Abstract
Purpose
This article reviews the pathophysiology, clinical relevance, and therapy of the catabolic response to surgical stress.
Principle findings
The key clinical features of perioperative catabolism are hyperglycemia and loss of body protein, both metabolic consequences of impaired insulin function. Muscle weakness and (even moderate) increases in perioperative blood glucose are associated with morbidity after major surgery. Although the optimal glucose concentration for improving clinical outcomes is unknown, most medical associations recommend treatment of random blood glucose > 10 mmol·L−1. Neuraxial anesthesia blunts the neuroendocrine stress response and enhances the anabolic effects of nutrition. There is evidence to suggest that the avoidance of preoperative fasting prevents insulin resistance and accelerates recovery after major abdominal surgery.
Conclusions
Current anticatabolic therapeutic strategies include glycemic control and perioperative nutrition in combination with optimal pain control and the avoidance of preoperative starvation. All these elements are part of Enhanced Recovery After Surgery (ERAS) programs.
Résumé
Objectif
Cet article examine la physiopathologie, la pertinence clinique et le traitement de la réponse catabolique au stress chirurgical.
Constatations principales
Les principales caractéristiques cliniques du catabolisme périopératoire sont l’’hyperglycémie et la perte de protéines corporelles, deux conséquences métaboliques de la perturbation de la fonction insulinique. Une faiblesse musculaire et des augmentations (même modérées) du glucose sanguin en périopératoire sont associées à une morbidité après des interventions chirurgicales majeures. Bien que la concentration optimale du glucose pour l’’amélioration des aboutissements cliniques soit inconnue, la plupart des sociétés médicales recommandent le traitement aléatoire de la glycémie > 10 mmol·L−1. L’’anesthésie neuraxiale émousse la réponse au stress neuroendocrinien et favorise les effets anaboliques de la nutrition. Des données probantes suggèrent que l’’évitement du jeûne préopératoire prévient la résistance à l’’insuline et accélère la récupération après chirurgie abdominale majeure.
Conclusions
Les stratégies thérapeutiques actuelles anticataboliques incluent le contrôle glycémique et la nutrition périopératoire en association avec un contrôle optimal de la douleur et l’’évitement du jeûne préopératoire. Tous ces éléments font partie des programmes de Récupération rapide après la chirurgie (RRAC).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients undergoing major surgery are exposed to metabolic and endocrine alterations in carbohydrate, protein, and insulin metabolism, often summarized as the catabolic response. Preventing stress in an effort to minimize this catabolic response to surgery represents one key mechanism on which perioperative programs such as the Enhanced Recovery After Surgery (ERAS) concept are based.
This narrative describes the pathophysiology, clinical relevance, and treatment of perioperative catabolism.
The catabolic response to surgery: pathophysiology and clinical relevance
Pathophysiology
Glucose metabolism
Characteristic features of glucose metabolism are increased rates of glucose production combined with decreased peripheral glucose utilization, which result in hyperglycemia.
The magnitude of hyperglycemia depends on the severity of surgical tissue trauma.
In fasting non-diabetic patients undergoing elective intraperitoneal operations, blood glucose levels typically increase to 7-10 mmol·L−1. During cardiac surgery, glycemia frequently exceeds 10 mmol·L−1 in non-diabetic patients and 15 mmol·L−1 in diabetic patients.1
This hyerglycemia is further aggravated by the intravenous administration of drugs diluted in dextrose (antibiotics, catecholamines, and nitroglycerin), blood products containing large amounts of glucose, and nutritional support. Importantly, an infusion of 5% dextrose 100 mL (= 5 g of glucose) doubles the circulating glucose in a 70-kg non-diabetic patient.2
Although the effect of surgical technique on perioperative catabolism has not been well studied, laparoscopic procedures may have less impact on glucose metabolism than the open approach. Patients undergoing laparoscopic colon resection showed better glucose utilization when compared with laparotomy, possibly mediated through the reduction of tissue trauma, mitigation of inflammatory responses, and the preservation of insulin sensitivity.3,4
The choice of anesthetic drugs also affects glucose homeostasis. In contrast to propofol, high doses of opioids,1 and neuraxial techniques, inhalational agents have been shown to accentuate the hyperglycemic response to surgery.5,6
The administration of corticosteroids for the prevention of postoperative nausea and vomiting, even in small doses, further exacerbates hyperglycemia in non-diabetic patients.7,8
Unexpectedly large numbers of patients show abnormal glucose homeostasis before surgery. In a prospective study in 500 patients presenting for elective procedures, 26% of previously undiagnosed patients showed blood glucose levels in the impaired-fasting glucose or diabetic range.9,10
Protein catabolism
Typical features of protein catabolism are stimulated rates of protein breakdown and amino acid oxidation which lead to a net loss of body protein.11-13
Metabolically healthy patients lose 40-80 g of nitrogen after elective abdominal surgery, equivalent to 1.2-2.4 kg of wet skeletal muscle.14 Patients with burns or sepsis experience daily losses of up to 800 g of muscle mass. Protein loss in type 2 diabetic patients after colorectal cancer surgery has been shown to be 50% greater than in non-diabetics.15
Muscle wasting occurs early and rapidly during the first week of critical illness and is more severe among patients with multi-organ failure.12 Significant muscle weakness and physical disability can persist for more than five years after injury and critical illness.16,17
There is no evidence to suggest that the magnitude of catabolic changes in elderly patients differs from those in younger adults. Age, however, may be associated with reduced muscle mass and a decreased capacity to utilize nutrients. Older patients may, therefore, be more vulnerable to protein catabolism.18
While older inhaled anesthetics, such as halothane, decrease protein breakdown and synthesis in humans, the impact of modern agents (desflurane, sevoflurane) on protein metabolism is unknown. Intravenous anesthetics (fentanyl, midazolam, propofol) have been shown to have no influence.19-21
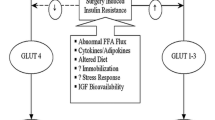
Insulin resistance
Insulin resistance can be defined as any condition whereby a normal concentration of insulin produces a subnormal biological response.
