Abstract
Purpose
Venous congestion is a pathophysiologic state that can result in organ dysfunction, particularly acute kidney injury (AKI). We sought to evaluate the feasibility of performing a definitive observational study to determine the impact of venous congestion quantified using point-of-care ultrasound (POCUS) in patients with septic shock.
Methods
We conducted a prospective observational feasibility study at two intensive care units (ICUs). We recruited adult patients with septic shock within 12 hr of ICU admission. Using the validated Venous Excess Ultrasound Score (VEXUS), we quantified venous congestion on day 1 and day 3 of ICU admission. The primary feasibility outcome was successful completion rate of the two VEXUS scores. We performed a survival analysis to quantify the hazard of renal replacement therapy (RRT).
Results
We enrolled 75 patients from January 2022 to January 2023. The success rate of completion for VEXUS scans was 94.5% (95% confidence interval [CI], 89.5 to 97.6). Severe venous congestion was present in 19% (14/75) of patients on ICU admission day 1 and in 16% (10/61) of patients on day 3. Venous congestion on ICU admission may be associated with a higher risk of requiring RRT (unadjusted hazard ratio, 3.35; 95% CI, 0.94 to 11.88; P = 0.06).
Conclusions
It is feasible to conduct a definitive observational study exploring the association between venous congestion quantified with POCUS and clinical outcomes in patients with septic shock. We hypothesize that venous congestion may be associated with an increased hazard of receiving RRT.
Résumé
Objectif
La congestion veineuse est un état physiopathologique qui peut entraîner un dysfonctionnement des organes, en particulier une insuffisance rénale aiguë (IRA). Nous avons cherché à évaluer la faisabilité de la réalisation d’une étude observationnelle définitive pour déterminer l’impact de la congestion veineuse quantifiée à l’aide de l’échographie ciblée (POCUS) chez des patient·es en choc septique.
Méthode
Nous avons réalisé une étude de faisabilité observationnelle prospective dans deux unités de soins intensifs (USI). Nous avons recruté des patient·es adultes souffrant d’un choc septique dans les 12 heures suivant leur admission aux soins intensifs. À l’aide du score VEXUS (score d’échographie de l’excès veineux) validé, nous avons quantifié la congestion veineuse au jour 1 et au jour 3 de leur admission aux soins intensifs. Le principal critère de faisabilité était le taux de réussite des deux scores VEXUS. Nous avons réalisé une analyse de survie pour quantifier le risque de thérapie de substitution rénale (TSR).
Résultats
Nous avons recruté 75 patient·es de janvier 2022 à janvier 2023. Le taux de réussite des scores VEXUS était de 94,5 % (intervalle de confiance [IC] à 95 %, 89,5 à 97,6). Une congestion veineuse sévère était présente chez 19 % (14/75) des patient·es au jour 1 d’admission aux soins intensifs et chez 16 % (10/61) des patient·es au jour 3. La congestion veineuse lors de l’admission aux soins intensifs peut être associée à un risque plus élevé de nécessiter une TSR (rapport de risque non ajusté, 3,35; IC 95 %, 0,94 à 11,88; P = 0,06).
Conclusion
Il est possible de mener une étude observationnelle définitive explorant l’association entre la congestion veineuse quantifiée par POCUS et les devenirs cliniques chez les patient·es en choc septique. Nous émettons l’hypothèse que la congestion veineuse peut être associée à un risque accru de recevoir une thérapie de substitution rénale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Septic shock results in mortality rates as high as 40% and is a leading cause of intensive care unit (ICU) admissions.1,2,3,4 Although early and aggressive intravenous fluid administration is recommended by clinical practice guidelines as a cornerstone treatment for septic shock, the optimal volume of intravenous fluid beyond the initial resuscitation and the timing for deresuscitation (i.e., stopping fluids and initiating diuresis) remain uncertain. Given the mounting evidence that suggests iatrogenic volume overload can precipitate organ injury,5,6,7 defining these endpoints are an important research priority to advance the care of patients in septic shock.
