Abstract
Purpose
Core body temperature has been extensively investigated as a thereuptic target in care after cardiac arrest. Nevertheless, the integrity of thermoregulation in patients after cardiac arrest has not been well studied. We sought to evaluate whether low spontaneous body temperature after cardiac arrest is associated with increased death and a worse neurologic outcome, and whether patients with low spontaneous body temperature exhibit features suggestive of impaired thermoregulation.
Methods
We conducted a single-centre retrospective cohort study. We included all adult patients who underwent temperature control with hypothermia after cardiac arrest between 1 January 2014 and 30 June 2020. The primary exposure was low spontaneous core body temperature (< 35 °C) at initiation of hypothermia therapy. The primary outcome was in-hospital death and the secondary outcome was poor neurologic outcomes at discharge.
Results
Five hundred and ninety-seven adult patients, comprising both in- and out-of-hospital cardiac arrests, were included. Patients with low spontaneous body temperature also had slightly lower average temperature, and more frequent transient but controlled breakthrough fever episodes in the first 24 hr. In the multivariable logistic regression analysis, low spontaneous body temperature was associated with higher odds of in-hospital death (odds ratio, 2.9; 95% confidence interval, 1.9 to 4.2; P < 0.001).
Conclusion
In this single-centre retrospective cohort study, low spontaneous core body temperature was associated with poor outcomes in patients after cardiac arrest. Patients with low spontaneous body temperature also exhibited features suggestive of impaired thermoregulation. Further research is needed to determine whether body temperature upon presentation reflects the robustness of the patient’s underlying physiology and severity of brain insult after a cardiac arrest.
Résumé
Objectif
La température corporelle centrale a fait l’objet d’études approfondies en tant que cible thérapeutique dans les soins après un arrêt cardiaque. Néanmoins, l’intégrité de la thermorégulation après un arrêt cardiaque n’a pas été bien étudiée. Nous avons cherché à évaluer si une température corporelle spontanément basse après un arrêt cardiaque était associée à une augmentation de la mortalité et à une issue neurologique plus grave, et si les individus ayant une température corporelle spontanément basse présentaient des caractéristiques suggérant une altération de la thermorégulation.
Méthode
Nous avons mené une étude de cohorte rétrospective monocentrique. Nous avons inclus tou·tes les patient·es adultes ayant bénéficié d’un contrôle de température lors d’une hypothermie après un arrêt cardiaque entre le 1er janvier 2014 et le 30 juin 2020. L’exposition principale était une température corporelle centrale spontanément basse (< 35 °C) au début du traitement de l’hypothermie. Le critère d’évaluation principal était le décès à l’hôpital, et le critère d’évaluation secondaire était de mauvaises issues neurologiques à la sortie de l’hôpital.
Résultats
Cinq cent quatre-vingt-dix-sept patient·es adultes, ayant subi des arrêts cardiaques à l’hôpital ou hors de l’hôpital, ont été inclus·es. Les patient·es ayant une température corporelle spontanément basse avaient également une température moyenne légèrement plus basse et des épisodes de fièvre paroxystique transitoires mais contrôlés plus fréquents au cours des premières 24 heures. Dans l’analyse de régression logistique multivariée, une température corporelle spontanément basse était associée à une probabilité plus élevée de décès à l’hôpital (rapport de cotes, 2,9; intervalle de confiance à 95 %, 1,9 à 4,2; P < 0,001).
Conclusion
Dans cette étude de cohorte rétrospective monocentrique, une température corporelle centrale spontanément basse a été associée à de mauvais devenirs après un arrêt cardiaque. Les patient·es présentant une température corporelle spontanément basse présentaient également des caractéristiques suggérant une altération de la thermorégulation. D’autres recherches sont nécessaires pour déterminer si la température corporelle lors de la présentation reflète la robustesse de la physiologie sous-jacente des patient·es et la gravité de la lésion cérébrale après un arrêt cardiaque.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Neurologic injury comprises 46% of all-cause mortality in patients admitted to the intensive care unit (ICU) after cardiac arrest.1 In 2002, two landmark research studies found that neurologic outcomes may be improved by cooling the patient to 32–34 °C for 12–24 hr.2,3 Since then, temperature control with hypothermia has been incorporated in the treatment algorithm for adult patients after cardiac arrest,4,5,6 guided by protocols on patient selection and optimal cooling temperatures.7,8,9
Apart from treating temperature as a therapeutic end point, Yoshimura et al. and Benz–Woerner et al. found that lower core body temperatures at the time of hospital admission, rapid achievement of the target temperature, and longer duration of passive rewarming were associated with increased mortaliy.10,11 These results suggest that the integrity of thermoregulation characterized by core body temperature at hospital admission, and ability to avoid poikiothermia, may reflect the patient’s underlying physiologic robustness and severity of brain insult after a cardiac arrest.
