo assay revealed that at the highest dose, rats infected with H. diminuta, showed a higher reduction of worm and EPG counts as compared to mice infected with S. obvelata. In a similar study by Nath and Yadav (2015), the leaf extract of Hibiscus rosa-sinensis showed promising anticestodal efficacy. Interestingly in a similar study by Gogoi and Yadav (2016), the methanolic extract of Caesalpinia bonducella showed a better in vivo nematicidal efficacy. In another report by Sapaat et al. (2012), papaya seeds were tested for anthelmintic efficacy against H. diminuta and there was a reduction in EPG as well as worm counts in a dose-dependent manner. This may be due to the various phytochemicals present in each of these plant parts which bring about such an effect. In a study by Vijaya et al. (2018) on in vitro and in vivo anthelmintic efficacy of two phytochemicals, ursolic acid and betulinic acid against S. obvelata, the result showed a dose-dependent efficacy. Therefore it may be stated that phytochemicals play an active role in a plant’s efficacy and hence, phytochemical tests on S. sesban var. bicolor were performed.
Phytochemical tests revealed the presence of alkaloids, glycosides, flavanoids, terpenoids, tannins, phlobatannins and saponins. Other studies, however, did not show the presence of saponin (Mythili and Ravindhran 2012). The presence of alkaloids, flavonoids, terpenoids, tannins, phlobatannins, saponins and glycosides has also been reported in a study on the phytochemistry of the genus S. sesban by Kumar et al. (2014). According to Bauri et al. (2015), phenolic compounds, flavonoids and tannins interfere with the energy generation mechanism and/or the glycoprotein of the cell surface/cuticle of parasites leading to their death. Likewise, alkaloids have also been reported to act on the central nervous system of parasites and lead to their paralysis. The active column fraction can be further studied to find the active compound followed by its characterization. This active compound can further undergo preclinical tests before it can be applied to human use.
In conclusion, the findings of the present study show that S. sesban var. bicolor leaf extract possesses significant anthelmintic anticestodal efficacy although the effect was comparatively less in nematode, and validates its traditional claims as an anthelmintic among the Santhal tribe. Therefore, the findings of this study bear relevance with regard to the fact that a large majority of the people in this region consume pork and beef in their staple diets. The consumption of these meats poses a threat for cestode infection in this region. Thus the traditional use of this plant against cestode infection will be useful in the traditional medicine of the Santhal tribe.
Availability of data and material
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Athanasiadou S, Githiori J, Kyriazakis I (2007) Medicinal plants for helminth parasite control: facts and fiction. Animal 1:1392–1400
Bauri RK, Tigga MN, Kullu SS (2015) A review on use of medicinal plants to control parasites. Indian J Nat Prod Resour 6(4):268–277
Bøgh HO, Andreassen J, Lemmich J (1996) Anthelmintic usage of extracts of Embelia schimperi from Tanzania. J Ethnopharmacol 50(1):35–42
Debela E, Tolera A, Eik LO, Salte R (2012) Condensed tannins from Sesbania sesban and Desmodium intortum as a means of Haemonchus contortus control in goats. Trop Anim Health Prod 44:1939–1944
Deori K, Yadav AK (2016) Anthelmintic effects of Oroxylum indicum stem bark extract on juvenile and adult stages of Hymenolepis diminuta (Cestoda), an in vitro and in vivo study. Parasitol Res 115(3):1275–1285
Devi JAI, Muthu AK (2015) Isolation and characterization of active components derived from whole plant of Saccharum spontaneum (Linn.). Pharm Lett 7(8):197–203
Dorsaz AC, Hostettmann M, Hostettmann K (1988) Molluscicidal saponins from Sesbania sesban. Planta Med 54(3):225–227