While there are multiple potential mechanisms by which excessive fluid resuscitation can cause organ injury, high central venous pressure underpins many of these pathways. High venous pressures precipitate endothelial dysfunction, interstitial edema, and organ congestion.8,9,10 Moreover, high venous pressures impair oxygen delivery to susceptible organs by reducing the perfusion pressure across vascular beds. Although high venous pressure has been associated with acute kidney injury (AKI) in critically ill patients, heterogeneity in patients’ physiology and measurement error precludes us from using central venous pressure to adequately guide fluid administration and guide deresuscitation.10,11
Aided by its portability, safety, and ability to provide real-time anatomic and physiologic data,10,12 point-of-care ultrasound (POCUS) has emerged as a useful tool to help tailor resuscitation strategies for patients with septic shock. Recent studies have shown that POCUS can quantify venous congestion by interrogating the size of the inferior vena cava (IVC) and blood flow patterns of the hepatic vein (HV), portal vein (PV), and intrarenal veins (IRV).10,13,14 Using these findings, one can grade the severity of venous congestion using a validated score called the Venous Excess Ultrasound Score (VEXUS).10,13,14 Higher VEXUS scores are associated with organ dysfunction, particularly AKI, in a broad cohort of critically ill patients,10,14,15,16,17 although its prevalence and significance for patients with septic shock remains unknown. Determining whether venous congestion is associated with clinical outcomes in septic shock could provide clinicians with an important bedside tool to guide intravenous fluid administration.
The primary objective of this study was to evaluate the feasibility of a larger observational study that will determine the association between venous congestion quantified using POCUS and clinical outcomes in patients with septic shock. The secondary objectives were to determine the prevalence of venous congestion in septic shock, describe the natural history of venous congestion on day 1 and day 3 of ICU admission, and explore the association between venous congestion and outcomes in patients with septic shock.
Methods
We conducted a pilot prospective multicentre cohort study of patients with septic shock admitted to the ICU. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement18 provided guidance on the design and reporting of the study. The Western Health Sciences Research Ethics Board in London, ON, Canada approved the conduct of this study (REB# 120202).
Setting
We recruited patients at the Critical Care Trauma Center (CCTC) at Victoria Hospital and the Medical Surgical Intensive Care Unit (MSICU) at University Hospital in London, ON, Canada. The CCTC is a 32-bed ICU that cares for patients with trauma and general medical and surgical issues. The MSICU is a 30-bed ICU that cares for patients with medical, surgical, neurosurgical, and transplant-related issues.
Patient enrolment
We enrolled adult patients (age ≥ 18 yr) with septic shock within 12 hr of ICU admission, defined as sepsis (according to the Sepsis-3 definition) with hypotension requiring vasoactive medications, as well as any one of the following features of end-organ dysfunction at the time of eligibility assessment: 1) serum lactate greater than or equal to 2.2 mmol·L−1, 2) AKI of at least Acute Kidney Injury Network stage I on ICU admission or decreased urine output (≤ 0.5 mL·kg−1·hr−1 for more than two hours after admission), 3) altered mental status, defined as a Glasgow Coma Scale < 13, or 4) the need for invasive mechanical ventilation.
We excluded patients who had any limitations on their wishes for life support interventions (e.g., do not resuscitate order) at the time of ICU admission, had a previous liver transplant, or with pre-existing end-stage renal disease requiring renal replacement therapy (RRT).
Venous congestion ultrasound
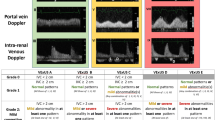
Trained ultrasound operators performed venous congestion ultrasound within 24 hr of ICU admission (day 1) and on ICU admission day 3. The venous congestion ultrasound evaluates four vessels: the IVC, HV, PV, and IRV. Measurements from these four vessels informed the validated VEXUS score.10,13,14 The VEXUS score ranges from 0 to 3, and scores of 2 or 3 represent severe congestion. The Electronic Supplementary Material eAppendix provides an overview of image acquisition and interpretation procedures for the VEXUS score.