The current literature investigating the integrity of thermoregulation in relation to patient outcomes is limited because studies on the topic are scarce, raising concerns about the reproducibility of the results. Furthermore, these studies often have small sample sizes, which further limits their generalizability. Previous studies have postulated that a low spontaneous body temperature might indicate impaired thermoregulation; however, these studies have not thoroughly explored additional physiologic parameters, such as temperature patterns, which could provide further evidence to support this postulation.
We hypothesized that low spontaneous body temperature may be associated with increased in-hospital death after return of spontaneous circulation (ROSC) in patients with cardiac arrest. We sought to examine the association between low spontaneous core temperature at the time of hypothermia initiation and in-hospital death. In doing so, we defined impaired thermoregulation as a spontaneous core temperature of < 35 °C at the time of temperature control with hypothermia initiation, which was based on the finding of previous studies conducted by Benz–Woerner et al. and den Hartog et al.11,12 We also hypothesized that the temporal temperature patterns would differ between patients with impaired and intact thermoregulation. Thus, we aimed to explore the 24-hr temporal temperature patterns of impaired thermoregulation such as transient breakthrough temperature > 37.5 °C during hypothermia therapy (lasting 48 hr).
Methods
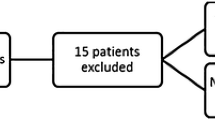
We conducted a single-centre retrospective cohort study that assessed the relationship between low spontaneous core temperature at the time of hypothermia initiation (primary exposure) and outcomes in both in- and out-of-hospital patients with cardiac arrest who underwent temperature control with hypothermia after ROSC. The primary outcome was in-hospital death. After obtaining approval from the research ethics board (REB: 116485; Western University, London, ON, Canada; principal investigator, Jason Chui), we screened 840 patient records and extracted data from 597 patients who achieved ROSC and received temperature control with hypothermia between 1 January 2014 and 30 June 2020.
Study cohort
We identified study patients through an electronic database maintained by the London Health Sciences Centre (LHSC), London, ON, Canada. We excluded adult (age ≥ 18 yr old) patients who had ROSC after in- or out-of-hospital cardiac arrest and received temperature control with hypothermia between 1 January 2014 and 30 June 2020. We chose the study start time, 1 January 2014, to coincide with the implementation of electronic orders for hypothermia therapy at our institution. We excluded patients if either primary exposure or primary outcome were not available. We considered a minimum of 24-hr temperature data to be necessary to describe the temperature pattern that could appropriately characterize the patients’ thermoregulation status. Therefore, we excluded patients if hypothermia therapy was not initiated at our institution, if hypothermia therapy occurred during transfer between hospitals, or if hypothermia therapy was terminated because life support was withdrawn within 24 hr.
Routine temperature control with hypothermia care at our institution allows for a core body temperature of 32–34 °C to be maintained for 24 hr and continuous core body temperature monitoring with hourly documentation. Cooling was achieved with a cooling blanket and nonheated airway humidification; however, if this was insufficient, patients could be given 500 mL of refrigerated normal saline (0.9%) solution intravenously over 15 min as needed. After 24 hr of cooling, passive rewarming was initiated with a target temperature of 37.5 °C within six hours. If this was not achieved, active rewarming with a warming blanket could be initiated. Hyperthermia prevention (> 37.5 °C) was maintained from 24–96 hr post cooling.