Much of the impairment of insulin function can be explained by the stress-induced increase of so-called counter-regulatory hormones (cortisol, glucagon, catecholamines). These hormones exert catabolic effects, either directly or indirectly, by inhibiting insulin secretion and/or counteracting its peripheral action. The relationship shown between the time course of perioperative interleukin-6 plasma concentrations and insulin resistance suggests that inflammatory mediators (cytokines) are also involved.22 The impairment of tissue insulin sensitivity is particularly severe in skeletal muscle, quantitatively the most important organ for insulin-mediated glucose uptake.23
The magnitude of whole-body insulin resistance is most pronounced on the day after surgery (up to 70% reduction) and lasts for about three weeks after uncomplicated elective abdominal operations. It has been linked primarily to the invasiveness of surgery.24,25 Other factors may also contribute, such as the duration of trauma,26 bed rest and immobilization,27 type of anesthesia and analgesia,28,29 nutrition and preoperative fasting,30,31 blood loss,25 physical status, and post-surgery rehabilitation.32
Clinical relevance
Glucose metabolism
Even moderate increases in blood glucose are associated with adverse outcomes. Patients with cardiovascular, infectious, and neurological problems are particularly sensitive to changes in glycemia.33-37 In critically ill patients, mortality is positively correlated with increasing glucose levels > 5 mmol·L−1,38 and in patients undergoing cardiovascular procedures, hyperglycemia was associated with increased mortality and organ dysfunction.39 Patients with fasting glucose levels > 7 mmol·L−1 or random blood glucose levels > 11.1 mmol·L−1 on general surgical wards had an 18-fold increase in in-hospital mortality, a longer stay, and a greater risk of infection.33
Acute changes in glucose levels may facilitate the development of post-traumatic chronic pain. In a chronic post-ischemia pain animal model, hyperglycemia occurred at the time of injury, and strict glycemic control reduced mechanical and cold allodynia.40
Marked fluctuations in blood glucose may be harmful, independent of the absolute mean glucose level.41-43 There is no consistent definition of glycemic variability, and several metrics, e.g., the coefficient of variation of blood glucose levels or the glycemic lability index, have been used in critical illness.41,44 It is also unclear whether variations within the normal glycemic range or periods of significant hypo- and hyperglycemia are problematic.
There is recent evidence to suggest that the quality of preoperative glycemic control is clinically important. Elevated levels of hemoglobin A1c, an indicator of glucose control in the preceding three months, were found to be predictive of complications after abdominal and cardiac surgery.45-48
In non-cardiac non-vascular patients, preoperative blood glucose levels > 11.1 mmol·L−1 were associated with a 2.1-fold higher risk in 30-day all-cause mortality and a four-fold higher risk of 30-day cardiovascular mortality.49 In a large cohort of 61,536 consecutive elective non-cardiac surgery patients, poor preoperative glycemic control was related to adverse in-hospital outcomes and one-year mortality.50
Protein catabolism
Because protein represents structural and functional components, erosion of lean tissue delays wound healing, compromises immune function, and diminishes muscle strength after surgery.51,52 The ensuing muscle weakness prolongs mechanical ventilation, inhibits coughing, and impedes mobilization, thereby causing morbidity and complicating convalescence.53,54 The length of time for return of normal physiologic function after discharge from the hospital is related to the extent of lean body loss during hospitalization.54
Significant mortality occurs after critically ill patients are discharged from the intensive care unit (ICU) and hospital.16 Many of these deaths are ascribed to the loss of muscle mass, inadequate physical activity, muscle weakness, and the inability to mobilize.
Insulin resistance
Studies performed in a small number of patients over a six-year period in Sweden (1990-1996) report a correlation between the postoperative decrease in the patient’s insulin sensitivity and length of hospital stay.25 In a larger cardiac surgery patient population, intraoperative insulin resistance was associated with clinical outcome.45 Independent of the patient’s diabetic state, for every 20% decrease in insulin sensitivity, the risk of serious complications (mortality, myocardial failure, stroke, dialysis, and infection) more than doubled.45 These findings support the assumption that, perioperatively, acute alterations in glucose homeostasis, i.e., the “diabetes of the injury”, predict adverse events better than the presence or absence of diabetes mellitus itself.
Anticatabolic strategies
The clinically important question is whether the catabolic stress response is a mere reflection of the severity of the underlying disease or whether the complications associated with catabolism can be reduced by its prevention or treatment. Hence, a number of strategies have been designed to minimize catabolic illness and, ideally, enhance outcome.55
Such therapies include the administration of endocrine (growth hormone,56,57 insulin,58 glucagon-like peptide-1,59 steroids) or other agents (ß-blocker),60 glycemic control,61 various types of anesthesia (epidural anesthesia, intravenous opioids),28,62 as well as nutritional support, in particular the provision of specific nutrients such as polyols (xylitol, sorbitol), fructose,63-65 and amino acids (glutamine, arginine, branched chain amino acids,66-68 α-ketoanalogues).69
Due to lack of effectiveness (branched chain amino acids, glutamine,70 α-ketoanalogues, intravenous opioids), unavailability in North America (polyols, fructose), cost and side effects (growth hormone, steroids, xylitol),71,72 only two treatment modalities are presently used in clinical practice – glycemic control and nutritional support.
Glycemic control
Pioneering observational studies conducted in diabetic patients undergoing open heart surgery in Portland, Oregon, USA, showed reduced morbidity and mortality with improved glycemic management.73 In 2001, the Leuven-I study showed superior outcomes with maintenance of normoglycemia, i.e., a mean blood glucose of 4.4-6.1 mmol·L−1.61
In critically ill patients, predominantly after cardiac procedures, mortality decreased by 50%. Besides saving lives, normoglycemia reduced the risk of infection, acute renal failure, liver dysfunction, peripheral neuropathy, muscle weakness, and anemia.
Several limitations of this unblinded trial raised concerns about the wider applicability of the study; these included the early administration of a relatively large amount of calories within the first 24 hr of admission, an unusually high mortality rate in the control group, and a treatment effect exceeding that of previous ICU studies. More importantly, recent large randomized clinical trials were unable to reproduce these benefits.74-78
The Leuven-II trial75 and the VISEP study,76 both using the Leuven insulin protocol and achieving mild hyperglycemia, reported a frequency of severe hypoglycemic episodes (< 2.2 mmol·L−1) in 18.7% and 17% of their respective study populations. In the NICE-SUGAR study, 74% of patients receiving intensive therapy had an episode of mild hypoglycemia, while in 7%, the hypoglycemia was severe.74 Post hoc analysis showed that hypoglycemia was associated with mortality.77 The Glucontrol trial was stopped prematurely because of a high rate of unintended protocol violations and a 9% incidence of hypoglycemia.78
A review of the effect of glycemic control on the incidence of surgical site infections was inconclusive because of the small number of studies (n = 5), the heterogeneity in patient populations, the definitions of outcome measures, and the fact that glycemic targets were different and/or were not achieved.79
A meta-analysis of studies performed in cardiac surgery suggested a lower mortality and risk of arrhythmias with tight perioperative glycemic control.80 In contrast, more recent studies in cardiac patients failed to show any benefit but reported an increased incidence of hypoglycemia.81-84 All these studies were small and some were retrospective.
