Abstract
Purpose
Improvement in delivery of perioperative care depends on the ability to measure outcomes that can direct meaningful changes in practice. We sought to identify and provide an overview of perioperative quality indicators specific to the practice of anesthesia in noncardiac surgery.
Source
We conducted an umbrella review (a systematic review of systematic reviews) according to Joanna Briggs Institute methodology. We included systematic reviews examining perioperative indicators in patients ≥ 18 yr of age undergoing noncardiac surgery. Our primary outcome was any quality indicator specific to anesthesia. Indicators were classified by the Donabedian system and perioperative phase of care. The quality of systematic reviews was assessed using AMSTAR 2 criteria. Level of evidence of quality indicators was stratified by the Oxford Centre for Evidence-Based Medicine Classification.
Principal Findings
Our search returned 1,475 studies. After removing duplicates and screening of abstracts and full texts, 23 systematic reviews encompassing 3,164 primary studies met our inclusion criteria. There were 330 unique quality indicators. Process indicators were most common (n = 169), followed by outcome (n = 114) and structure indicators (n = 47). Few identified indicators were supported by high-level evidence (45/330, 14%). Level 1 evidence supported indicators of antibiotic prophylaxis (1a), venous thromboembolism prophylaxis (1a), postoperative nausea/vomiting prophylaxis (1b), maintenance of normothermia (1a), and goal-directed fluid therapy (1b).
Conclusion
This umbrella review highlights the scarcity of perioperative quality indicators that are supported by high quality evidence. Future development of quality indicators and recommendations for outcome measurement should focus on metrics that are supported by level 1 evidence. Potential targets for evidence-based quality-improvement programs in anesthesia are identified herein.
Study registration
PROSPERO (CRD42020164691); first registered 28 April 2020.
Résumé
Objectif
L’amélioration de la prestation des soins périopératoires dépend de la capacité de mesurer les résultats qui peuvent orienter des changements significatifs dans la pratique. Nous avons cherché à identifier et à fournir une vue d’ensemble des indicateurs périopératoires de qualité spécifiques à la pratique de l’anesthésie en chirurgie non cardiaque.
Sources
Nous avons mené une revue d’ensemble (une revue systématique des revues systématiques) selon la méthodologie de l’Institut Joanna Briggs. Nous avons inclus des revues systématiques examinant les indicateurs périopératoires chez les patient·es âgé·es de 18 ans ou plus bénéficiant d’une chirurgie non cardiaque. Notre critère d’évaluation principal était tout indicateur de qualité spécifique à l’anesthésie. Les indicateurs ont été classés en fonction du système de Donabedian et de la phase périopératoire des soins. La qualité des revues systématiques a été évaluée à l’aide des critères AMSTAR 2. Le niveau de donnée probante des indicateurs de qualité a été stratifié selon l’Oxford Centre for Evidence-Based Medicine Classification.
Constatations principales
Notre recherche a permis de trouver 1475 études. Après avoir éliminé les doublons et examiné les résumés et les textes intégraux, 23 revues systématiques englobant 3164 études primaires ont répondu à nos critères d’inclusion. Il y avait 330 indicateurs de qualité uniques. Les indicateurs de processus étaient les plus courants (n = 169), suivi des indicateurs de résultats (n = 114) et des indicateurs de structure (n = 47). Peu d’indicateurs identifiés étaient étayés par des données probantes de haut niveau (45/330, 14 %). Les données probantes de niveau 1 ont confirmé les indicateurs de l’antibioprophylaxie (1a), de la prophylaxie pour la thromboembolie veineuse (1a), de la prophylaxie postopératoire pour les nausées/vomissements (1b), du maintien de la normothermie (1a) et de la fluidothérapie ciblée (1b).
Conclusion
Cet examen d’ensemble met en évidence la rareté des indicateurs périopératoires de qualité qui sont étayés par des données probantes de haute qualité. L’élaboration future d’indicateurs de qualité et de recommandations pour la mesure des résultats devrait être axée sur des paramètres étayés par des données probantes de niveau 1. Les cibles potentielles des programmes d’amélioration de la qualité de l’anesthésie fondés sur des données probantes sont identifiées dans le présent manuscrit.