Exposure variables
We defined low spontaneous body temperature as a spontaneous core temperature of < 35 °C at the time of hypothermia initiation, which was based on the previous finding that an admission temperature of < 35 °C was associated with lower survival.1,2 Impaired thermoregulation was defined as the inability of autonomic and behavioural responses to maintain body temperature within the physiologic range; however, it is not an operative definition in clinical practice.10 Therefore, we first defined all patients with a spontaneous core temperature of < 35 °C at the time of hypothermia initiation to have impaired thermoregulation, and then further characterized additional features of thermoregulation like the average and variation in body temperature and the presence and duration of breakthrough fever. We chose a duration of 24 hr to allow for adequate description of the temperature pattern and to minimize missing temperature data once the maintenance phase of hypothermia therapy was completed. The goal was to determine if these indirect measures of impaired thermoregulation would support our definition based on spontaneous core body temperature at the time of hypothermia initiation. We defined breakthrough fever as any spike in body temperature > 37.5 °C. Average body temperature during hypothermia therapy was defined as the average hourly core body temperature from initiation of hypothermia therapy up to 48 hr after. The hourly core body temperature was obtained via either esophageal, bladder, or pulmonary artery catheter measurements.
Outcome variables
The primary outcome was in-hospital death. We used death occurring in the hospital to minimize the amount of missing data. The cause of death was obtained from the medical certificate of death. Neurologic-related death such as brain injury or brain death was listed as the primary cause of death on the death certificate (Electronic Supplementary Material [ESM] eTable 1).
The secondary outcomes included neurologic outcome at institutional discharge, ICU length of stay, and hospital length of stay. The neurologic outcome was collected at the time of hospital discharge (i.e., discharge from either acute care, long-term care, or rehabilitation facilities in continuity with the encounter for cardiac arrest management). We scored the neurologic status using a dichotomized form of the Cerebral Performance Category (CPC) score, where a score of ≤ 2 was defined as good neurologic status and a score > 2 was defined as poor neurologic status4,9 (ESM eTable 2).
Data collection
We retrieved patient demographics from electronic patient records. We manually collected cardiac arrest characteristics, patient body temperature during hypothermia therapy (up to 48 hr), method of temperature measurement, and outcome variables from paper charts. Patient temperature data were taken from the time of hypothermia therapy initiation to 48 hr later, thus allowing for capture of the rewarming phase (i.e., 24 hr after cooling). We collected and managed study data using REDCap® (Vanderbilt University, Nashville, TN, USA) electronic data capture tools. Attempts to minimize interrater variability during manual data extraction were accomplished by allowing a data extraction learning period with two patient records and data verification, providing a standardized data extraction protocol and training to each of the research assistants, and creating an open-door policy for questions (ESM eTable 1 provides guidelines to resolve contentious variables encountered during data collection). Additionally, each REDCap instrument had a maximum of two investigators performing independent, nonoverlapping data extraction, with primary outcomes being extracted by only one investigator. We performed double data extraction for 10% of randomly selected patients or patients with known data collection errors.
Statistical analysis
We summarized patient demographics, cardiac arrest characteristics, body temperature during hypothermia therapy, and outcomes. We also summarized the hourly body temperature during hypothermia therapy, hypothermia therapy characteristics, and outcomes for both patients with initial spontaneous temperature of < 35 °C or initial spontaneous temperature of ≥ 35 °C. We constructed a directed acyclic graph (DAG) to identify confounders in the regression analysis based on the recommendations from the critical care society (ESM eAppendix 1).13 We controlled age, diabetes, baseline CPC score, renal replacement therapy, cirrhosis, out-of-hospital cardiac arrest, witness cardiac arrest, bystander cardiopulmonary resuscitation (CPR), drug overdose, trauma, and winter season (November to February) in the analysis using the causal model method. Other variables examined were either not confounders or did not have enough supporting evidence. Further explanation of confounder selection is summarized in the DAG.
We then performed multivariable logistic regression analysis for in-hospital death with control for the aforementioned 11 confounders identified in the DAG. The strength of association was quantified by odds ratios (ORs) and 95% confidence intervals (CIs).
We further used a multivariable Cox proportional hazard model to assess the association between the survival time of patients and low spontaneous body temperature < 35 °C. Time-to-event analysis was performed to assess the probability of survival from hypothermia therapy initiation to either death or censoring at 180 days after hypothermia therapy initiation, whichever came first. We constructed a Kaplan–Meier survival graph to compare the survival probability between low and normal spontaneous body temperature. We further used log-rank test to assess difference of survival between two groups.