Hence, to date, the optimal glucose level for enhancing clinical outcomes is unknown.
This uncertainty is reflected by the diversity of published recommendations concerning glycemic control in critically ill and surgical patients (Table).85-89
It is particularly interesting that most “glycemic control studies” after Leuven-I were unable to establish and preserve a normal blood glucose level.74,76,78 Therefore, the conclusions that studies failing to reach this target reported regarding the clinical benefits of normoglycemia are questionable. While most associations still recommend treatment of random glucose levels > 10 mmol·L−1 (Table), a large clinical trial is warranted to identify the ideal blood glucose concentration during and after major surgery.
According to conventional protocols, blood glucose is frequently monitored, and insulin is titrated to a glycemic range. These “insulin sliding scales” are reactive, allow hyperglycemia to occur before therapy is initiated, take hours to be effective, and sometimes fail to establish target glycemia.61,90
In the critically ill, many regimens have evolved into algorithms requiring difficult and, at times, impractical calculations. Unless computerized, they are time-consuming and error prone. The interaction between glucose homeostasis and the neuroendocrine/inflammatory consequences of surgery is so complex that optimal glucose control cannot be achieved by occasional blood glucose measurements followed by reactive adjustments of insulin administration. Using a preemptive infusion of large doses of insulin, together with glucose infused at a variable rate to maintain a blood glucose of 4.0-6.0 mmol·L−1 (glucose insulin administration while maintaining normoglycemia = GIN therapy), it was possible to preserve consistent normoglycemia during open heart and major abdominal cancer surgery.91
While maintenance of normoglycemia and avoidance of large variations in glycemia are metabolically important, insulin per se possesses non-metabolic properties with potential benefits for surgical patients, especially those with cardiovascular disease. Protocols using supraphysiological doses of insulin in patients undergoing coronary artery bypass grafting showed anti-inflammatory and cardioprotective effects, as reflected by lower tumour necrosis factor α, interleukin 6, interleukin 8, and troponin levels.92 These immunological changes were associated with a decreased requirement for inotropic support93 and echocardiographic signs94 of improved global myocardial function.93
Nutrition
Critical illness and the immediate period after abdominal surgery are characterized by semistarvation due to anorexia and/or restricted oral food intake. Unless amino acids and calories are provided in amounts sufficient to match ongoing demands, rapid net loss of lean tissue ensues. Hence, the primary goal of nutrition support is to attenuate protein wasting by optimizing nutrient delivery within the constraints of organ function.
The provision of hyper-, iso-, or hypocaloric amounts of energy, with or without protein, is a therapeutic modality that has traditionally been used to achieve this goal in surgical patients. Hyperalimentation, i.e., the administration of large hypercaloric amounts of energy and amino acids, is the only traditional strategy to induce anabolism after surgery.95,96 Overfeeding, however, has been abandoned in clinical practice because of serious adverse effects (hyperglycemia,39,97-99 respiratory distress,100 liver dysfunction).101,102 The provision of isocaloric amounts of glucose and amino acids improves nitrogen balance95 and attenuates protein losses after surgery, but it fails to produce a positive nitrogen balance, i.e., anabolism.95,103 Meta-analyses in surgical patients concluded that concepts of hyper- and isocaloric intravenous feeding have no overall clinical benefit and, in fact, may even be harmful, i.e. increase the rate of infectious complications and cardiovascular morbidity.104,105
In North America, provision of hypocaloric glucose at a dose below the patient`s actual energy requirement is still being used to “feed” patients after elective abdominal surgery. Nevertheless, the anticatabolic properties of hypocaloric glucose observed in healthy fasting subjects do not apply to patients after surgical trauma. A glucose infusion of 150-200 g·day−1, administered either alone95,106-108 or with amino acids,95,106 has no impact on nitrogen balance after surgery. Despite its inability to prevent protein catabolism, perioperative hypocaloric nutrition may have clinical benefits, such as a reduced infectious complications and length of hospitalization.109
Although little is known about the clinical advantage of early enteral nutrition after elective major surgery, some protocols, including ERAS programs advocate early recommencement of oral food intake. According to the results of one clinical trial, patients receiving preoperative carbohydrates and complete enteral feeding immediately after colorectal surgery remained normoglycemic and maintained a positive protein balance.110 Early enteral nutrition after major rectal cancer surgery has been shown to be safe and associated with less ileus and anastomotic leakage.111 More recent studies focused on the effects of immune-enhancing nutrients, such as n-3 fatty acids, arginine, and nucleotides. One meta-analysis using preoperative immunonutrition reported a decrease in total complications and infections when compared with no or standard therapy.112 Another study failed to show the superiority of preoperative immunonutrition over the use of standard oral supplements.113 In selected surgical cancer populations (head and neck, pancreas), perioperative immunonutrition may be beneficial.114-116
Some methodological problems, encountered in earlier reports, may explain the limited effectiveness of hypocaloric nutritional concepts in surgical patients. These include the disregard of type and quality of analgesia, lack of individualization of nutrition support, inadequate assessment of catabolism before surgery, underestimation of preoperative starvation and disregard of hyperglycemia.