Enregistrement de l’étude
PROSPERO (CRD42020164691); premier enregistrement le 28 avril 2020.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Anesthesia care occupies a unique scope of practice in medicine, as it facilitates the safe delivery of surgical care and postoperative pain management.1,2,3 Since its inception, anesthesiology has driven improvements in the quality and safety of surgical care. There has been a 97% decrease in anesthesia-related deaths since the 1940s and the current mortality risk attributable to anesthesia for surgical inpatients is approximately 1 in 100,000.4
Improvement in health care delivery depends on the ability to measure outcomes that direct meaningful changes in health systems.5,6,7,8 This process requires derivation of valid and usable quality indicators specific to the area of health care delivery. Quality indicators are commonly categorized using the Donabedian framework, which classifies indicators as structure (resources and capacity), process (health care providers’ actions during delivery of care), and outcome (impact of health care service or intervention on health status).9,10 Classification systems aid in communicating the relevance and significance of quality indicators but do not provide insight into the level of evidence on which they are based. In the USA, the gold standard for evidence-based health care quality measurement are the National Quality Forum (NQF)-endorsed quality measures.11,12
Within the field of anesthesiology, the Anesthesia Quality Institute (AQI) was established in 2010. The AQI has implemented a number of quality initiatives (e.g., National Anesthesia Clinical Outcomes Registry, Anesthesia Incident Reporting System)13 to improve delivery of anesthesia care. Similar efforts in the UK include the development of the Perioperative Quality Improvement Programme in 2016, with the goal of collecting perioperative information such as complications and patient-reported outcomes in patients undergoing major noncardiac surgery.14 These registries have driven improvements in large-scale measurement of health care delivery but their ability to affect meaningful clinical change remains unknown. Furthermore, the measures used to improve health care delivery are often derived from expert consensus rather than systematic reviews or prospective evaluation. Many anesthesia-specific indicators are not NQF-endorsed.11 Ultimately, it remains unclear which quality indicators should be used to drive improvements in perioperative patient outcomes from an anesthesiology perspective.
Our objective was to conduct an umbrella review (a systematic review of systematic reviews15) to identify and synthesize systematic reviews that provide an overview of anesthesia-attributable quality indicators in noncardiac surgery. As multiple up-to-date systematic reviews of quality indicators were available, an umbrella review design was effective and efficient, with the advantages of allowing a broad overview of indicators while supporting a detailed synthesis to identify consistent recommendations that can inform care.16 The umbrella review design also allowed identification of important gaps requiring future scholarship. We further planned to synthesize our objectives according to the Donabedian framework to align our findings with a key quality improvement framework. Ultimately, our goal was to provide findings that could highlight robust quality indicators that should be routinely considered in clinical practice, while identifying key knowledge gaps to inform further research and development in anesthesia quality.
Methods
The present umbrella review was conducted in accordance with best practice methodology recommended by the Joanna Briggs Institute (JBI).17 The protocol was registered a priori with the International Prospective Register of Systematic Reviews (PROSPERO); CRD42020164691. This manuscript adheres to the applicable Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.18
Literature search
We developed a comprehensive systematic search with the assistance of an information specialist. Our search strategy adhered to the Peer Review of Electronic Search Strategies (PRESS) checklist, an evidence-based set of guidelines used by librarians and information specialists to evaluate search strategies.19 We applied the search to MEDLINE, Embase, CINAHL, and Cochrane databases for articles published from database inception to 25 January 2022. We included electronic publications ahead of print, in-process, and other nonindexed citations. No language restrictions were applied. We combined keywords for quality indicators with surgery-, anesthesia-, and perioperative-specific keywords and the term “systematic review” (see Electronic Supplementary Material [ESM] eAppendix for full search strategy).
Study selection
We included systematic reviews examining perioperative quality indicators in patients ≥ 18 yr of age undergoing noncardiac surgery. We excluded studies if the reviews 1) included mixed populations of medical or surgical patients where perioperative indicators could not be separated from other types, 2) focused on surgical, nursing, or critical care quality indicators, 3) did not meet all eleven criteria of the JBI critical appraisal checklist17 (see ESM eTable 1 for full checklist).
Screening
Title and abstract screening was performed in duplicate by two independent reviewers (F. N., G. L.). Disagreements were reviewed and resolved by consensus with a senior team member (G. H.). If uncertainty remained, the full text of the study was reviewed. Full texts were also reviewed independently and in duplicate for consensus; disagreements were resolved with a third reviewer. The screening process was completed using DistillerSR (Evidence Partners, Ottawa, ON, Canada), a web-based systematic review platform.
Risk of bias assessment
Risk of bias assessment of the included systematic reviews was performed by adaptation of an existing risk of bias assessment tool.20 As there is no specific tool available to assess the risk of bias in systematic reviews of quality indicators, we used the AMSTAR 2 (revised A MeaSurement Tool to Assess systematic Reviews)21 criteria that our team determined to be applicable to reviews of quality indicators (see ESM eTable 2 for all AMSTAR 2 criteria). AMSTAR 2 denotes criteria as critical or noncritical. Critical criteria relevant to our study were criteria 2 (protocol registration prior to commencement of review), 4 (adequacy of the literature search), and 7 (justification for excluding individual studies). Noncritical criteria that were also assessed were 1, 3, 5, 6, and 16. Criteria 8–15 did not apply to our included studies.