Ultrasound operators used a curvilinear probe with an abdominal imaging preset. If the curvilinear probe did not produce adequate images, a phased array probe was used. We obtained images on a Mindray M9 machine (Mindray Bio-Medical Electronics Co., Ltd., Shenzen, China) and archived them in QPath (Telexy Healthcare Inc., Vancouver, BC, Canada), a privacy-compliant cloud-based archiving system. Ultrasound operators used electrocardiogram gating to correlate the Doppler tracings to the cardiac cycle and obtained measurements during end-expiration.
Ultrasound operators included fellows or attending physicians with experience performing Doppler ultrasound as part of their routine POCUS practice. Each operator underwent a two-hour didactic training session, and their first scan was directly supervised by one of the investigators (R. P.). Two experts (R. P. and J. B.) reviewed all POCUS scans independently to ensure that the acquisition was correct. We used the interpretation of the VEXUS operator in our final analysis. We quantified the interrater agreement between the two experts using an unweighted kappa.
Blinding practices
In keeping with previous recommendations for blinding practices during POCUS research,19 ultrasound operators performing venous congestion measurements were not blinded to the patient's clinical information. Nevertheless, the clinical team caring for the patient was blinded to the venous congestion ultrasound results to not influence the natural history of venous congestion, and occurrence of secondary outcomes that may be influenced by clinicians’ management decisions (e.g., initiation of RRT). The outcome assessors were blinded to the VEXUS results.
Data collection
We extracted data from the electronic medical record, paper chart, and the Critical Care Information System (CCIS). Baseline characteristics and patient demographics included age, sex, body mass index, admission multiorgan dysfunction score, pre-existing comorbidities, source of sepsis, creatinine, lactate, white blood cell, hemoglobin, and platelet count at the closest time of ICU admission. We also collected data on the cumulative fluid balance on day 1 and day 3 (at the time the VEXUS scan was performed), highest norepinephrine equivalent requirements on day 1, and the need for invasive or noninvasive mechanical ventilation at the time the VEXUS scan was performed. For cumulative fluid balance on day 1 and day 3, we determined the pre-ICU fluid balance in several different ways depending on the location of the patient before ICU admission. For patients from the emergency department (ED), we included the entire ED fluid balance. For patients from the ward, we looked at 72 hr of pre-ICU cumulative fluid balance. For patients from the operating room, we looked at the intraoperative fluid balance. We did not include fluid balance at outside hospitals for direct transfers.
At both participating sites, critical care practitioners routinely perform focused bedside echocardiography on most patients admitted to the ICU with shock to assess for biventricular function, valvulopathy, and to quantify cardiac output. If a cardiac POCUS or echocardiogram was performed within 12 hr of the VEXUS measurements, we collected data on left ventricular (LV) function, right ventricular (RV) size and function, moderate or severe tricuspid regurgitation, the tricuspid annular plane systolic excursion (TAPSE), RV S’ velocity on tissue Doppler imaging (TV S’) (cm·sec−1), and stroke volume determined through LV outflow tract velocity time integral and cardiac output (L·min−1). We defined LV dysfunction as a visual assessment of ejection fraction of < 55%. Right ventricular dysfunction was determined by qualitative assessment, or a TV S’ < 11 cm·sec−1 or a TAPSE < 17 mm.
Outcomes
The primary outcome was the successful completion of VEXUS scans on day 1 and day 3 for patients still in hospital (i.e., not discharged or deceased). We defined successful completion as adequate image acquisition to interpret the VEXUS score for the patient, as determined by a VEXUS expert (R. P.). Other feasibility outcomes included recruitment rate (number of patients recruited per month) and consent rate (total number of patients consented divided by the number of patients approached for consent). We set feasibility targets of recruitment rate of one patient per site per month, more than 80% of VEXUS scans completed, and less than 10% of patients declined consent. We obtained secondary outcome data from the electronic medical record and the CCIS database. The secondary outcomes included major adverse kidney events at 30 days (MAKE − 30, composite of death, use of RRT, or Creatinine ≥ 200% baseline at 30 days), use of RRT in the first 30 days, duration of vasoactive medications (vasopressors or inotropes) and mechanical ventilation, ICU length of stay, and 30-day mortality.