El-Bahy NM, Bazh EKA (2015) Anthelmintic activity of ginger, curcumin, and praziquentel against Raillietina cesticillus (in vitro and in vivo). Parasitol Res 114:2427–2434
El-Emam MA, Mahmoud SS, Bayaumy FE (2015) Potential role of mefloquine (antimalarial drug) and methanol extract of Chenopodium ambrosioides and Sesbania sesban in mice infected with Schistosoma mansoni. Asian Pac J Trop Dis 5(8):608–613
Evans WC (1989) Trease and Evans’ pharmacognosy, 13th edn. Bailliere Tindall, London, pp 176–180
Gogoi S, Yadav AK (2016) In vitro and in vivo anthelmintic effects of Caesalpinia bonducella (L.) Roxb. leaf extract on Hymenolepis diminuta (Cestoda) and Syphacia obvelata (Nematoda). J Intercult Ethnopharmacol 5(4):427–433
Göhl B (1982) Les aliments du bétail sous les tropiques. FAO, Division de Production et Santé Animale, Roma
Gomase P, Gomase P, Anjum S, Shakil S, Shahnavaj KM (2012) Sesbania sesban Linn: a review on its ethnobotany, phytochemical and pharmacological profile. Asain J Biomed Pharma Sci 2(12):10–14
Goswami S, Mishra KN, Singh RP, Singh P, Singh P (2016) Sesbania sesban, a plant with diverse therapeutic benefits: an overview. J Pharma Res Edu 1(1):111–121
Gueye S, Diop MT, Seck D, Sembene M (2011) Biochemical fractions activity of Annona senegalensis Pers. extract leaves to protect groundnut against the seed-beetle Caryedon serratus Ol. (Coleoptera, Chrysomelidea, Bruchinae). IJPAES 1(2):122–130
Gutteridge RC (1993) The perennial Sesbania species. http://www.fao.org/ag/AGP/AGPC/doc/Publicat/Guttshel/x5556e08.html. Accessed 11 Aug 2018
Harborne JB (1973) Phytochemicals methods. Chapman and Hall, London, p 113
Ibrahim AM (1992) Anthelmintic activity of some Sudanese medicinal plants. Phyto Res 6(3):155–157
Kamel EG, El-Emam MA, Mahmoud SSM, Fouda FM, Bayaumy FE (2011) Parasitological and biochemical parameters in Schistosoma mansoni-infected mice treated with methanol extract from the plants Chenopodium ambrosioides, Conyza dioscorides and Sesbania sesban. Parasitol Int 60:388–392
Kathiresh M, Devi PS, Saravanakumar M (2012) Bioactive compounds in Sesbania sesban flower and its antioxidant and antimicrobial activity. J Pharm Res 5(1):390–393
Kohli DV (1988) A new saponin, stigmasta-5,24(28),-diene-3β-O-β-D-galactopyranoside, from seeds of Sesbania sesban. Fitoterapia 59:478–479
Kozan E, Küpeli E, Yesilada E (2006) Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J Ethnopharmacol 108(2):211–216
Kumar S, Bajwa BS, Kumar N (2014) Physico-chemical and phytochemical investigation of plant Sesbania sesban. Res J Pharm Biol Chem Sci 5(1):110–117
Limsay RP, Jangde CR, Jahan A, Uma PS (2014) In vitro anthelmintic activity of hydroethanolic and aqueous Sesbania sesban, Pers. leaf extract against Moneizia expansa and Paramphistomes. Int J Pharm Pharm Sci 6(2):504–505
Mani RP, Pandey A, Goswami S, Tripathi P, Kumudhavalli V, Singh AP (2011) Phytochemical screening and in vitro evaluation of antioxidant activity and antimicrobial activity of the leaves of Sesbania sesban (L) Merr. Free Radic Antioxid 3(1):66–69
Meade TM, Watson J (2014) Characterization of rat pinworm (Syphacia muris) epidemiology as a means to increase detection and elimination. J Am Assoc Lab Anim Sci 53(6):661–667
Murugan M, Muniyappan N (2018) Bio-inspired synthesis of silver nanoparticles using Sesbania sesban and their pharmacological applications. J Bionanoscience 12(2):211–219
Mythili T, Ravindhran R (2012) Phytochemical screening and antimicrobial activity of Sesbania sesban (L.) Merr. Asian J Pharm Clin Res 5(4):179–182
Nath P, Yadav AK (2015) Anticestodal properties of Hibiscus rosa-sinensis L. (Malvaceae): an in vitro and in vivo study against Hymenolepis diminuta (Rudolphi, 1819), a zoonotic tapeworm. J Parasit Dis 40(4):1261–1265
Nigussie Z, Alemayehu G (2013) Sesbania sesban (L.) Merrill: potential uses of an underutilized multipurpose tree in Ethiopia. Afr J Plant Sci 7(10):468–475
