Abstract
Hydatidosisis a parasitic disease caused by the larval stage of Echinococcus granulosus with different genotypes, and major complications in vital organs such as liver, lungs and, brain. Also, this parasite can infect animals and cause economic damages. Recently, some investigations indicated that the genetic variation of the parasite affects the antigenic, immunogenic and pathogenic features. Therefore, present study conducted to genotyping of the E. granulosus larva based on mitochondrial cox1 gene in livestock in the endemic areas of Markazi province, Iran. In this study, 49 hydatid cysts samples collected from 36 sheep, 11 goats and 2 cattle from different slaughterhouses of Markazi province in central part of Iran, 2017. The mitochondrial cox1 gene was amplified and genotyping were accomplished using sequence analysis. The sequencing analysis indicated that the main genotype G1 (61%) and G3 (37%) were identified. Also, one of the samples shows similarity with the G2 (2%) genotype. The results showed the statistically significant differences between the genotypes in different livestock (P < 0.05). This study indicated that the main genotypes of E. granulosus in Markazi province are G1 and G3 which are related to dog/sheep strain. Therefore, parasite control in dogs and sheep can reduce the risk of transmission of infection to humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydatidosis is a parasitic disease caused by the larval stage of Echinococcus granulosus (Fadakar et al. 2015). The Hydatidosis prevalence has been raised in recent decades. The geographical distribution of the disease is depended on its host, for instance sheep as interface Host. The E. granulosus prevalence is higher in Moderate climate such as, Mediterranean, Asia, China, Russia and the north of the Africa (Grosso et al. 2012). This disease can cause the major complications in vital organs such as, liver, lungs and, brain. Also, this parasite can infect animals and cause economic damages (Rokni 2009). There are different investigations conducted in Iran for assessment of phonotypical and genotypic features of E. granulosus by using mitochondrial cox1 and Nad1 genes (Dousti et al. 2013; Mitra Sharbatkhori et al. 2011). The investigations introduce ten genotypes for this parasite which they called as G1 to G10 (Sharbatkhori et al. 2011; Nejad et al. 2010). The inducted studies showed that the major genotype in Iran and also the worldwide is the G1 (Harandi et al. 2002; Kinkar et al. 2018).
Recent investigations indicated that the genetic variation of the parasite affects the antigenic, immunogenic and pathogenic features (Kinkar et al. 2018; Eckert et al. 2001; Gholami et al. 2011; Romig et al. 2015). The parasite dominate genotype is different in the geographical regions which it indicate the importance of the genotyping the parasite in different geographical regions (Eckert et al. 2001; Gholami et al. 2011; Romig et al. 2015; Kinkar et al. 2018). The genotyping of the parasite can be important in prevention of transmission and the treatment in human populations. In a previous study, the high prevalence of the Echinococcosis was reported in livestock in Markazi province in the central part of Iran (Ghasemikhah et al. 2015).
Therefore, present study conducted to genotyping of the E. granulosus larval stage based on mitochondrial cox1 gene in livestock in the endemic areas of Markazi province, Iran.
Materials and methods
Sampling and sample preparation
Totally, 100 hydatid cyst specimens were collected from the slaughter houses in Markazi province, located in central part of Iran in 2017. The specimen enrolled into the current cross-sectional study was 49 hydatid cysts from 36 sheep, 11 goat and 2 cattle isolates from different slaughterhouses which confirmed macroscopically and microscopically. Also, this project supported by Ethical Committee of Hamadan University of Medical Sciences (Code no: P.16.35.6.399).
Nucleic acids extraction
DNA was extracted from the samples by using the commercial DNA extraction kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. Evaluation of purified nucleic acids was performed by using Nano Drop ND-1000® spectrophotometry in 260 nm (Thermo Fisher Scientific Inc., Waltham, MA, USA). Extraction product DNA was kept at − 20 °C.