Sample size and statistical analyses
The sample size was based on achieving adequate precision around estimates for feasibility.20 We would deem a larger observational study feasible if the lower bound of the 95% confidence interval (CI) for completeness of venous congestion scans was 80%. Assuming a preliminary estimate of 90% and a margin of error of 10%, 75 patients would afford us 95% confidence that the venous congestion scan completion rate was at least 80%.20
We used IBM SPSS for Windows version 26 (IBM Corp., Armonk, NY, USA) and R version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria) software for data analysis. We present results of statistical tests as two-sided and considered a P value < 0.05 statistically significant. For continuous variables, we used mean and standard deviation to summarize data with normal distributions, and median and interquartile range [IQR] for nonnormally distributed data. Counts and percentages represented categorical variables. We classified patients with severe venous congestion (VEXUS score of 2 or 3) on day 1 of ICU admission as congested, while those with VEXUS scores of 0 or 1 were classified as not congested. We compared the baseline characteristics and outcomes of patients who were congested to those who were not congested using the Student’s t test, Mann–Whitney U test (for nonparametric variables), or Chi square test. We excluded missing data from any analyses without imputation. We had a complete data set for feasibility metrics, for which 95% CIs were calculated.
We used the Fine and Gray model for an unadjusted estimate of the hazard ratio (HR) of RRT, and then adjusted for the competing risk of death. We present the cumulative incidence function for the cumulative incidence renal replacement from the day of ICU admission to day 30. For this time-to-event analysis, we excluded four patients who had been started on intermittent RRT prior to admission to the ICU and completion of the VEXUS scan.
Results
From January 2022 to January 2023, we screened 340 patients and enrolled 75 patients in this feasibility study. Figure 1 presents an overview of screening and enrolment procedures. The overall completion rate for VEXUS scans was 95% (95% CI, 90 to 98). Portal veins, HV, and IRV Doppler could not be obtained in three, two, and two patients respectively (in these cases, the VEXUS score was calculated from the remaining Dopplers). The kappa between the two experts for VEXUS interpretation was excellent at 0.95 (95% CI, 0.90 to 1.0). Table 1 provides an overview of the feasibility outcomes.
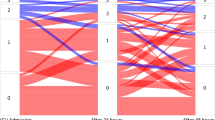
On day 1 of ICU admission, 19% (14/75) of patients showed venous congestion, while 16% (10/61) of patients showed venous congestion on day 3. Figure 2 provides the natural history of venous congestion. The distributions of VEXUS scores on day 1 and day 3 are provided in Fig. 3. We recruited 6.25 patients per month between the two sites. The consent rate for eligible patients was 100% (95% CI, 94 to 100).
VEXUS scores on day 1 and day 3. On day 1, 33 patients had a VEXUS score of 0, 28 patients had a VEXUS score of 1, eight patients had a VEXUS score of 2, and six patients had a VEXUS score of 3. On day 3, 30 patients had a VEXUS score of 0, 21 patients had a VEXUS score of 1, four patients had a VEXUS score of 2, six patients had a VEXUS score of 3, and 14 patients did not have a score because no repeat scan was completed (i.e., deceased, unable to complete in timeframe).
We found no statistically significant differences in age, sex, basal metabolic index, or multiorgan dysfunction score between patients with and without congestion. Patients with venous congestion were more likely to have a prior history of coronary artery disease (43% vs 7%; P = 0.02) and atrial fibrillation (50% vs 10%; P = 0.002), but we found no differences in the rate of chronic kidney disease, diabetes, chronic obstructive pulmonary disease, congestive heart failure, stroke, or cirrhosis. Similarly, we found no important differences between groups in the source of sepsis, admission serum lactate, hematology serum blood tests on ICU admission day 1, and need for mechanical ventilation. Table 2 provides an overview of the demographics and baseline characteristics for patients with and without venous congestion.