The risk of bias assessment was completed in duplicate by two independent reviewers. Discrepancies were resolved by consensus. Included studies were assigned an overall level of confidence as “high” (0–1 noncritical weakness), “moderate” (> 1 noncritical weakness), “low” (one critical flaw with or without a noncritical weakness), or “critically low” (more than one critical flaw with or without noncritical weaknesses) according to the AMSTAR 2 criteria as outlined in Box 2.17
Data extraction
The primary outcome was any perioperative quality care indicator that could be directly attributed to the practice of anesthesiology. For example, the appropriate administration, timing, and dose of prophylactic antibiotics by an anesthesiologist was considered to be an anesthetic indicator, while the development of surgical site infection was considered to be multifactorial. Data were extracted from full text using a prespecified and piloted data collection form. The data extraction was completed using forms created with Airtable (San Francisco, CA, USA), a spreadsheet-database platform. Quality indicators were collected from systematic reviews by an individual reviewer, unless a systematic review included more than ten indicators, in which that study was reviewed in duplicate to ensure no indicators were missed. All indicators were reviewed in duplicate after being collected from the original systematic review. Discrepancies or uncertainties were discussed with a senior author. Where applicable, the surgical subspeciality focus of an included quality indicator was extracted and categorized as reported in the included review. All indicators were classified into the Donabedian framework per the original systematic review, or by the review team if not previously classified. Along with classification by Donabedian domain, each indicator was also classified according to the most relevant perioperative phase of care (preoperative, intraoperative, postoperative). An indicator was classified as being “perioperative” if it was relevant to all three perioperative phases of care. If an indicator could be applied to multiple (> 1) but not all perioperative phases, it was also classified into the perioperative phase, which was used to classify any indicator that could not be uniquely classified into the preoperative, intraoperative, or postoperative phases. The classification process was performed in duplicate by two authors (F. N., G. L.). Any disagreements or uncertainty were reviewed and resolved in consensus with a senior team member (G. H.). If an indicator was assigned to multiple domains by the original authors, reviewers determined the most relevant domain through consensus.
Using these two frameworks allowed for the creation of an evidence matrix where indicators could be concurrently mapped by Donabedian domain within the context of the perioperative journey. To address overlapping data between systematic reviews, data were extracted from all included systematic reviews and duplicate quality indicators were removed during data synthesis, as outlined in the methodology of performing umbrella reviews.22
Level of evidence
We also collected the Oxford Centre for Evidence-Based Medicine (OCEBM) level of evidence23 for each indicator using the OCEBM 2009 criteria; this was the most used system in the included studies. Levels of evidence were extracted as reported for each indicator in each included systematic review. If included reviews did not state the OCEBM level of evidence, the level of evidence was set as missing (i.e., we did not assign our own level of evidence if none was reported).
Results
Included studies
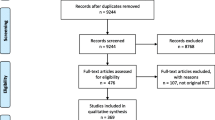
Our systematic search returned 1,475 citations. After duplicate removal and title and abstract screening, 92 citations advanced to full-text review; in the end, 23 systematic reviews were included. The oldest included systematic review was from 2005 and the most recent review was from 2019. The most common reason for excluding a citation from our full-text review was lack of anesthesia indicators (n = 24), focus on nursing, surgical, or intensive care unit indicators (n = 10), or duplicate articles (n = 7). Three citations were excluded for not meeting all eleven of the JBI criteria.24,25,26 A PRISMA flowchart outlining the search results is shown in Fig. 1.
Study characteristics
A description of the 23 included reviews27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 with a summary of key findings is shown in Table 1. The 23 included systematic reviews synthesized data from a total of 3,164 primary studies. The most common country of publication was the USA (11/23). Most included systematic reviews used the Donabedian quality framework (13/23).
Risk of bias
Using the AMSTAR 2 tool, the overall quality of included reviews was “high” (n = 0), “moderate” (n = 1), “low” (n = 7), or “critically low” (n = 15). Specific critical AMSTAR 2 domains leading to decreased overall confidence were 1) the lack of explicit statements that review methods were established a priori, 2) failing to outline the search strategy, or 3) failing to list the excluded studies and reasons for exclusion. Methodological quality ratings for each included review are summarized in ESM eTable 3.
Quality indicators
In total, 330 unique perioperative quality indicators were collected from the included systematic reviews (full list in ESM eTable 4). One hundred of the 330 quality indicators were specific to a surgical subspecialty, with orthopedic (n = 27) and colorectal surgery (n = 21) having the most specialty-specific indicators. Donabedian process indicators occurred with the highest frequency (n = 169; 51% of indicators), followed by outcome indicators (n = 114; 35%), and structure indicators (n = 47; 14%). Most indicators applied to the intraoperative phase of care (n = 135; 41%), followed by postoperative (n = 75; 23%), preoperative (n = 57; 17%), and perioperative (n = 63; 19%) phases. Overall structure, process, or outcome quality indicator distribution organized by perioperative phase of care is shown in Fig. 2.
Structure indicators (Table 2) were identified across all perioperative phases. Level 2 evidence supported preoperative multidisciplinary care, while the strongest evidence (level 1b) supported intraoperative quality indicators (i.e., that anesthetists performing regional techniques should be trained in ultrasound-guided techniques) and perioperative indicators (i.e., the need for protocols to manage perioperative complications and comorbidities). No postoperative recommendations were supported by high-level evidence.
Process indicators (Table 2) were also present across all phases of perioperative care, but were predominantly focused on pre- and intraoperative phases. Within the preoperative phase of care, there was high-quality evidence (level 1a) supporting the use of prophylactic antibiotics prior to surgical incision, use of thromboembolism prophylaxis, and avoidance of premedication or sedation prior to procedures. Four other preoperative processes also achieved level 1b evidence.