Mitochondrial PCR amplification
A conventional-PCR (cPCR) performed for detection of E. granulosus by mitochondrial cox1gene. The forward primer (JB3) (TTT TTT GGG CAT CCT GAG GTT TAT) and reverse (JB4.5) (TAA AGA AAG AAC ATA ATG AAA ATG) were used by 450 bp amplicon sizes (Bowles et al. 1992). The reaction mix contains 0.5 µM of template DNA or controls, 0.5 mM of dNTP mix, 1 µM of each primer, 1.5 µM MgCl2, and 0.5 units/µl of Taq DNA polymerase (Fermentas GmbH, Germany), 2 µl PCR buffer and sterilized DW added to round out the total volume to 15 µl for each reaction. A Bio-Rad thermo cycler (T100™ Thermal Cycler) was used for heating programs. Thermo cycler heating protocol was included 5 min at 95 °C, 40 cycles of the 60 s at 90 °C, 30 s at 60 °C, 120 s at 72 °C and one final extension step at 72 °C for 10 min. Also, PCR products gel electrophoresis was performed by Tris-Boric Acid-EDTA 1 × buffer system and the 1.5% agarose gel stained by Ethidium bromide for visualization the UV.
Sequencing
The cox1-PCR products were sequenced employing the same primers conducted in the primary PCR. The obtained data were aligned by the reference genotypes of E. granulosus in Gene bank to determine the genotypes using CLC Main Workbench 5 software
Statistical analysis
Statistical analyses and the basic descriptive and frequency variables performed by SPSS version 16 software (SPSS Inc., Chicago, IL, USA). Statistical significance was considered with P values < 0.05.
Results
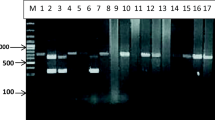
The PCR amplification of 49 hydatid cyst samples have shown 450 bp band for cox1 gen and genotypes were determined based on sequencing (Fig. 1). The result indicated that the main genotype in of E. granulosus in this study was G1. The G1 isolates prevalence in this study was 61% and follows by G3 37% in samples. The additional data of genotypes are shown in Table 1.
Also, in this study the prevalence of G1 genotype of E. granulosushave shown 57.5% in sheep, 81.8% in goats. Significant difference was found in the different livestock and genotypes (P < 0.05), but no significant differences with the involved organ (Table 1). The majority of the hydatid cyst samples isolates in this study obtained from liver (40 samples) (P > 0.05).
Discussion
This study is conducted to determined genotypes by mitochondrial cox1 gene of E. granulosus larval stage isolates from sheep, goats and cattle in the Markazi province, central part of Iran. The main reason for using mitochondrial genes is this genes conservation (Kinkar et al. 2017). The result of the current study indicated that the main genotype in of E. granulosus larval by cox1 gene is G1. The G1 isolates prevalence in this study was 61% and follows by G3 37% in our samples. In the similar study conducted by Shahnazi et al., in 2014 indicated that the major genotype of the E. granulosus in Esfahan, Iran is the G1 genotype (Shahnazi et al. 2011). Also, study in Isfahan confirmed the G1 genotype of the E. granulosus in 74.2% of samples and induce this genotype as major genotype in this province. In the other study by Dousti et al., in 2014 G1 reported as the main genotype in Ilam, Iran (Dousti et al. 2013). The results in our study confirm these data and indicate the importance of the G1 genotype in Iran. Also, the differences in the reported prevalence are due to the difference in the samples in these studies.
In our study, the major genotype of E. granulosus was G1 in sheep, goat isolates and cattle isolates G2 and G3 genotype. Also, in compare to study in the north of Iran it is been showed the 100% of sheep isolates were G1 (Fadakar et al. 2015). The conducted results from these study confirmers our result in the association of the livestock and genotype. Furthermore, in the study conducted in 2016 by Hizem et al. has shown that there is no association between the infected organ and the E. granulosus infected genotype. Also, there was no difference between the infected organ and parasite genotype. The result from present study also confirmers this finding (Hizem et al. 2016).