Anesthesia and analgesia
Neuraxial anesthesia
Segmental blockade of nociceptive signals at the spinal cord level provides the most effective pain relief after intraperitoneal procedures. Apart from optimal pain control, neuraxial blockade has anticatabolic effects that may contribute to better outcome.117
Epidural and intrathecal administration of local anesthetics prevents or blunts the neuroendocrine stress response, which results in improved insulin sensitivity with a positive influence on glucose and protein catabolism.118 By attenuating insulin resistance and facilitating exogenous glucose utilization, neuraxial techniques reduce the amount of energy that is required to maintain protein balance. If the energy load of parenteral feeding can be decreased, use can be made of peripheral veins and hyperglycemia can be avoided. Epidural analgesia together with the perioperative infusion of hypocaloric glucose (200 g·day−1) has been shown to minimize the oxidative loss of protein after colorectal surgery, thereby saving muscle mass at a rate of 100 g·day−1.119 The extent of protein sparing was greater than that previously achieved with other pharmacological and nutritional interventions, including growth hormone, glutamine, and total parenteral nutrition.56,66,120 In addition, patients receiving epidural analgesia could be rendered anabolic by supplementing hypocaloric glucose with amino acids.121
Opioids
High-dose opioid anesthesia attenuates most of the endocrine and metabolic responses to surgery, but it is used rarely for procedures of short and intermediate duration.122 Newer short-acting narcotics, such as sufentanil, alfentanil, and remifentanil, prevent intraoperative catabolism, also when used at a smaller dose. Postoperative catabolic changes, however, are either unaffected or even more pronounced.28
Individualization of nutritional support
Nutrition is typically prescribed on the basis of body weight and/or estimations of the patient’s energy expenditure (EE). Use of body weight as the sole reference does not account for variations in body fat and lean tissue, the main determinant of whole-body energy consumption.123 The Harris-Benedict equation is a formula commonly used to predict EE in surgical patients;124 however, marked differences between measured and calculated EE have been reported, with measured amounts from 50-150% of the predicted EE value.125-127 Indirect calorimetry allows direct measurement of the patient’s EE and prompt adjustment to individual nutritional needs.
Assessment of catabolism before surgery
In order to evaluate the efficacy of nutritional support, the patient’s baseline catabolic state must be quantified because sarcopenia is related to postoperative morbidity and mortality.128,129 A significant association exists between the degree of preoperative catabolism and the anabolic effect of nutrition, with catabolic patients benefitting the most.121 These more recent observations support the previous substantiation of superior outcomes in perioperatively fed malnourished patients.130
Many clinical and biochemical indices have been used to characterize the nutritional status of surgical patients, but all techniques have limitations.131-133 Anthropometric and body composition measurements need to be treated with caution in subjects who are dehydrated and/or have edema or ascites.131 Serum proteins are pathophysiological markers influenced by factors other than malnutrition or catabolism.131,134
Protein economy in surgical patients was often expressed as nitrogen balance, i.e. the difference between the body’s total nitrogen intake and its total nitrogen loss; however, retention of nitrogen within the body and underestimation of nitrogen excretion in urine and other routes (feces, skin, and wound secretion) invariably lead to false positive values.135,136
Novel tracer methods using metabolic substrates (glucose, amino acids) labelled with stable isotopes (2H, 13C, 15N) are considered the technique of choice for the global assessment of catabolism in humans and its relation to protein and energy intake.137
They provide a dynamic picture about the kinetics of glucose and amino acids on the whole body (protein breakdown, oxidation and synthesis, glucose production and utilization) and the organ-tissue level.138-140
Preoperative fasting
Elective surgery has routinely been performed after overnight fasting to minimize the risk of aspiration. Under certain conditions, such as a high risk of aspiration or preoperative bowel preparation, fasting periods are long enough to deplete hepatic glycogen stores and, thereby, increase the demand for amino acids for gluconeogenesis rather than tissue repair.141-145 Animal studies have shown that coping with stress is improved when the animals enter the trauma fed, not fasting.142 Overnight treatment with glucose prevents postoperative decrease in insulin sensitivity30,146 and early loss of protein after gastrointestinal surgery147-149 and augments voluntary muscle function.150 Clinical studies conducted in small patient populations reported better outcomes with preoperative nutrition112,130,151-154 and emphasize that avoidance of fasting makes patients less susceptible to complications and may decrease hospital length of stay.27,154-156 In contrast, the results of a larger randomized controlled trial showed no significant benefit.157
A recent meta-analysis, however, suggests that preoperative administration of oral carbohydrates accelerates recovery after major abdominal surgery.158 The current ERAS guidelines, therefore, recommend the routine use of preoperative carbohydrate drinks.159
Hyperglycemia
Hyperglycemia per se has been shown to exacerbate protein catabolism during critical illness and, therefore, may blunt the anabolic response to feeding strategies that include glucose. In severely burned patients, net muscle protein catabolism increased proportionally with the level of blood glucose.160 After major surgery for cancer, hyperglycemia induced by parenteral nutrition was associated with muscle protein catabolism, while maintenance of normoglycemia restored a neutral protein balance.161
Similar observations were made more recently in patients receiving intensive care, particularly during the early stages of critical illness.162
Conclusion
In conclusion, more and more evidence suggests that reversal of the catabolic responses to surgery may be associated with better outcomes. It appears that there is significant potential for perioperative physicians to optimize surgical recovery by preserving glucose homeostasis and providing optimal pain control and perioperative nutrition.
Key points
-
Hyperglycemia, protein loss, and insulin resistance are important characteristics of the so-called catabolic response to surgery. All these features are associated with adverse outcomes.
-
The optimal glucose concentration for improving clinical outcomes is unknown. Most associations recommend treatment of random blood glucose > 10 mmol·L−1.
-
Glucose insulin administration, while maintaining normoglycemia (GIN) therapy, allows for maintenance of perioperative normoglycemia, even in patients undergoing major surgery. Furthermore, GIN reduces glycemic variability, allows for feeding while avoiding glucotoxicity, and has anti-inflammatory, cardioprotective, and inotropic effects.
-
Neuraxial anesthesia blunts the neuroendocrine stress response and results in improved insulin sensitivity with a positive influence on glucose and protein catabolism.
-
The avoidance of preoperative fasting with oral carbohydrate administration accelerates recovery after major abdominal surgery.
-
Enhanced Recovery After Surgery programs advocate early enteral feeding after colorectal surgery with potential clinical benefits.
References
Schricker T, Lattermann R, Schreiber M, Geisser W, Georgieff M, Radermacher P. The hyperglycaemic response to surgery: pathophysiology, clinical implications and modification by the anaesthetic technique. Clinical Intensive Care 1998; 9: 118-28.