Due to limited knowledge in guiding construction of the DAG, we repeated our logistic regression analysis based on P value methods for variable selection to ensure the robustness of our results. Twenty variables were included in the univariable analysis of P value method. We then used variables with P < 0.1 to construct two multivariable logistic regression models for death and poor neurologic outcomes and a multivariable Cox proportional hazard regression model.
A subgroup analysis of included patients who had withdrawal of life support was performed for both multivariable logistic regression analysis and multivariable Cox proportional hazard analysis.
We employed the E-value method to evaluate the potential impact of unmeasured confounding.14,15,16 The E-value method estimates the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome, while accounting for other confounding variables, to fully explain away the observed association between the exposure and outcome.14,15,16
We performed all statistical analyses using Stata version 14.0 (StataCorp LLC, College Station, TX, USA). A P value < 0.05 was considered statistically significant.
Results
Eight hundred and forty patients underwent temperature control with hypothermia after ROSC between 1 January 2014 and 30 June 2020. We excluded 154 patients because life support was withdrawn within 24 hr of targeted temperature management initiation (i.e., no temperature data for 24 hr), 74 because hypothermia therapy was not initiated at LHSC, 14 because no temperature data were available, and one because of conflicting time data (where hypothermia therapy was initiated before the date of cardiac arrest). Therefore, 597 patients were included in our final analysis (ESM eAppendix 2). This cohort was predominately male (69%) with a mean (standard deviation [SD]) age of 61 (16) yr. Most patients (94%) had a good baseline neurologic status before cardiac arrest. Regarding cardiac arrest characteristics, 61% and 75% of patients experienced an out-of-hospital cardiac arrest and witnessed arrest, respectively. Bystander CPR was initiated in 64% of patients. Most (59%) presented with nonshockable rhythm, while only 19% of patients with cardiac arrest had an identifiable ST-elevation myocardial infarction on the electrocardiogram. Other baseline characteristics for the cohort of 597 patients are summarized in Table 1.
Of the 597 patients, 209 had low initial spontaneous core body temperature < 35 °C and 388 had an initial spontaneous core body temperature ≥ 35 °C. The mean (SD) spontaneous temperature at the time of hypothermia therapy initiation was 33.9 (0.9) °C in the low spontaneous core body temperature group, and 36.3 (0.8) °C in the group with spontaneous core body temperature ≥ 35 °C. The hourly body temperature between the two groups is shown in Fig. 1. The mean (SD) body temperature during hypothermia therapy in participants with low spontaneous core body temperature was slightly lower than that in the group with spontaneous core body temperature ≥ 35 °C (34.6 [1.0] °C and 35.0 [1.0] °C , respectively; Table 2). Patients with spontaneous core body temperature ≥ 35 °C had more frequent breakthrough fever (29% and 18%, respectively); however, the average cumulative duration of fever was short and was not different between two groups (Table 2). The short duration of fever in both groups may reflect the aggressive control of fever in our unit.
Hourly body temperature during targeted temperature management. The graph shows the hourly body temperature in two groups 48 hr since TTM initiation. The upper and lower whiskers represent the 75th and 25th percentiles of hourly temperature. The midline in the box represents the median hourly temperature. The upper and lower adjacent lines show the upper and lower adjacent values. The dots represent outliners. The mean (standard deviation) time from cardiac arrest to TTM initiation was 5.4 (10.5) hr.
TTM = targeted temperature management
In-hospital death
Three hundred and twenty-five of 597 patients (54%) died in the hospital. The cause of death was specified in 313 patients, 227 of whom (73%) died of neurologic causes, 32 (10%) of cardiovascular causes, and 54 (17%) of other causes (Table 2).
In the multivariable logistic regression, low spontaneous body temperature was associated with increased in-hospital death (OR, 2.9; 95% CI, 1.9 to 4.2; P < 0.001) (Table 3) with control of 11 confounders.
From the Kaplan–Meier plot, the median duration of time to death or censoring was 24 days. Most deaths occurred shortly after hypothermia therapy initiation, after which the mortality rate reached a plateau 30 days after hypothermia therapy initiation. The probability of death was significantly greater in the low spontaneous core body temperature group than in the group with spontaneous core body temperature ≥ 35 °C (log-rank test, P < 0.001) (Fig. 2). In the multivariate Cox proportional hazard regression analysis, low spontaneous body temperature was positively associated with increased in-hospital death (hazard ratio [HR], 1.4; 95% CI, 1.2 to 1.7; P < 0.001).