With respect to hemodynamic variables, we found no important differences in the highest dose of norepinephrine equivalents between patients with and without venous congestion. Patients with venous congestion were more likely to receive inotropic agents on day 1 (36% vs 11%; P = 0.03). Patients without congestion had a higher median positive fluid balance on day 1 (2,514 mL vs 1,296 mL; P = 0.09) and day 3 (5,510 mL vs 279 mL; P = 0.09) of ICU admission. Patients who were not congested on day 1 but became congested on day 3 had a higher median positive fluid balance compared with patients who were congested on day 1 but became noncongested on day 3 (9,200 mL vs − 368 mL; P = 0.13). Table 3 provides an overview of hemodynamic and resuscitation data.
Patients with venous congestion on day 1 of ICU admission were more likely to exhibit LV systolic dysfunction (43% vs 13%; P = 0.01), RV systolic dysfunction (57% vs 10%; P < 0.001), RV dilation (64% vs 21%; P = 0.006), and lower TV S’ (8.6 vs 14.0; P = 0.009). The rates of moderate or severe tricuspid regurgitation, stroke volume, cardiac output, or TAPSE were similar between groups. Table 4 provides a comparison of the echocardiographic data based on venous congestion.
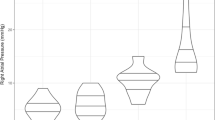
We did not find any important differences in MAKE-30, use of RRT at 30 days, vasopressor days, duration of mechanical ventilation, ICU length of stay, or 30-day mortality between patients with and without congestion. Patients with venous congestion may show an increased risk for requiring RRT (unadjusted HR, 3.35; 95% CI, 0.94 to 11.88; P = 0.06), but this finding was limited by CIs that crossed no effect (Fig. 4). Table 5 presents the secondary outcomes of patients with and without venous congestion.
Discussion
This pilot multicentre prospective cohort study takes the first step to understanding the prevalence, natural history, and associated outcomes in patients with septic shock and venous congestion. Based on the VEXUS scan completion rate, recruitment rate, and consent rate, it is feasible to undertake a larger observational study to address important knowledge gaps to determine whether venous congestion is an important physiologic marker to guide intravenous fluid administration. We also hypothesize that venous congestion may be associated with a higher risk of requiring RRT, but we require larger prospective studies to fully understand this relationship.
The derivation and validation of VEXUS occurred in a cardiac surgical population, where venous congestion was strongly associated with the development of AKI on day 7.10,15 Furthermore, markers of venous congestion are associated with longer duration of life support therapies and complications in cardiac surgical populations.21 Despite these findings, the biological thresholds for harm have not yet been established for venous congestion in patients with septic shock. It is conceivable that the threshold for venous congestion-induced organ injury may be lower in septic shock patients with endothelial dysfunction and capillary leak syndromes; however, this has yet to be determined. Future research should focus on determining the thresholds at which venous congestion is harmful for patients with septic shock.
In this study, nearly 20% of patients showed venous congestion on day 1 of ICU admission, which is slightly lower than the 40% seen in cardiac surgical populations.10,15,16 We also observed that venous congestion is a dynamic process; a number patients who were congested on day 1 were no longer congested on day 3 and vice versa. Interestingly, patients with congestion on day 1 had a lower fluid balance on day 1 and day 3. One explanation may be that certain patients show a lower threshold to develop venous congestion because of cardiac dysfunction. In our cohort, patients with venous congestion were also more likely to have biventricular failure. Another explanation is that patients with congestion had other markers of fluid overload that led to clinicians giving less fluid. Nevertheless, patients who transitioned from being congested on day 1 to not congested on day 3 had a more negative fluid balance on day 3, while patients who were not congested on day 1 but became congested on day 3 had a more positive fluid balance on day 3. Although this study was not powered to statistically ascertain these differences, the results raise the possibility that venous congestion may be dynamic and modifiable through control of intravascular volume. Previous findings show a large absolute difference, but statistical significance was not reached probably because the number of patients was small. These findings may also challenge the common practice of initiating or titrating diuretics based on 24-hr fluid balance alone.