Schricker T, Lattermann R, Wykes L, Carli F. Effect of i.v. dextrose administration on glucose metabolism during surgery. JPEN J Parenter Enteral Nutr 2004; 28: 149-53.
Kuntz C, Wunsch A, Bay F, Windeler J, Glaser F, Herfarth C. Prospective randomized study of stress and immune response after laparoscopic vs conventional colonic resection. Surg Endosc 1998; 12: 963-7.
Carli F, Galeone M, Gzodzic B, et al. Effect of laparoscopic colon resection on postoperative glucose utilization and protein sparing: an integrated analysis of glucose and protein metabolism during the fasted and fed States using stable isotopes. Arch Surg 2005; 140: 593-7.
Horber FF, Krayer S, Miles J, Cryer P, Rehder K, Haymond MW. Isoflurane and whole body leucine, glucose, and fatty acid metabolism in dogs. Anesthesiology 1990; 73: 82-92.
Horber FF, Krayer S, Rehder K, Haymond MW. Anesthesia with halothane and nitrous oxide alters protein and amino acid metabolism in dogs. Anesthesiology 1988; 69: 319-26.
Eberhart LH, Graf J, Morin AM, et al. Randomised controlled trial of the effect of oral premedication with dexamethasone on hyperglycaemic response to abdominal hysterectomy. Eur J Anaesthesiol 2011; 28: 195-201.
Lukins MB, Manninen PH. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg 2005; 100: 1129-33.
Hatzakorzian R, Bui H, Carvalho G, Shan WL, Sidhu S, Schricker T. Fasting blood glucose levels in patients presenting for elective surgery. Nutrition 2011; 27: 298-301.
Abdelmalak B, Abdelmalak JB, Knittel J, et al. The prevalence of undiagnosed diabetes in non-cardiac surgery patients, an observational study. Can J Anesth 2010; 57: 1058-64.
Cuthbertson DP. Post-shock metabolic response. Lancet 1942; 239: 433-7.
Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013; 310: 1591-600.
Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inverse regulation of protein turnover and amino acid transport in skeletal muscle of hypercatabolic patients. J Clin Endocrinol Metab 2002; 87: 3378-84.
Kinney JM, Elwyn DH. Protein metabolism and injury. Annu Rev Nutr 1983; 3: 433-66.
Schricker T, Gougeon R, Eberhart L, et al. Type 2 diabetes mellitus and the catabolic response to surgery. Anesthesiology 2005; 102: 320-6.
Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003; 348: 683-93.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304: 1787-94.
Morais JA, Chevalier S, Gougeon R. Protein turnover and requirements in the healthy and frail elderly. J Nutr Health Aging 2006; 10: 272-83.
Schricker T, Klubien K, Carli F. The independent effect of propofol anesthesia on whole body protein metabolism in humans. Anesthesiology 1999; 90: 1636-42.
Essen P, McNurlan MA, Wernerman J, Vinnars E, Garlick PJ. Uncomplicated surgery, but not general anesthesia, decreases muscle protein synthesis. Am J Physiol 1992; 262(3 Pt 1): E253-60.
Rennie MJ, MacLennan PA. Protein turnover and amino acid oxidation: the effect of anaesthesia and surgery. In: Garrow JS, Halliday D, editors. Substrate and Energy Metabolism in Man. London: John Libbey; 1985: 213-21.
Thorell A, Loftenius A, Andersson B, Ljungqvist O. Postoperative insulin resistance and circulating concentrations of stress hormones and cytokines. Clin Nutr 1996; 15: 75-9.
DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981; 30: 1000-7.
Thorell A, Efendic S, Gutniak M, Haggmark T, Ljungqvist O. Insulin resistance after abdominal surgery. Br J Surg 1994; 81: 59-63.
Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care 1999; 2: 69-78.
Tsubo T, Kudo T, Matsuki A, Oyama T. Decreased glucose utilization during prolonged anaesthesia and surgery. Can J Anaesth 1990; 37: 645-9.
Nygren J, Thorell A, Efendic S, Nair KS, Ljungqvist O. Site of insulin resistance after surgery: the contribution of hypocaloric nutrition and bed rest. Clin Sci (Lond) 1997; 93: 137-46.
Schricker T, Carli F, Schreiber M, et al. Propofol/sufentanil anesthesia suppresses the metabolic and endocrine response during, not after, lower abdominal surgery. Anesth Analg 2000; 90: 450-5.
Uchida I, Asoh T, Shirasaka C, Tsuji H. Effect of epidural analgesia on postoperative insulin resistance as evaluated by insulin clamp technique. Br J Surg 1988; 75: 557-62.
Nygren JO, Thorell A, Soop M, et al. Perioperative insulin and glucose infusion maintains normal insulin sensitivity after surgery. Am J Physiol 1998; 275(1 Pt 1): E140-8.
Wang ZG, Wang Q, Wang WJ, Qin HL. Randomized clinical trial to compare the effects of preoperative oral carbohydrate versus placebo on insulin resistance after colorectal surgery. Br J Surg 2010; 97: 317-27.
Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology 2008; 108: 506-23.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87: 978-82.
Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773-8.
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001; 32: 2426-32.
McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care 2005; 28: 810-5.
Lepore G, Formentano A, Boselli L, Nosari I. Clinical significance of admission hyperglycemia in critical non-diabetic patients after head injury or subarachnoid hemorrhage. Diabet Nutr 1998; 11: 175-8.
Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 2003; 78: 1471-8.
Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc 2005; 80: 862-6.
Ross-Huot MC, Laferriere A, Gi CM, Khorashadi M, Schricker T, Coderre TJ. Effects of glycemic regulation on chronic postischemia pain. Anesthesiology 2011; 115: 614-25.
Deane AM, Horowitz M. Dysglycaemia in the critically ill - significance and management. Diabetes Obes Metab 2013; 15: 792-801.
Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006; 105: 244-52.
Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 2008; 36: 3008-13.
Mackenzie IM, Whitehouse T, Nightingale PG. The metrics of glycaemic control in critical care. Intensive Care Med 2011; 37: 435-43.
Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 2010; 95: 4338-44.
Halkos ME, Puskas JD, Lattouf OM, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg 2008; 136: 631-40.
Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg 2009; 96: 1358-64.
O’Sullivan CJ, Hynes N, Mahendran B, et al. Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg 2006; 32: 188-97.
Noordzij PG, Boersma E, Schreiner F, et al. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol 2007; 156: 137-42.