Probability of survival up to 180 days after targeted temperature management initiation. The graph shows the probability of survival up to 180 days after TTM initiation among patients classified into two groups. Data were censored as either death occurred or reaching the last day of follow-up. The graph shows high probability of survival in patients who initial spontaneous body temperature ≥ 35 °C during TTM.
TTM = targeted temperature management
Secondary outcomes
A poor neurologic outcome at hospital discharge was found in 32% of patients. In the multivariable logistic regression analysis, low spontaneous body temperature was associated with increased risk of a poor neurologic outcome (OR, 1.7; 95% CI, 1.0 to 2.4; P < 0.001) (ESM eTable 4).
The mean (SD) ICU length of stay for patients with low spontaneous core body temperature was shorter than that for patients with a spontaneous core body temperature ≥ 35 °C (10 [16] and 18 [51] days, respectively). Nevertheless, this difference was related to the higher ICU mortality rate in patients with low spontaneous core body temperature. There was no statistically significant difference in hospital length of stay between the two groups for the participants who survived until ICU discharge. The time-to-event analysis also showed no difference in the time to hospital discharge between the two groups for the participants who survived until ICU discharge (log-rank test, P = 0.2) (ESM eFigure).
Ancillary analysis
The repeated multivariable logistic regression analysis and Cox proportional hazard regression analyses using the P value method for confounder selection yielded similar results to using the causal model (or DAG) method (ESM eTables 3–5). Using the P value method of confounder selection for multivariable logistic regression, patients with low spontaneous body temperature were associated with more in-hospital death (OR, 2.7; 95% CI, 1.8 to 4.0; P < 0.0001) (ESM eTables 3 and 4). In the Cox proportional hazard regression analyses using the P value method of confounder selection, low spontaneous body temperature was associated with increased in-hospital death (HR, 1.4; 95% CI, 1.2 to 1.6; P < 0.001) (ESM eTable 5).
The subgroup analysis revealed that the presence or absence of withdrawal of life support did not influence the association between initial spontaneous body temperature and in-hospital death (ESM eTable 6).
The calculated E-value for the association between initial spontaneous body temperature (exposure) and in-hospital death (primary outcome) was 2.8. This E-value represents the magnitude of unmeasured confounding required to invalidate the observed association. In other words, an unmeasured confounder would need to influence the spontaneous body temperature (exposure) and in-hospital death (outcome) by 2.8-fold, while also controlling other covariates. Given the context of this study, it is possible but unlikely that a variable capable of exerting such a substantial impact on both exposure and outcome exists that could significantly alter the study results. The details of calculation are presented in ESM eTable 7.
Discussion
Our study found that a low spontaneous body temperature < 35 °C at hypothermia therapy initiation was associated with increased death and worsened neurologic outcome in patients after cardiac arrest. Our results were consistent with those shown by prior studies,10,11,12,17 where low admission body temperature was associated with either higher mortality or poorer neurologic outcomes. Nevertheless, our study is the first to show that both death and poor neurologic outcomes are impacted by low spontaneous body temperature in the same data set. The consistency of our results with previous findings supports the validity of the observed association. Furthermore, our time-to-death analysis suggests that the majority of deaths occurred within the first 15 days of ICU admission, providing new insights for future outcome measurements.
Our results also suggest that patients with low spontaneous body temperature exhibited features indicative of impaired thermoregulation. Therefore, our findings support the postulation that the thermoregulatory function may be associated with the outcomes of patients after cardiac arrest. From a pathophysiologic standpoint, a low spontaneous core body temperature may reflect the robustness of the patients’ underlying physiology, especially after having undergone a period of global ischemia,18,19 or may reflect the degree of neurologic injury. Literature involving patients with ruptured cerebral aneurysms has shown that low admission body temperature was associated with delayed cerebral infraction. Traumatic brain injury patients with low admission body temperature were also shown to have lower admission Glasgow coma scale ratings.20 Furthermore, either extremely low or high brain temperatures in patients with traumatic brain injury were shown to be associated with poor long-term neurologic outcomes.20 Taken together, low spontaneous body temperature may reflect the severity of cerebral injury and systemic injury secondary to cardiac arrest. Nevertheless, it remains unknown whether low spontaneous body temperature is solely a poor prognosticator or if it holds potential as a therapeutic target for future interventions.