While some clinicians may view venous congestion as an independent physiologic entity, some of our findings challenge this notion. Patients with venous congestion were more likely to exhibit biventricular dysfunction. Is venous congestion independently associated with worsening organ dysfunction or is it a downstream consequence of RV pathology? We hypothesize that multiple factors contribute to venous congestion. Patient-related factors, such as pre-existing comorbidities (e.g., diastolic dysfunction, pulmonary hypertension), disease-related factors (e.g., acute biventricular dysfunction, acidosis, hypoxemia), and treatment-related factors (e.g., intravenous fluid administration, mechanical ventilation, positive end expiratory pressure) may all converge to precipitate venous congestion in susceptible patients. Future studies should explore the interplay between cardiovascular dysfunction and venous congestion to determine which physiologic entities are the best candidates for targeted resuscitation strategies. We hypothesize that the management of venous congestion requires more than just the administration of diuretics and the withholding of intravenous fluids. Rather, clinicians need to employ a multimodal approach to treat RV and/or LV dysfunction, pulmonary hypertension, and intrabdominal hypertension that could be resulting in organ-level congestion.
This study has several important limitations. First, given the feasibility objectives, it was not powered to explore clinical outcomes. Therefore, all outcome data should be considered exploratory. Furthermore, the study was not powered to perform multivariable regression to determine whether venous congestion was independently associated with clinical outcomes. Second, missing venous congestion measurements on day 3 limit conclusions evaluating the evolution of venous congestion during the resuscitation phase. Some of these data were missing because of logistics, as day 3 fell on a Saturday or Sunday so providers were not available to perform the repeat VEXUS scan. With increasing depth of VEXUS experience and dedicated knowledge translation efforts, we hope to improve this to ensure feasibility of a definitive trial. Third, VEXUS was not initially derived nor validated in the septic shock population. Although VEXUS has construct validity, the thresholds for severity of venous congestion may not optimally discriminate which patients will experience harm.
Conclusion
The results of this study indicate that a larger prospective multicentre cohort study to determine whether venous congestion is independently associated with clinical outcomes in septic shock is feasible. Furthermore, this study raises the notion that venous congestion may be a modifiable risk factor as it appears that differences in fluid balances between day 1 and day 3 are associated with changes in congestion status for patients. Finally, venous congestion was associated with biventricular dysfunction on echocardiography and pre-existing cardiovascular disease. These findings suggest that venous congestion results from an interplay between cardiovascular status, respiratory status, and fluid balance, and is likely not an independent physiologic entity. Given the increasing organic adoption of VEXUS in clinical practice, there is an increasing sense of urgency to understand this complex physiologic process to optimize its safe and effective implementation into practice.
References
Brown RM, Semler MW. Fluid management in sepsis. J Intensive Care Med 2019; 34: 364–73. https://doi.org/10.1177/0885066618784861
Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372: 1301–11. https://doi.org/10.1056/nejmoa1500896
Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a Resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA 2019; 321: 654–64. https://doi.org/10.1001/jama.2019.0071
Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med 2014; 370: 1683–93. https://doi.org/10.1056/nejmoa1401602
Silversides JA, Fitzgerald E, Manickavasagam US, et al. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med 2018; 46: 1600–7. https://doi.org/10.1097/ccm.0000000000003276
Messmer AS, Zingg C, Müller M, Gerber JL, Schefold JC, Pfortmueller CA. Fluid overload and mortality in adult critical care patients—a systematic review and meta-analysis of observational studies. Crit Care Med 2020; 48: 1862–70. https://doi.org/10.1097/ccm.0000000000004617
Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol 2016; 17: 109. https://doi.org/10.1186/s12882-016-0323-6
Pouska J, Tegl V, Astapenko D, Cerny V, Lehmann C, Benes J. Impact of intravenous fluid challenge infusion time on macrocirculation and endothelial glycocalyx in surgical and critically ill patients. Biomed Res Int 2018; 2018: 8925345. https://doi.org/10.1155/2018/8925345
Milford EM, Reade MC. Resuscitation fluid choices to preserve the endothelial glycocalyx. Crit Care 2019; 23: 77. https://doi.org/10.1186/s13054-019-2369-x
Beaubien-Souligny W, Rola P, Haycock K, et al. Quantifying systemic congestion with point-of-care ultrasound: development of the venous excess ultrasound grading system. Ultrasound J 2020; 12: 16. https://doi.org/10.1186/s13089-020-00163-w
Sun R, Guo Q, Wang J, et al. Central venous pressure and acute kidney injury in critically ill patients with multiple comorbidities: a large retrospective cohort study. BMC Nephrol 2022; 23: 83. https://doi.org/10.1186/s12882-022-02715-9
Lee CW, Kory PD, Arntfield RT. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care 2016; 31: 96–100. https://doi.org/10.1016/j.jcrc.2015.09.016
Rola P, Miralles-Aguiar F, Argaiz E, et al. Clinical applications of the venous excess ultrasound (VExUS) score: conceptual review and case series. Ultrasound J 2021; 13: 32. https://doi.org/10.1186/s13089-021-00232-8
Bhardwaj V, Vikneswaran G, Rola P, et al. Combination of inferior vena cava diameter, hepatic venous flow, and portal vein pulsatility index: venous excess ultrasound score (VEXUS score) in predicting acute kidney injury in patients with cardiorenal syndrome: a prospective cohort study. Indian J Crit Care Med 2020; 24: 783–9. https://doi.org/10.5005/jp-journals-10071-23570
Beaubien-Souligny W, Benkreira A, Robillard P, et al. Alterations in portal vein flow and intrarenal venous flow are associated with acute kidney injury after cardiac surgery: a prospective observational cohort study. J Am Heart Assoc 2018; 7: e009961. https://doi.org/10.1161/jaha.118.009961
Benkreira A, Beaubien-Souligny W, Mailhot T, et al. Portal hypertension is associated with congestive encephalopathy and delirium after cardiac surgery. Can J Cardiol 2019; 35: 1134–41. https://doi.org/10.1016/j.cjca.2019.04.006
Spiegel R, Teeter W, Sullivan S, et al. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit Care 2020; 24: 615. https://doi.org/10.1186/s13054-020-03330-6
von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–8. https://doi.org/10.1136/bmj.39335.541782.ad
Prager R, Wu K, Bachar R, et al. Blinding practices during acute point-of-care ultrasound research: the BLIND-US meta-research study. BMJ Evid Based Med 2020; 26: 110–1. https://doi.org/10.1136/bmjebm-2020-111577
Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010; 10: 1. https://doi.org/10.1186/1471-2288-10-1
Denault A, Couture EJ, De Medicis É, et al. Perioperative Doppler ultrasound assessment of portal vein flow pulsatility in high-risk cardiac surgery patients: a multicentre prospective cohort study. Br J Anaesth 2022; 129: 659–69. https://doi.org/10.1016/j.bja.2022.07.053
Author contributions
Ross Prager and John Basmaji contributed to the conception, design, and implementation of the research; acquisition, analysis and interpretation of the data; and preparation and writing of the manuscript. Michelle Y. S. Wong contributed to data acquisition, data analysis, data interpretation, and manuscript preparation. Robert Arntfield, Ian Ball, Kimberley Lewis, and Bram Rochwerg contributed to the interpretation of the data and manuscript preparation.
Disclosures
No authors have conflicts of interest to declare.
Funding statement
A resident PSI Foundation (North York, ON, Canada) research grant was awarded to support this work.
Editorial responsibility
This submission was handled by Dr. Patricia S. Fontela, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prager, R., Arntfield, R., Wong, M.Y.S. et al. Venous congestion in septic shock quantified with point-of-care ultrasound: a pilot prospective multicentre cohort study. Can J Anesth/J Can Anesth 71, 640–649 (2024). https://doi.org/10.1007/s12630-024-02717-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-024-02717-1