Abdelmalak BB, Knittel J, Abdelmalak JB, et al. Preoperative blood glucose concentrations and postoperative outcomes after elective non-cardiac surgery: an observational study. Br J Anaesth 2014; 112: 79-88.
Chandra RK. Nutrition, immunity, and infection: present knowledge and future directions. Lancet 1983; 1: 688-91.
Windsor JA, Hill GL. Weight loss with physiologic impairment. A basic indicator of surgical risk. Ann Surg 1988; 207: 290-6.
Watters JM, Clancey SM, Moulton SB, Briere KM, Zhu JM. Impaired recovery of strength in older patients after major abdominal surgery. Ann Surg 1993; 218: 380-90; discussion 390-3.
Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg 1982; 69: 417-9.
Satyanarayana R, Klein S. Clinical efficacy of perioperative nutrition support. Curr Opin Clin Nutr Metab Care 1998; 1: 51-8.
Ponting GA, Halliday D, Teale JD, Sim AJ. Postoperative positive nitrogen balance with intravenous hyponutrition and growth hormone. Lancet 1988; 1: 438-40.
Kupfer SR, Underwood LE, Baxter RC, Clemmons DR. Enhancement of the anabolic effects of growth hormone and insulin-like growth factor I by use of both agents simultaneously. J Clin Invest 1993; 91: 391-6.
Woolfson AM, Heatley RV, Allison SP. Insulin to inhibit protein catabolism after injury. N Engl J Med 1979; 300: 14-7.
Combes J, Borot S, Mougel F, Penfornis A. The potential role of glucagon-like peptide-1 or its analogues in enhancing glycaemic control in critically ill adult patients. Diabetes Obes Metab 2011; 13: 118-29.
Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med 2001; 345: 1223-9.
van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001; 345: 1359-67.
Schricker T. The catabolic response to surgery: how can it be modified by the anesthesiologist? Can J Anesth 2001; 48(1 Suppl): R67-71.
Schricker T, Kugler B, Trager K, Anhaupl T, Vogt J, Georgieff M. Effects of xylitol on carbohydrate and protein metabolism after trauma and during sepsis. Nutr Hosp 1993; 8: 471-8.
de Lorenzo Garcia. A, Culebras JM, Zarazaga A, Rodriguez Montes JA. Carbohydrates—no glucose—in parenteral nutrition. A fading concept? (Spanish). Nutr Hosp 1996; 11: 17-28.
Felbinger T, Suchner U, Schmitz J. Carbohydrates and polyols during hypercaloric parenteral feeding of the critically ill patient - A prospective, randomized clinical comparison of glucose, glucose-xylitol, fructose-glucose-xylitol (German) Anästhesiol Intensivmed 2000; 41: 509-18.
Stehle P, Zander J, Mertes N, et al. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet 1989; 1: 231-3.
Sandstedt S, Jorfeldt L, Larsson J. Randomized, controlled study evaluating effects of branched chain amino acids and alpha-ketoisocaproate on protein metabolism after surgery. Br J Surg 1992; 79: 217-20.
Kern KA, Bower RH, Atamian S, Matarese LE, Ghory MJ, Fischer JE. The effect of a new branched chain—enriched amino acid solution on postoperative catabolism. Surgery 1982; 92: 780-5.
Sapir DG, Stewart PM, Walser M, et al. Effects of alpha-ketoisocaproate and of leucine on nitrogen metabolism in postoperative patients. Lancet 1983; 1: 1010-4.
Heyland D, Muscedere J, Wischmeyer PE, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 2013; 368: 1489-97.
Takala J, Ruokonen E, Webster NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med 1999; 341: 785-92.
Peiffer J, Danner E, Schmidt PF. Oxalate-induced encephalitic reactions to polyol-containing infusions during intensive care. Clin Neuropathol 1984; 3: 76-87.
Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: the Portland Diabetic Project. Endocr Pract 2006; 12(Suppl 3): 22-6.
NICE-SUGAR Study Investigators; Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283-97.
Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354: 449-61.
Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008; 358: 125-39.
NICE-SUGAR Study Investigators; Finfer S, Liu B, Chittock DR, et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108-18.
Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009; 35: 1738-48.
Kao LS, Meeks D, Moyer VA, Lally KP. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev 2009; 3: CD006806.
Haga KK, McClymont KL, Clarke S, et al. The effect of tight glycaemic control, during and after cardiac surgery, on patient mortality and morbidity: a systematic review and meta-analysis. J Cardiothorac Surg 2011; 6: 3.
Lazar HL, McDonnell MM, Chipkin S, Fitzgerald C, Bliss C, Cabral H. Effects of aggressive versus moderate glycemic control on clinical outcomes in diabetic coronary artery bypass graft patients. Ann Surg 2011; 254: 458-63; discussion 463-4.
Bhamidipati CM, LaPar DJ, Stukenborg GJ, et al. Superiority of moderate control of hyperglycemia to tight control in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2011; 141: 543-51.
Lazar HL. Glycemic control during coronary artery bypass graft surgery. ISRN Cardiol 2012; 2012: 292490.
Agus MS, Steil GM, Wypij D, et al. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med 2012; 367: 1208-19.
Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg 2009; 87: 663-9.
Ryden L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013; 34: 3035-87.
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee; Cheng AY. Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can J Diabetes 2013; 37(suppl 1): S1-3.
Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165-228.
American Diabetes Association. Standards of Medical Care in Diabetes—2014. Diabetes Care 2014; 37 Suppl 1: S14-80.
Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012; 97: 16-38.
Carvalho G, Moore A, Qizilbash B, Lachapelle K, Schricker T. Maintenance of normoglycemia during cardiac surgery. Anesth Analg 2004; 99: 319-24.
Albacker T, Carvalho G, Schricker T, Lachapelle K. High-dose insulin therapy attenuates systemic inflammatory response in coronary artery bypass grafting patients. Ann Thorac Surg 2008; 86: 20-7.
Carvalho G, Pelletier P, Albacker T, et al. Cardioprotective effects of glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing coronary artery bypass grafting. J Clin Endocrinol Metab 2011; 96: 1469-77.
Sato H, Hatzakorzian R, Carvalho G, et al. High-dose insulin administration improves left ventricular function after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011; 25: 1086-91.