Integrity of thermoregulation is difficult to assess in practice. Our operative definition of low spontaneous body temperature at the time of hypothermia therapy initiation allows clinical application of our findings. The patients with intact thermoregulation were more likely to have higher average body temperature during hypothermia therapy and more frequent transient breakthrough fever. It is important to reiterate that the breakthrough fever in this study was transient and was similar in terms of duration in both groups. Therefore, our results did not imply presence of breakthrough fever was associated with better outcomes. Instead, the presence of transient but controlled breakthrough fever episodes may indicate the physiologic ability to mount a fever response and may indirectly reflect on the robustness of the patients’ physiology. Despite our findings suggesting that low spontaneous body temperature may indicate impaired thermoregulation, it is important to consider that various factors, such as environmental temperature, can contribute to both low body temperature and poor outcomes. Therefore, the causal relationship between impaired thermoregulation and low spontaneous body temperature remains speculative.
Limitations
This study is limited by its retrospective, single-centre design but the sample size is considerable. Although we employed two approaches to account for confounders, several important confounders impacting poor outcomes such as duration of cardiac arrest, time to CPR, APACHE II score, cumulative no-flow states and low-flow states were not included because consistent documentation was lacking. To address these unmeasured confounders in our models, we calculated the E-value to quantify their potential impact. Despite our best efforts, there may still be unmeasured confounders that could have affected the certainty of the associations. Additionally, although our findings suggest that a low spontaneous body temperature may indicate impaired thermoregulation, causal inference in this regard remains uncertain. Furthermore, we only included patients who underwent hypothermia therapy because these patients had mandatory hourly temperature measurements as per our institutional policy. Thus, our results may not be applicable to patients who require temperature management for the prevention of hypothermia, such as those who regain complete consciousness after resuscitation or present with brief cardiac arrest that allows for early extubation. One hundred and fifty-four patients who had withdrawal of life support within 24 hr of hypothermia therapy initiation were excluded because of incomplete hourly temperature data to characterize the status of thermoregulation. The exclusion of these patients may have introduced selection bias; however, our subgroup analysis of time to in-hospital death did not find any differences between subgroups (ESM eTable 6). We did not explore the reasons for withdrawal of life support nor did we include the neuro-prognostication data as it was beyond the scope of this study. The method of temperature measurement, such as esophageal, bladder, or other routes, was not consistently documented. Consequently, it was not possible to analyze its effect. Nevertheless, based on our clinical experience, the predominant method of measuring body temperature was through the use of an esophageal temperature probe. Lastly, although the temperature targets for therapeutic hypothermia in many institutes have been changed from 32–34 °C to 36 °C, the external validity of our results should not be limited because a previous clinical trial7 showed that the temperature target of hypothermia therapy does not influence death or poor neurologic outcomes.
Conclusion
In this single-centre retrospective cohort study, low spontaneous core body temperature was associated with poor outcomes in patients after cardiac arrest. Body temperature is not only a therapeutic target in patients who experience cardiac arrest but may also reflect the severity of the initial injury and/or the robustness of the patient’s residual physiology. Because of the limitations of this study, future research is needed to confirm and expand on our findings.