For process indicators in the intraoperative phase of care, the strongest evidence (level 1a) supported the use of sterile technique for central venous line insertion, avoidance of systemic morphine intraoperatively, and the presence of a trained anesthetist throughout the operative period. Three intraoperative level 1b process indicators and five level 2 process indicators were also identified.
Perioperatively, level 1a evidence supported an indicator of continuation of chronic beta blockers and maintenance of normothermia. Three further quality indicators achieved level 1b evidence and three indicators achieved level 2 evidence. The highest level of evidence achieved by postoperative process quality indicators was level 2b, specifically the use of a multimodal approach to optimize gut function.
Outcome indicators (Table 2) were most frequently reported in the intraoperative phase of care; however, no outcome indicators were supported by high-level evidence (i.e. OCEBM level 1 or 2). Most outcome indicators related to failure at the process level (i.e., failure of a regional technique, airway incident, hypothermia) or medical and surgical complications.
Overall, 88 of the 330 indicators had an OCEBM level of evidence assigned based on the original systematic review. Of these, 45 indicators included in our study were supported by level 1 or 2 evidence. Most indicators supported by level 1 or 2 evidence were process indicators (n = 40) and were specific to the preoperative phase of care (n = 16). The quality indicators supported by the highest evidence are highlighted in Table 3.
Discussion
In the present umbrella review of 23 systematic reviews that reported 330 anesthesia quality indicators from 3,164 primary studies, we synthesized available indicators as structures, processes, or outcomes organized by the applicable perioperative phase of care. By situating indicators from recent and up-to-date systematic reviews within the Donabedian quality framework across the perioperative period, supported by the underlying quality of evidence for each indicator, we provide a map of quality indicators that can be used to monitor anesthesia quality and drive improvement. Our map further identifies gaps among available indicators that should be addressed through further scholarship. High-level evidence supports current monitoring of important perioperative structures related to staff training and clinical management protocols, and processes associated with antibiotic and thromboembolic prophylaxis, sterile procedural techniques, presence of trained anesthesia providers, maintenance of normothermia, and beta-blocker continuation. A clear gap appears to exist in outcome-quality indicators, as none were supported by high-quality evidence, which highlights a key focus for anesthesia quality initiatives moving forward.
Improving the quality and outcomes of perioperative care is a long-established priority and a strength of the field of anesthesiology. Over the past 80 years, quality and safety measures have allowed for anesthesia-related deaths to decrease by more than 97%.4 Concurrent with these remarkable improvements in safety, the formal field of quality improvement has also emerged, led by earlier pioneers such as Codman, who focused on adequate measurements of long-term patient outcomes, and Donabedian who codified the need to consider the related impacts of structures and processes of care on the end results experienced by patients.50 Such efforts have been taken up in recent decades in anesthesia-specific quality organizations such as the USA-based AQI13 and UK-based Perioperative Quality Improvement Programme initiatives,14 which have worked towards improving the assessment and delivery of high quality perioperative care.
Based on the findings of our umbrella review, substantial effort has been expended in the field to develop quality indicators to drive further improvement in anesthesia care. Since 2005, at least 23 systematic reviews of quality indicators have been published, which synthesized findings from more than 3,000 original studies. Taken together, this has led to over 300 quality indicators that have been recommended at least once. While a common saying in health care improvement is that “we cannot improve what we can’t measure,”51 clinicians, quality leads, administrators and other stakeholders require high-quality information to choose relevant and evidence-based quality indicators from the hundreds that have been described. Our synthesis suggests that at least five structures and 40 process indicators are supported by high-quality evidence (i.e., OCEBM level 1 or 2). These indicators should likely be considered for regular monitoring in many settings, recognizing that applicability may vary by surgical specialty or perioperative setting. Our review found that quality indicators with the strongest evidence were for antibiotic prophylaxis and venous thromboembolism (VTE) prophylaxis, including their appropriate selection and timely administration, which coincide with national consensus guidelines for antibiotic and VTE prophylaxis proposed by the NQF,52,53 as well as national and international guidelines by the Centers for Disease Control and Prevention and the World Health Organization.54,55 By highlighting indicators supported by high-level evidence, we propose potential evidence-based targets for further guidelines to be developed that can direct the measurement, assessment, and improvement of health care quality metrics within the field of anesthesiology. Concurrently, the lack of high-quality evidence supporting other core areas of anesthesia management, such as monitoring and reversal of neuromuscular blockade, highlight the continued need to develop a high-certainty evidence base for routine anesthesia care.
Several indicators that were classified as OCEBM level 1 evidence in their original systematic review could be applied to all cases of noncardiac surgery, but there were some that could be patient- or surgery-specific. For example, avoiding the use of routine preoperative medication or sedation and avoiding the intraoperative use of systemic morphine were indicators supported by level 1a evidence in an included systematic review,29 without additional information in the included review regarding their applicability as quality indicators for specific patient populations, types of surgery, or other clinical contexts. This is an acknowledged limitation of umbrella reviews, which necessitates that clinicians interpret and apply this evidence synthesis through the lens of clinical experience and expertise. For example, while high-certainty randomized controlled trial evidence shows no efficacy for preoperative sedation with lorazepam in older patients,56 this cannot be directly generalized to provision of midazolam in the operating room immediately prior to or as part of induction of general anesthesia. Therefore, some indicators may be suggesting that the premedication and systemic morphine should be used judiciously and tailored to specific patient populations rather than used routinely and in all patients. This highlights the importance of developing specific, objective, and contextualized quality indicators in the perioperative period.