Pandhare RB, Sangameswaran B, Mohite PB, Khanage SG (2011) Antidiabetic activity of aqueous leaf extract of Sesbania sesban (L.) Merr. in Streptozotocin induced diabetic rats. Avicenna J Med Biotechnol 3(1):37–43
Sapaat A, Satrija F, Mahsol HH, Ahmad AH (2012) Anthelmintic activity of papaya seeds on Hymenolepis diminuta infections in rats. Trop Biomed 29(4):508–512
Singh B, Sharma R, Kalia AN, Kumar V (2017) Isolation and identification of chemical compounds from stem and roots of Sesbania sesban. Ann Plant Sci 6(10):1718–1719
Sofowora A (1993) Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd, Ibadan, p 289
Subramanian K, Kalava SV (2014) Anti-inflammatory potential of aqueous extract of Sesbania sesban (L.) Merr. Int J Pharm Pharm Sci 6(2):301–304
Swargiary A, Roy B (2015) In vitro anthelmintic efficacy of Alpinia nigra and its bioactive compound, astragalin against Fasciolopsis buski. Int J Pharm Pharm Sci 7(10):30–35
Tandon V, Pal P, Roy B, Rao HSP, Reddy KS (1996) In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant of Shillong, India. Parasitol Res 83:492–498
Tangpu V, Temjenmongla, Yadav AK (2006) Anticestodal property of Strobilanthes discolor: an experimental study in Hymenolepis diminut-rat model. J Ethnopharmacol 105(3):459–463
Tatiya AU, Dande PR, Mutha RE, Surana SJ (2013) Effect of saponins from of Sesbania sesban L. (Merr) on acute and chronic inflammation in experimental induced animals. J Biol Sci 13(3):123–130
Vijaya, Yadav AK (2016) In vitro anthelmintic assessment of selected phytochemicals against Hymenolepis diminuta, a zoonotic tapeworm. J Parasit Dis 40(3):1082–1086
Vijaya, Yadav AK, Gogoi S (2018) In vitro and in vivo anthelmintic efficacy of two pentacyclic triterpenoids, ursolic acid and betulinic acid against mice pinworm, Syphacia obvelata. J Parasit Dis 42(1):144–149
World Health Organization (2015) Investing to overcome the global impact of neglected tropical diseases: third WHO report on neglected diseases. WHO Press, Geneva
Yadav AK, Tangpu V (2012) Anthelmintic activity of ripe fruit extract of Solanum myriacanthum Dunal (Solanaceae) against experimentally induced Hymenolepis diminuta (Cestoda) infections in rats. Parasitol Res 110(2):1047–1053
Acknowledgements
The authors are thankful to the Department of Zoology, North-Eastern Hill University for providing necessary facilities. Due thanks is also acknowledged to Dr. Larisha Lyndem for allowing us to carry out in vivo experiments in the Parasitology Lab, Centre for Advanced Studies in Zoology, Visva Bharati University, Shantiniketan. The authors also wish to thank Dr. Saptarshi Roy and Bidisha Ukil, Parasitology Lab, Centre for Advanced Studies in Zoology, Visva Bharati University, Shantiniketan for their assistance while performing this study. Thanks is also due to the University Grants Commission, New Delhi for award of a fellowship.
Funding
No funding was received from any organization to carry out this work.
Author information
Authors and Affiliations
Contributions
All the authors have contributed equally to this study.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
All experiments on rats and mice were done after due approval from the Institutional Animal Ethics Committee (Animal Models) of North-Eastern Hill University, Shillong (Vide, Member Secretary, IEC, NEHU, dated December 4, 2014).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
All data and materials as well as software application or custom code support their published claims and comply with field standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soren, A.D., Chen, R.P. & Yadav, A.K. In vitro and in vivo anthelmintic study of Sesbania sesban var. bicolor, a traditionally used medicinal plant of Santhal tribe in Assam, India. J Parasit Dis 45, 1–9 (2021). https://doi.org/10.1007/s12639-020-01267-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01267-9