Lopez-Hellin J, Baena-Fustegueras JA, Schwartz-Riera S, Garcia-Arumi E. Usefulness of short-lived proteins as nutritonal indicators in surgical patients. Clin Nutr 2002; 21: 119-25.
Nordenstrom J, Askanazi J, Elwyn DH, et al. Nitrogen balance during total parenteral nutrition: glucose vs. fat. Ann Surg 1983; 197: 27-33.
Anderson RE, Klerdal K, Ivert T, Hammar N, Barr G, Owall A. Are even impaired fasting blood glucose levels preoperatively associated with increased mortality after CABG surgery? Eur Heart J 2005; 26: 1513-8.
Ouattara A, Lecomte P, Le Manach Y, et al. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology 2005; 103: 687-94.
Visser L, Zuurbier CJ, Hoek FJ, et al. Glucose, insulin and potassium applied as perioperative hyperinsulinaemic normoglycaemic clamp: effects on inflammatory response during coronary artery surgery. Br J Anaesth 2005; 95: 448-57.
Askanazi J, Rosenbaum SH, Hyman AI, Silverberg PA, Milic-Emili J, Kinney JM. Respiratory changes induced by the large glucose loads of total parenteral nutrition. JAMA 1980; 243: 1444-7.
Guenst JM, Nelson LD. Predictors of total parenteral nutrition-induced lipogenesis. Chest 1994; 105: 553-9.
Schloerb PR, Henning JF. Patterns and problems of adult total parenteral nutrition use in US academic medical centers. Arch Surg 1998; 133: 7-12.
Shaw JH, Wolfe RR. An integrated analysis of glucose, fat, and protein metabolism in severely traumatized patients. Studies in the basal state and the response to total parenteral nutrition. Ann Surg 1989; 209: 63-72.
Heyland DK, MacDonald S, Keefe L, Drover JW. Total parenteral nutrition in the critically ill patient: a meta-analysis. JAMA 1998; 280: 2013-9.
Heyland DK, Montalvo M, MacDonald S, Keefe L, Su XY, Drover JW. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg 2001; 44: 102-11.
Greenberg GR, Marliss EB, Anderson GH, et al. Protein-sparing therapy in postoperative patients. Effects of added hypocaloric glucose or lipid. N Engl J Med 1976; 294: 1411-6.
Askanazi J, Carpentier YA, Jeevanandam M, Michelsen CB, Elwyn DH, Kinney JM. Energy expenditure, nitrogen balance, and norepinephrine excretion after injury. Surgery 1981; 89: 478-84.
Wolfe RR, O’Donnell TF Jr, Stone MD, Richmand DA, Burke JF. Investigation of factors determining the optimal glucose infusion rate in total parenteral nutrition. Metabolism 1980; 29: 892-900.
Jiang H, Sun MW, Hefright B, Chen W, Lu CD, Zeng J. Efficacy of hypocaloric parenteral nutrition for surgical patients: a systematic review and meta-analysis. Clin Nutr 2011; 30: 730-7.
Soop M, Carlson GL, Hopkinson J, et al. Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br J Surg 2004; 91: 1138-45.
Boelens PG, Heesakkers FF, Luyer MD, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann Surg 2014; 259: 649-55.
Burden S, Todd C, Hill J, Lal S. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev 2012; 11: CD008879.
Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg 2014; DOI:10.1016/j.jamcollsurg.2014.06.016.
Hamza N, Darwish A, O’Reilly DA, et al. Perioperative enteral immunonutrition modulates systemic and mucosal immunity and the inflammatory response in patients with periampullary cancer scheduled for pancreaticoduodenectomy: a randomized clinical trial. Pancreas 2014; DOI:10.1097/MPA.0000000000000222.
Plank LD, Mathur S, Gane EJ, et al. Perioperative immunonutrition in patients undergoing liver transplantation: a randomized double-blind trial. Hepatology 2014; DOI:10.1002/hep.27433.
Vidal-Casariego A, Calleja-Fernandez A, Villar-Taibo R, Kyriakos G, Ballesteros-Pomar MD. Efficacy of arginine-enriched enteral formulas in the reduction of surgical complications in head and neck cancer: a systematic review and meta-analysis. Clin Nutr 2014; DOI:10.1016/j.clnu.2014.04.020.
Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ 2000; 321: 1493.
Donatelli F, Vavassori A, Bonfanti S, et al. Epidural anesthesia and analgesia decrease the postoperative incidence of insulin resistance in preoperative insulin-resistant subjects only. Anesth Analg 2007; 104: 1587-93.
Schricker T, Meterissian S, Wykes L, Eberhart L, Lattermann R, Carli F. Postoperative protein sparing with epidural analgesia and hypocaloric dextrose. Ann Surg 2004; 240: 916-21.
Ward HC, Halliday D, Sim AJ. Protein and energy metabolism with biosynthetic human growth hormone after gastrointestinal surgery. Ann Surg 1987; 206: 56-61.
Schricker T, Wykes L, Meterissian S, et al. The anabolic effect of perioperative nutrition depends on the patient’s catabolic state before surgery. Ann Surg 2013; 257: 155-9.
Giesecke K, Klingstedt C, Ljungqvist O, Hagenfeldt L. The modifying influence of anaesthesia on postoperative protein catabolism. Br J Anaesth 1994; 72: 697-9.
Moore FD, Olesen K, McMurrey J. The Body Cell Mass and its Supporting Environment: Body Composition in Health and Disease. Philadelphia: WB Saunders Company; 1963 .
Harris J, Benedict F. A Biometric Study of Basal Metabolism in Man. Publication no. 279. Washington DC: Carnegie Institution; 1919.
Makk LJ, McClave SA, Creech PW, et al. Clinical application of the metabolic cart to the delivery of total parenteral nutrition. Crit Care Med 1990; 18: 1320-7.
Knox LS, Crosby LO, Feurer ID, Buzby GP, Miller CL, Mullen JL. Energy expenditure in malnourished cancer patients. Ann Surg 1983; 197: 152-62.
Shaw-Delanty SN, Elwyn DH, Askanazi J, Iles M, Schwarz Y, Kinney JM. Resting energy expenditure in injured, septic, and malnourished adult patients on intravenous diets. Clin Nutr 1990; 9: 305-12.