Some of conducted studies and their result about the genotypes of E. granulosus in Iran is summarized in Table 2. Khademvatan et al. (2018) in a review study on 73 documents from Iran different regions, indicated that the major genotypes are G1, G6 and, G3 which they have been reported in 320, 13 and 7 cases respectively (Khademvatan et al. 2018). Also, Sharafi et al., in 2014 investigated the genotypes prevalence of E. granulosus in Iran. Their result shows that G1, G2, G3 and G6 are the most common genotypes in humans in Iran (Sharafi et al. 2014). The differences in these studies and the present study could be justified by differences in samples and sample size. Our present study indicated that the main genotypes in Markazi province are G1 and G3 which are related to sheep strain. In the life cycle of these genotypes, the dog is the definitive host and livestock are known as intermediate host. Therefore, parasite control in dogs and sheep can reduce the risk of transmission of infection to humans. Investigations conducted in this field show the needs for the further studies about the association of the genotypes and different types of livestock. The major limitation of this study could be the sample size.
References
Bowles J, Blair D, McManus DP (1992) Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol 54(2):165–173
Dousti M, Abdi J, Bakhtiyari S, Mohebali M, Mirhendi S, Rokni M (2013) Genotyping of hydatid cyst isolated from human and domestic animals in Ilam Province, Western Iran using PCR-RFLP. Iran J Parasitol 8(1):47–52
Eckert J, Gemmell M, Meslin FX, Pawlowski ZS, World Health Organization (2001) WHO/OIE manual on echinococcosis in humans and animals: a public health problem of global concern. In: Eckert J et al (eds) World Organisation for Animal Health, Paris. http://www.who.int/iris/handle/10665/42427
Fadakar B, Tabatabaei N, Borji H, Naghibi A (2015) Genotyping of Echinococcus granulosus from goats and sheep indicating G7 genotype in goats in the Northeast of Iran. Vet Parasitol 214(1–2):204–207. https://doi.org/10.1016/j.vetpar.2015.09.029
Gezehegn D, Abay M, Tetemke D, Zelalem H, Teklay H, Baraki Z, Medhin G (2017) Prevalence and factors associated with intestinal parasites among food handlers of food and drinking establishments in Aksum Town, Northern Ethiopia. BMC Public Health 17(1):819. https://doi.org/10.1186/s12889-017-4831-5
Ghasemikhah R, Shahdoust M, Sarmadian H, Rezaei M, Ghorbanzadeh B, Gorji A, Zare-Bidaki M (2015) Echinococcosis in livestock slaughtered in arak industrial abattoir in Central Iran during 2006 to 2012. West Indian Med J. https://doi.org/10.7727/wimj.2015.153
Gholami S, Irshadullah M, Mobedi I (2011) Rostellar hook morphology of larval Echinococcus granulosus isolates from the Indian buffalo and Iranian sheep, cattle and camel. J Helminthol 85(3):239–245. https://doi.org/10.1017/S0022149X10000520
Gholami S, Sosari M, Fakhar M, Sharif M, Daryani A, Hashemi M, Vahadi M (2012) Molecular characterization of Echinococcus granulosus from hydatid cysts isolated from human and animals in Golestan Province, North of Iran. Iran J Parasitol 7(4):8–16
Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A (2012) Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol 18(13):1425–1437. https://doi.org/10.3748/wjg.v18.i13.1425