Once quality indicators have been identified, it is important to develop methods of measuring them appropriately so that they can be applied towards improving patient outcomes. Importantly, the adoption of electronic health records has allowed meaningful patient data to be collected more easily and on a larger scale.57 For quality improvement departments, it will be important to prioritize measurement of quality indicators that can feasibly and consistently be collected, as this will vary by hospital system and electronic health record. By highlighting quality indicators of the highest level of evidence, we propose several possible indicators that can be used by anesthesiology departments moving forward. Collection of data into nationwide databases such as NACOR13 developed by the AQI allows for the broad evaluation of indicators, with the potential for use in large-scale health care analytics that can drive health care quality improvement using comprehensive evidence.
The identification and measurement of quality indicators is not enough to improve health care delivery—measurement of these indicators needs to encourage change in the actions of health care providers, a process that requires initiatives that can provide feedback and re-evaluation. Feedback should be continuous and should occur over an extended time, two features that have been shown to be influential on physicians’ acceptance of feedback.58 Within our own hospital, we have explored the use of a performance assessment tool that collected postoperative outcome data (e.g., postoperative nausea, vomiting, and pain) that was sent as feedback to anesthetists.59 The majority of participants in this initiative found that it would be an effective tool for professional development but further studies will be required to explore how the feedback can be directly used to change outcomes within our institution. Other groups have used methods such as monthly feedback systems or peer audits to direct change60 and have shown the ability to create clinically significant differences in process measures of quality after the implementation of these feedback systems. The focus on process measures can support high levels of adherence and change in clinician behaviour to improve care, as process measures are directly under the purview of health care providers.61 While determining which quality indicators are important to measure, the continual reassessment of their use based on feedback will be vital in ensuring that they will properly improve patient care.
Ultimately, while optimization of health care structures and processes is an important goal, the primary measure of success in perioperative care should reflect the outcomes that patients experience. This makes it notable that none of the outcome measures synthesized were supported by high-quality evidence. Moving forward, collaboration between quality improvement experts and researchers working to optimize outcomes in clinical research could represent one pathway to achieve a consistent and high-quality set of patient outcome indicators. Efforts such as the Standardized Endpoints for Perioperative Medicine (StEP) group and Core Outcomes Measures in Perioperative and Anaesthetic Care (COMPAC) initiative represent key building blocks.62 Nevertheless, in addition to inputs from clinicians, quality leads and researchers, patients should also be directly involved to ensure relevance of any measures to the individuals who ultimately receive anesthesia care. Further efforts to causally link improvements in structure and process indicators to these key outcomes should also be prioritized.
Our review has several limitations. First, no risk of bias tool exists that is specific to qualitative umbrella reviews examining quality indicators. To ensure we approached risk of bias assessment in a structured manner, we used the AMSTAR 2 tool with unapplicable criteria omitted to evaluate the systematic reviews included in our umbrella review. As such, the risk of bias assessment may not assess all relevant qualities of included studies as no specific tool exists for this type of assessment.
Second, our umbrella review search was limited to systematic reviews published in bibliographic databases. There may be quality indicators which are housed in other settings such as guidelines, websites, or hospital policies, which have not been included in systematic reviews. Initiatives from other groups such as StEP or COMPAC that have examined quality indicators in anesthesiology would not be captured in our study if their work was not published in a systematic review.
Additionally, overlap in data across systematic reviews is a known problem in umbrella reviews.22 Although we tried to overcome this by removing duplicate quality indicators, it is likely that some of the reviews included in our umbrella review drew from similar or same sources. Further analysis into the degree of overlap could provide additional insight into whether disproportionate consideration is being made towards indictors for which an established evidence base is already present.
Working in the perioperative realm, there is significant overlap between the work of anesthesiologists, surgeons, nurses, and other clinicians. Delineating clinical indicators as purely attributable to anesthesia or surgery is difficult in the collaborative setting of perioperative medicine and is an acknowledged challenge in the field.63 For example, the development of a surgical site infection can be considered attributable to anesthesia, surgery, or potentially jointly attributable. While we were unable to identify any qualitative evidence that could proportionally attribute surgical site infections to surgeons vs anesthesiologists, we acknowledge that this indicator could be considered an anesthetic, surgical or combined quality indicator. Moving forward, future efforts to develop quality indicator sets will likely need to address methods to navigate quality metrics that are not clearly attributable to only one group of clinical actors.
Lastly, heterogeneity existed across the systematic reviews included in our study. Seven of 23 included studies used a form of expert consensus to determine quality indicators, using a Delphi, modified Delphi, or Research and Development/University of California, Los Angeles method.64 Only a subset of our included methods included this approach while the others were systematic reviews of existing quality indicators, therefore introducing heterogeneity into our data.