Hasselager R, Gogenur I. Core muscle size assessed by perioperative abdominal CT scan is related to mortality, postoperative complications, and hospitalization after major abdominal surgery: a systematic review. Langenbecks Arch Surg 2014; 399: 287-95.
Jagoe RT, Goodship TH, Gibson GJ. The influence of nutritional status on complications after operations for lung cancer. Ann Thorac Surg 2001; 71: 936-43.
Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med 1991; 325: 525-32.
Downs JH, Haffejee A. Nutritional assessment in the critically ill. Curr Opin Clin Nutr Metab Care 1998; 1: 275-9.
Hoffer LJ, Bistrian BR. Appropriate protein provision in critical illness: a systematic and narrative review. Am J Clin Nutr 2012; 96: 591-600.
Allison SP. Malnutrition, disease, and outcome. Nutrition 2000; 16: 590-3.
Allison SP, Lobo DN, Stanga Z. The treatment of hypoalbuminaemia. Clin Nutr 2001; 20: 275-9.
Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol 1980; 238: E473-9.
Prelack K, Dwyer J, Yu YM, Sheridan RL, Tompkins RG. Urinary urea nitrogen is imprecise as a predictor of protein balance in burned children. J Am Diet Assoc 1997; 97: 489-95.
Berg A, Rooyackers O, Bellander BM, Wernerman J. Whole body protein kinetics during hypocaloric and normocaloric feeding in critically ill patients. Crit Care 2013; 17: R158.
Lattermann R, Carli F, Wykes L, Schricker T. Perioperative glucose infusion and the catabolic response to surgery: the effect of epidural block. Anesth Analg 2003; 96: 555-62.
Lattermann R, Carli F, Wykes L, Schricker T. Epidural blockade modifies perioperative glucose production without affecting protein catabolism. Anesthesiology 2002; 97: 374-81.
Schricker T, Wykes L, Carli F. Epidural blockade improves substrate utilization after surgery. Am J Physiol Endocrinol Metab 2000; 279: E646-53.
Dahn MS, Mitchell RA, Lange MP, Smith S, Jacobs LA. Hepatic metabolic response to injury and sepsis. Surgery 1995; 117: 520-30.
Stadler M, Nuyens V, Seidel L, Albert A, Boogaerts JG. Effect of nutritional status on oxidative stress in an ex vivo perfused rat liver. Anesthesiology 2005; 103: 978-86.
Esahili AH, Boija PO, Ljungqvist O, Rubio C, Ware J. Twenty-four hour fasting increases endotoxin lethality in the rat. Eur J Surg 1991; 157: 89-95.
Ljungqvist O, Boija PO, Esahili H, Larsson M, Ware J. Food deprivation alters liver glycogen metabolism and endocrine responses to hemorrhage. Am J Physiol 1990; 259(5 Pt 1): E692-8.
Ljungqvist O, Jansson E, Ware J. Effect of food deprivation on survival after hemorrhage in the rat. Circ Shock 1987; 22: 251-60.
Ljungqvist O, Thorell A, Gutniak M, Haggmark T, Efendic S. Glucose infusion instead of preoperative fasting reduces postoperative insulin resistance. J Am Coll Surg 1994; 178: 329-36.
Crowe PJ, Dennison A, Royle GT. The effect of pre-operative glucose loading on postoperative nitrogen metabolism. Br J Surg 1984; 71: 635-7.
Lopez-Hellin J, Baena-Fustegueras JA, Vidal M, Riera SS, Garcia-Arumi E. Perioperative nutrition prevents the early protein losses in patients submitted to gastrointestinal surgery. Clin Nutr 2004; 23: 1001-8.
Schricker T, Meterissian S, Lattermann R, et al. Anticatabolic effects of avoiding preoperative fasting by intravenous hypocaloric nutrition: a randomized clinical trial. Ann Surg 2008; 248: 1051-9.
Henriksen MG, Hessov I, Dela F, Hansen HV, Haraldsted V, Rodt SA. Effects of preoperative oral carbohydrates and peptides on postoperative endocrine response, mobilization, nutrition and muscle function in abdominal surgery. Acta Anaesthesiol Scand 2003; 47: 191-9.
Von Meyenfeldt MF, Meijerink WJ, Rouflart MM, Builmaassen MT, Soeters PB. Perioperative nutritional support: a randomized clinical trial. Clin Nutr 1992; 11: 180-6.
Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr 2000; 24: 7-14.
Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. Br J Surg 2005; 92: 415-21.
Bozzetti F. Rationale and indications for preoperative feeding of malnourished surgical cancer patients. Nutrition 2002; 18: 953-9.
Nygren J, Soop M, Thorell A, Efendic S, Nair KS, Ljungqvist O. Preoperative oral carbohydrate administration reduces postoperative insulin resistance. Clin Nutr 1996; 17: 65-70.
Yagci G, Can MF, Ozturk E, et al. Effects of preoperative carbohydrate loading on glucose metabolism and gastric contents in patients undergoing moderate surgery: a randomized, controlled trial. Nutrition 2008; 24: 212-6.
Mathur S, Plank LD, McCall JL, et al. Randomized controlled trial of preoperative oral carbohydrate treatment in major abdominal surgery. Br J Surg 2010; 97: 485-94.
Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr 2013; 32: 34-44.
Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg 2013; 37: 259-84.
Gore DC, Chinkes DL, Hart DW, Wolf SE, Herndon DN, Sanford AP. Hyperglycemia exacerbates muscle protein catabolism in burn-injured patients. Crit Care Med 2002; 30: 2438-42.
Biolo G, De Cicco M, Lorenzon S, et al. Treating hyperglycemia improves skeletal muscle protein metabolism in cancer patients after major surgery. Crit Care Med 2008; 36: 1768-75.
Hsu CW, Sun SF, Lin SL, Huang HH, Wong KF. Moderate glucose control results in less negative nitrogen balances in medical intensive care unit patients: a randomized, controlled study. Crit Care 2012; 16: R56.
Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian Critical Care Nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract 2014; 29: 29-43.
McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009; 33: 277-316.
Acknowledgements
Thomas Schricker and Ralph Lattermann are supported by the Canadian Institutes of Health Research (CIHR).
Conflicts of interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schricker, T., Lattermann, R. Perioperative catabolism. Can J Anesth/J Can Anesth 62, 182–193 (2015). https://doi.org/10.1007/s12630-014-0274-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-014-0274-y