Hajialilo E, Harandi MF, Sharbatkhori M, Mirhendi H, Rostami S (2012) Genetic characterization of Echinococcus granulosus in camels, cattle and sheep from the south-east of Iran indicates the presence of the G3 genotype. J Helminthol 86(3):263–270. https://doi.org/10.1017/s0022149x11000320
Haniloo A, Farhadi M, Fazaeli A, Nourian N (2013) Genotype characterization of hydatid cysts isolated from Zanjan using PCR-RFLP technique. ZUMS J 21(84):57–65
Harandi MF, Hobbs R, Adams P, Mobedi I, Morgan-Ryan U, Thompson R (2002) Molecular and morphological characterization of Echinococcus granulosus of human and animal origin in Iran. Parasitology 125(4):367–373
Hizem A, M’rad S, Oudni-M’rad M, Mestiri S, Hammedi F, Mezhoud H, Zakhama A, Mokni M, Babba H (2016) Molecular genotyping of Echinococcus granulosus using formalin-fixed paraffin-embedded preparations from human isolates in unusual tissue sites. J Helminthol 90(4):417–421. https://doi.org/10.1017/S0022149X15000516
Khademvatan S, Yousefi E, Rafiei A, Rahdar M, Saki J (2013) Molecular characterization of livestock and human isolates of Echinococcus granulosus from south-west Iran. J helminthol 87(2):240–244. https://doi.org/10.1017/S0022149X12000296
Khademvatan S, Majidiani H, Foroutan M, Tappeh KH, Aryamand S, Khalkhali H (2018) Echinococcus granulosus genotypes in Iran: a systematic review. J Helminthol 93(2):131–138. https://doi.org/10.1017/S0022149X18000275
Kinkar L, Laurimäe T, Sharbatkhori M, Mirhendi H, Kia EB, Ponce-Gordo F, Andresiuk V, Simsek S, Lavikainen A, Irshadullah M, Umhang G, Oudni-M’rad M, Acosta-Jamett G, Rehbein S, Saarma U (2017) New mitogenome and nuclear evidence on the phylogeny and taxonomy of the highly zoonotic tapeworm Echinococcus granulosus sensu stricto. Infect Genet Evol 52:52–58. https://doi.org/10.1016/j.meegid.2017.04.023
Kinkar L, Laurimäe T, Acosta-Jamett G, Andresiuk V, Balkaya I, Casulli A, Gasser RB, van der Giessen J, González LM, Haag KL, Zait H, Irshadullah M, Jabbar A, Jenkins DJ, Kia EB, Manfredi MT, Mirhendi H, M’rad S, Rostami-Nejad M, Oudni-M’rad M, Pierangeli NB, Ponce-Gordo F, Rehbein S, Sharbatkhori M, Simsek S, Soriano SV, Sprong H, Šnábel V, Umhang G, Varcasia A, Saarma U (2018) Global phylogeography and genetic diversity of the zoonotic tapeworm Echinococcus granulosus sensu stricto genotype G1. Int J Parasitol 48(9–10):729–742. https://doi.org/10.1016/j.ijpara.2018.03.006
Nejad MR, Mojarad EN, Norouzina M, Harandi MF (2010) Echinococcosis: based on molecular studies in Iran. Gastroenterol Hepatol Bed Bench 3(4):169–176. https://doi.org/10.22037/ghfbb.v3i4.116
Nikmanesh B, Mirhendi H, Ghalavand Z, Alebouyeh M, Sharbatkhori M, Kia E, Mohebali M, Rokni MB (2014) Genotyping of Echinococcus granulosus isolates from human clinical samples based on sequencing of mitochondrial genes in Iran, Tehran. Iran J Parasitol 9(1):20–27
Parsa F, Harandi MF, Rostami S, Sharbatkhori M (2012) Genotyping Echinococcus granulosus from dogs from Western Iran. Exp Parasitol 132(2):308–312. https://doi.org/10.1016/j.exppara.2012.07.010
Pezeshki A, Akhlaghi L, Sharbatkhori M, Razmjou E, Oormazdi H, Mohebali M, Meamar AR (2013) Genotyping of Echinococcus granulosus from domestic animals and humans from Ardabil Province, northwest Iran. J Helminthol 87(4):387–391. https://doi.org/10.1017/s0022149x1200051x
Rokni M (2009) Echinococcosis/hydatidosis in Iran. Iran J Parasitol 4(2):1–16