From an umbrella review that synthesized 23 systematic reviews of anesthesia quality indicators, we mapped 330 indicators across the perioperative period according to the Donabedian structure-process-outcome framework. Our review highlights high-quality indicators specific to anesthesia currently supported by level 1 evidence such as antibiotic prophylaxis, VTE prophylaxis, postoperative nausea and vomiting prophylaxis, maintenance of normothermia, perioperative beta-blocker management, and multidisciplinary care. These may represent useful and valuable targets for anesthesiology quality-improvement initiatives. Further development of quality indicators at the patient outcome level are required and should build upon patient-oriented and multistakeholder engagement.
References
American Society of Anesthesiologists. Standards for postanesthesia care; 2019. Available from URL: https://www.asahq.org/standards-and-practice-parameters/standards-for-postanesthesia-care (accessed October 2023).
Werner MU, Søholm L, Rotbøll-Nielsen P, Kehlet H. Does an acute pain service improve postoperative outcome? Anesth Analg 2002; 95: 1361–72. https://doi.org/10.1097/00000539-200211000-00049
Stamer UM, Liguori GA, Rawal N. Thirty-five years of acute pain services: where do we go from here? Anesth Analg 2020; 131: 650–6. https://doi.org/10.1213/ane.0000000000004655
Li G, Warner M, Lang BH, Huang L, Sun LS. Epidemiology of anesthesia-related mortality in the United States, 1999–2005. Anesthesiology 2009; 110: 759–65. https://doi.org/10.1097/aln.0b013e31819b5bdc
Dutton RP, Dukatz A. Quality improvement using automated data sources: the Anesthesia Quality Institute. Anesthesiol Clin 2011; 29: 439–54. https://doi.org/10.1016/j.anclin.2011.05.002
Berwick DM, Knapp MG. Theory and practice for measuring health care quality. Health Care Financ Rev 1987.
Agency for Healthcare Research and Quality. Section 4: Ways to approach the quality improvement process; 2017. Available from URL: https://www.ahrq.gov/cahps/quality-improvement/improvement-guide/4-approach-qi-process/index.html (accessed October 2023).
Kohn LT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, D.C.: National Academy Press; 2000.
Donabedian A. Evaluating the quality of medical care; 1966. Milbank Q 2005; 83: 691–729. https://doi.org/10.1111/j.1468-0009.2005.00397.x
Donabedian A. The quality of care. How can it be assessed? JAMA 1988; 260: 1743–8. https://doi.org/10.1001/jama.260.12.1743
National Quality Forum. Measure evaluation criteria; 2019; Available from URL: https://www.qualityforum.org/measuring_performance/submitting_standards/measure_evaluation_criteria.aspx (accessed October 2023).
Agency for Healthcare Research and Quality. Key questions when choosing health care quality measures; 2018. Available from URL: https://www.ahrq.gov/talkingquality/measures/measure-questions.html (accessed October 2023).
Anesthesia Quality Institute. 2021 QCDR measure specifications; 2021. Available from URL: https://www.aqihq.org/files/MIPS/2021/2021_QCDR_Measure_Book.pdf (accessed October 2023).
Gilhooly D, Moonesinghe SR. The Perioperative Quality Improvement Programme: improving outcomes. Br J Hosp Med (Lond) 2018; 79: 117. https://doi.org/10.12968/hmed.2018.79.2.117
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc 2015. https://doi.org/10.1097/xeb.0000000000000055
Belbasis L, Bellou V, Ioannidis JP. Conducting umbrella reviews. BMJ Med 2022; 1: e000071. https://doi.org/10.1136/bmjmed-2021-000071
Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. Chapter 10: Umbrella reviews; 2022. Available from URL: https://jbi-global-wiki.refined.site/space/MANUAL/4687363/Chapter+10%3A+Umbrella+reviews (accessed October 2023).
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. https://doi.org/10.1136/bmj.n71
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75: 40–6. https://doi.org/10.1016/j.jclinepi.2016.01.021
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 2-risk of bias assessment; synthesis, presentation and summary of the findings; and assessment of the certainty of the evidence. Syst Rev 2018; 7: 159. https://doi.org/10.1186/s13643-018-0784-8
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. https://doi.org/10.1136/bmj.j4008
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev 2017; 6: 231. https://doi.org/10.1186/s13643-017-0617-1
Howick J. Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). 2009 Available from URL: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed October 2023).