References
Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med 2004; 30: 2126–8. https://doi.org/10.1007/s00134-004-2425-z
Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346: 549–56. https://doi.org/10.1056/nejmoa012689
Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346: 557–563. https://doi.org/10.1056/nejmoa003289
Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122: S768–86. https://doi.org/10.1161/circulationaha.110.971002
Callaway CW, Donnino MW, Fink EL, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015; 132: S465–82. https://doi.org/10.1161/cir.0000000000000262
Howes D, Gray SH, Brooks SC, et al. Canadian guidelines for the use of targeted temperature management (therapeutic hypothermia) after cardiac arrest: a joint statement from the Canadian Critical Care Society (CCCS), Canadian Neurocritical Care Society (CNCCS), and the Canadian Critical Care Trials Group (CCCTG). Resuscitation 2016; 98: 48–63. https://doi.org/10.1016/j.resuscitation.2015.07.052
Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013; 369: 2197–206. https://doi.org/10.1056/nejmoa1310519
Hifumi T, Inoue A, Kokubu N, et al. Association between rewarming duration and neurological outcome in out-of-hospital cardiac arrest patients receiving therapeutic hypothermia. Resuscitation 2020; 146: 170–7. https://doi.org/10.1016/j.resuscitation.2019.07.029
Bray JE, Stub D, Bloom JE, et al. Changing target temperature from 33°C to 36°C in the ICU management of out-of-hospital cardiac arrest: a before and after study. Resuscitation 2017; 113: 39–43. https://doi.org/10.1016/j.resuscitation.2017.01.016
Yoshimura S, Kiguchi T, Irisawa T, et al. Association between initial body temperature on hospital arrival and neurological outcome among patients with out-of-hospital cardiac arrest: a multicenter cohort study (the CRITICAL study in Osaka, Japan). BMC Emerg Med 2022; 22: 84. https://doi.org/10.1186/s12873-022-00641-5
Benz-Woerner J, Delodder F, Benz R, et al. Body temperature regulation and outcome after cardiac arrest and therapeutic hypothermia. Resuscitation 2012; 83: 338–42. https://doi.org/10.1016/j.resuscitation.2011.10.026
Den Hartog AW, de Pont AC, Robillard LB, Binnekade JM, Schultz MJ, Horn J. Spontaneous hypothermia on intensive care unit admission is a predictor of unfavorable neurological outcome in patients after resuscitation: an observational cohort study. Crit Care 2010; 14: R121. https://doi.org/10.1186/cc9077
Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc 2019; 16: 22–8. https://doi.org/10.1513/annalsats.201808-564ps
Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019; 321: 602–3. https://doi.org/10.1001/jama.2018.21554
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167: 268–74. https://doi.org/10.7326/m16-2607
Blum MR, Tan YJ, Ioannidis JP. Use of E-values for addressing confounding in observational studies—an empirical assessment of the literature. Int J Epidemiol 2020; 49: 1482–94. https://doi.org/10.1093/ije/dyz261
Lyon RM, Richardson SE, Hay AW, Andrews PJ, Robertson CE, Clegg GR. Esophageal temperature after out-of-hospital cardiac arrest: an observational study. Resuscitation 2010; 81: 867–71. https://doi.org/10.1016/j.resuscitation.2010.03.017
Perkins GD, Callaway CW, Haywood K, et al. Brain injury after cardiac arrest. Lancet 2021; 398: 1269–78. https://doi.org/10.1016/s0140-6736(21)00953-3
Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: a review of therapeutic strategies. Circulation 2011; 123: 1428–35. https://doi.org/10.1161/circulationaha.110.988725
Sacho RH, Vail A, Rainey T, King AT, Childs C. The effect of spontaneous alterations in brain temperature on outcome: a prospective observational cohort study in patients with severe traumatic brain injury. J Neurotraum 2010; 27: 2157–64. https://doi.org/10.1089/neu.2010.1384
Author contributions
Annie Li contributed to study design, ethics application, data collection, data analysis, and manuscript preparation, and agrees to be accountable for all aspects of the work. Ahmed F. Hegazy contributed to study design, ethics application, and manuscript revision, and agrees to be accountable for all aspects of the work. Luis E. M. Vasquez, Lisa Liu, Alexandra M. Durocher, Andrea Vucetic, Arjun Patel, and Courtney Fleming contributed to data collection and manuscript revision, and agree to be accountable for all aspects of the work. Jason Chui contributed to study design, ethics application, data analysis, and manuscript preparation and agrees to be accountable for all aspects of the work.
Acknowledgments
We acknowledge Ms. Huifang Liu, Data Analyst at London Health Sciences Center, Canada and Mr. Jason Nagyszegi, Medical Clerk at London Health Sciences Center, Canada for patient identification and patient chart retrieval.
Disclosures
The authors declare no competing interests.
Funding statement
The author(s) received no financial support for the research, authorship, and/or publication of this work.
Prior conference presentations
An abstract was presented at the 2022 Canadian Anesthesiologists’ Society Annual Meeting (24–26 June, Halifax, NS, Canada).
Editorial responsibility
This submission was handled by Dr. Patricia S. Fontela, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, A., Hegazy, A.F., Vasquez, L.E.M. et al. Characterizing the impact of thermoregulation in patients after cardiac arrest: a retrospective cohort study. Can J Anesth/J Can Anesth 71, 629–639 (2024). https://doi.org/10.1007/s12630-024-02737-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-024-02737-x