Romig T, Ebi D, Wassermann M (2015) Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet Parasitol 213(3–4):76–84. https://doi.org/10.1016/j.vetpar.2015.07.035
Rostami S, Shariat Torbaghan S, Dabiri S, Babaei Z, Ali Mohammadi M, Sharbatkhori M, Fasihi Harandi M (2015) Genetic characterization of Echinococcus granulosus from a large number of formalin-fixed, paraffin-embedded tissue samples of human isolates in Iran. Am J Trop Med Hyg 92(3):588–594. https://doi.org/10.4269/ajtmh.14-0585
Sadjjadi SM, Mikaeili F, Karamian M, Maraghi S, Sadjjadi FS, Shariat-Torbaghan S, Kia EB (2013) Evidence that the Echinococcus granulosus G6 genotype has an affinity for the brain in humans. Int J Parasitol 43(11):875–877. https://doi.org/10.1016/j.ijpara.2013.06.008
Sadri A, Moshfe A, Doosti A, Ansari H, Abidi H, Ghorbani Dalini S (2012) Characterization of isolated hydatid cyst from slaughtered livestock in Yasuj industrial slaughterhouse by PCR-RFLP. Armaghane danesh 17(3):243–252
Shahnazi M, Hejazi H, Salehi M, Andalib AR (2011) Molecular characterization of human and animal Echinococcus granulosus isolates in Isfahan, Iran. Acta Trop 117(1):47–50. https://doi.org/10.1016/j.actatropica.2010.09.002
Shamsi M, Dalimi A, Khosravi A, Ghafarifar F (2015) Determination of genotype isolates of human and sheep hydatid cyst in Ilam. Sci J Ilam Univ Med Sci 23(2):111–119
Sharafi SM, Rostami-Nejad M, Moazeni M, Yousefi M, Saneie B, Hosseini-Safa A, Yousofi-Darani H (2014) Echinococcus granulosus genotypes in Iran. Gastroenterol Hepatol Bed Bench 7(2):82–88
Sharbatkhori M, Harandi MF, Mirhendi H, Hajialilo E, Kia EB (2011) Sequence analysis of cox1 and nad1 genes in Echinococcus granulosus G3 genotype in camels (Camelus dromedarius) from central Iran. Parasitol res 108(3):521–527. https://doi.org/10.1007/s00436-010-2092-7
Sharbatkhori M, Tanzifi A, Rostami S, Rostami M, Fasihi Harandi M (2016) Echinococcus granulosus sensu lato genotypes in domestic livestock and humans in Golestan province, Iran. Rev Inst Med Trop Sao Paulo 58:38. https://doi.org/10.1590/s1678-9946201658038
Acknowledgements
We are very grateful to Dr. Mohammad Amin Tabatabaiefar for her helpful consultation and comments on the manuscript.
Funding
Vice-chancellor for Research and Technology, Hamadan University of Medical Sciences, Hamadan, Iran (Grant No. 9511267049).
Author information
Authors and Affiliations
Contributions
This study was conducted by BA, AHM, BK, MF, MM, SG, ASP, and RG. Also, RG designed the study. Laboratory evaluation was performed by BA, AHM and BK. Manuscript preparation was conducted by MF, MM, SG, ASP and RG. BA and AHM participated in the data analysis. BA and RG performed the statistical analysis. All authors includes BA, AHM, BK, MF, MM, SG, ASP and RG read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There was not any conflict of interests by all authors.
Ethical standard
All experimental procedures were approved by the Ethics Committee of Arak University of Medical Sciences, Iran.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abedi, B., Maghsood, A.H., Khansarinejad, B. et al. Genotyping of Echinococcus granulosus isolates from livestock based on mitochondrial cox1 gene, in the Markazi province, Iran. J Parasit Dis 43, 592–596 (2019). https://doi.org/10.1007/s12639-019-01132-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-019-01132-4