Numan RC, Ten Berge M, Burgers JA, et al. Peri- and postoperative management of stage I-III non small cell lung cancer: which quality of care indicators are evidence-based? Lung Cancer 2016; 101: 129–36. https://doi.org/10.1016/j.lungcan.2016.06.007
Arora VM, McGory ML, Fung CH. Quality indicators for hospitalization and surgery in vulnerable elders. J Am Geriatr Soc 2007; 55: 347–58. https://doi.org/10.1111/j.1532-5415.2007.01342.x
Sauvegrain P, Chantry AA, Chiesa-Dubruille C, Keita H, Goffinet F, Deneux-Tharaux C. Monitoring quality of obstetric care from hospital discharge databases: a Delphi survey to propose a new set of indicators based on maternal health outcomes. PLoS One 2019; 14: e0211955. https://doi.org/10.1371/journal.pone.0211955
Debaun MR, Chen MJ, Bishop JA, Gardner MJ, Kamal RN. Orthopaedic trauma quality measures for value-based health care delivery: a systematic review. J Orthop Trauma 2019; 33: 104–10. https://doi.org/10.1097/bot.0000000000001372
Brett KE, Ritchie LJ, Ertel E, Bennett A, Knoll GA. Quality metrics in solid organ transplantation: a systematic review. Transplantation 2018; 102: e308–30. https://doi.org/10.1097/tp.0000000000002149
Chazapis M, Gilhooly D, Smith AF, et al. Perioperative structure and process quality and safety indicators: a systematic review. Br J Anaesth 2018; 120: 51–66. https://doi.org/10.1016/j.bja.2017.10.001
Nazerali RN, Finnegan MA, Divi V, Lee GK, Kamal RN. Quality measures in breast reconstruction a systematic review. Ann Plast Surg 2017; 79: 320–5. https://doi.org/10.1097/sap.0000000000001088
Tinmouth J, Kennedy EB, Baron D, et al. Colonoscopy quality assurance in Ontario: systematic review and clinical practice guideline. Can J Gastroenterol Hepatol 2014; 28: 251–74. https://doi.org/10.1155/2014/262816
Patwardhan M, Fisher DA, Mantyh CR, et al. Assessing the quality of colorectal cancer care: do we have appropriate quality measures? (A systematic review of literature). J Eval Clin Pract 2007; 13: 831–45. https://doi.org/10.1111/j.1365-2753.2006.00762.x
Leow JJ, Catto JW, Efstathiou JA, et al. Quality indicators for bladder cancer services: a collaborative review. Eur Urol 2020; 78: 43–59. https://doi.org/10.1016/j.eururo.2019.09.001
Saluja S, Mukhopadhyay S, Amundson JR, et al. Quality of essential surgical care in low- and middle-income countries: a systematic review of the literature. Int J Qual Health Care 2019; 31: 166–72. https://doi.org/10.1093/intqhc/mzy141
Haller G, Bampoe S, Cook T, et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine initiative: clinical indicators. Br J Anaesth 2019; 123: 228–37. https://doi.org/10.1016/j.bja.2019.04.041
Voeten SC, Krijnen P, Voeten DM, Hegeman JH, Wouters MW, Schipper IB. Quality indicators for hip fracture care, a systematic review. Osteoporos Int 2018; 29: 1963–85. https://doi.org/10.1007/s00198-018-4558-x
Aitken SJ, Naganathan V, Blyth FM. Aortic aneurysm trials in octogenarians: are we really measuring the outcomes that matter? Vascular 2016; 24: 435–45. https://doi.org/10.1177/1708538115597079
Querleu D, Planchamp F, Chiva L, et al. European society of gynaecologic oncology quality indicators for advanced ovarian cancer surgery. Int J Gynecol Cancer 2016; 26: 1354–63. https://doi.org/10.1097/igc.0000000000000767
Halverson AL, Sellers MM, Bilimoria KY, et al. Identification of process measures to reduce postoperative readmission. J Gastrointest Surg 2014; 18: 1407–15. https://doi.org/10.1007/s11605-013-2429-5
Dikken JL, Stiekema J, Van De Velde CJ, et al. Quality of care indicators for the surgical treatment of gastric cancer: a systematic review. Ann Surg Oncol 2013; 20: 381–98. https://doi.org/10.1245/s10434-012-2574-1
Borgaonkar MR, Hookey L, Hollingworth R, et al. Indicators of safety compromise in gastrointestinal endoscopy. Can J Gastroenterol 2012; 26: 71–8. https://doi.org/10.1155/2012/782790
Stelfox HT, Straus SE, Nathens A, Bobranska-Artiuch B. Evidence for quality indicators to evaluate adult trauma care: a systematic review. Crit Care Med 2011; 39: 846–59. https://doi.org/10.1097/ccm.0b013e31820a859a
Haller G, Stoelwinder J, Myles PS, McNeil J. Quality and safety indicators in anesthesia: a systematic review. Anesthesiology 2009; 110: 1158–75. https://doi.org/10.1097/aln.0b013e3181a1093b
McGory ML, Shekelle PG, Ko CY. Development of quality indicators for patients undergoing colorectal cancer surgery. J Natl Cancer Inst 2006; 98: 1623–33. https://doi.org/10.1093/jnci/djj438
McGory ML, Shekelle PG, Rubenstein LZ, Fink A, Ko CY. Developing quality indicators for elderly patients undergoing abdominal operations. J Am Coll Surg 2005; 201: 870–83. https://doi.org/10.1016/j.jamcollsurg.2005.07.009
Amanatullah DF, McQuillan T, Kamal RN. Quality measures in total hip and total knee arthroplasty. J Am Acad Orthop Surg 2019; 27: 219–26. https://doi.org/10.5435/jaaos-d-17-00283
Sun BJ, Kamal RN, Lee GK, Nazerali RS. Quality measures in ventral hernia repair: a systematic review. Hernia 2018; 22: 1023–32. https://doi.org/10.1007/s10029-018-1794-0
van Zelm R, Janssen I, Vanhaecht K, et al. Development of a model care pathway for adults undergoing colorectal cancer surgery: evidence-based key interventions and indicators. J Eval Clin Pract 2018; 24: 232–9. https://doi.org/10.1111/jep.12700
Patwardhan MB, Samsa GP, McCrory DC, et al. Cancer care quality measures: diagnosis and treatment of colorectal cancer. Evid Rep Technol Assess (Full Rep) 2006; (138): 1–116.
Donabedian A. The end results of health care: Ernest Codman’s contribution to quality assessment and beyond. Milbank Q 1989; 67: 233–56.
Berenson RA. If you can’t measure performance, can you improve it? JAMA 2016; 315: 645–6. https://doi.org/10.1001/jama.2016.0767
National Quality Forum. NQF-endorsed measures for surgical procedures, 2015–2017: final report; 2017. Available from URL: https://www.qualityforum.org/Projects/s-z/Surgery_Project_2015-2017/Final_Report.aspx (accessed October 2023).
National Quality Forum. Patient safety 2015 final report; 2016. Available from URL: https://www.qualityforum.org/Publications/2016/02/Patient_Safety_2015_Final_Report.aspx (accessed October 2023).
National Quality Forum. National voluntary consensus standards for prevention and care of venous thromboembolism: policy, preferred practices, and initial performance measure; 2006. Available from URL: https://www.qualityforum.org/Publications/2006/12/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Policy,_Preferred_Practices,_and_Initial_Performance_Measures.aspx (accessed October 2023).
Berriós-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017; 152: 784–91. https://doi.org/10.1001/jamasurg.2017.0904
Maurice-Szamburski A, Auquier P, Viarre-Oreal V, et al. Effect of sedative premedication on patient experience after general anesthesia: a randomized clinical trial. JAMA 2015; 313: 916–25. https://doi.org/10.1001/jama.2015.1108
Simpao AF, Ahumada LM, Rehman MA. Big data and visual analytics in anaesthesia and health care. Br J Anaesth 2015; 115: 350–6. https://doi.org/10.1093/bja/aeu552
Veloski J, Boex JR, Grasberger MJ, Evans A, Wolfson DB. Systematic review of the literature on assessment, feedback and physicians’ clinical performance: BEME Guide No. 7. Med Teach 2006; 28: 117–28. https://doi.org/10.1080/01421590600622665
Wheeler K, Baxter A, Boet S, Pysyk C, Bryson GL. Performance feedback in anesthesia: a post-implementation survey. Can J Anesth 2017; 64: 681–2. https://doi.org/10.1007/s12630-017-0860-x
McCormick PJ, Yeoh C, Vicario-Feliciano RM, et al. Improved compliance with anesthesia quality measures after implementation of automated monthly feedback. J Oncol Pract 2019; 15: e583–92. https://doi.org/10.1200/jop.18.00521
Evans SM, Lowinger JS, Sprivulis PC, Copnell B, Cameron PA. Prioritizing quality indicator development across the healthcare system: identifying what to measure. Intern Med J 2009; 39: 648–54. https://doi.org/10.1111/j.1445-5994.2008.01733.x
Myles PS, Grocott MP, Boney O, Moonesinghe SR. Standardizing end points in perioperative trials: towards a core and extended outcome set. Br J Anaesth 2016; 116: 586–9. https://doi.org/10.1093/bja/aew066
Hyder JA, Niconchuk J, Glance LG, et al. What can the national quality forum tell us about performance measurement in anesthesiology? Anesth Analg 2015; 120: 440–8. https://doi.org/10.1213/ane.0000000000000553
Linstone HA, Turoff M. The Delphi method: techniques and applications; 2002. Available from URL: http://www.foresight.pl/assets/downloads/publications/Turoff_Linstone.pdf (accessed October 2023).
Author contributions
Frederic Nguyen, Gary Liao, and Gavin M. Hamilton contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Daniel I. McIsaac and Manoj M. Lalu contributed to study conception and design; analysis and interpretation of data; and drafting the article. Christopher L. Pysyk contributed to study conception and design, and drafting the article.
Acknowledgements
The authors would like to thank Sascha Davis, and Risa Shorr, information specialists at The Ottawa Hospital, for their assistance with development of our search strategy.
Disclosures
None.
Funding statement
None.
Prior conference presentations
The abstract was presented as an e-Poster at the International Anesthesia Research Society (IARS) 2021 annual meeting (15–16 May, held virtually).
Editorial responsibility
This submission was handled by Dr. Stephan K. W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, F., Liao, G., McIsaac, D.I. et al. Perioperative quality indicators specific to the practice of anesthesia in noncardiac surgery: an umbrella review. Can J Anesth/J Can Anesth 71, 274–291 (2024). https://doi.org/10.1007/s12630-023-02671-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02671-4