Abstract
Purpose of Review
This review article seeks to summarize the existent literature regarding the use of anthracyclines (specifically doxorubicin) in the treatment of early-stage breast cancers, reviewing the clinically significant side effects of said therapy, and discussing new tools to risk stratify patients.
Recent Findings
The 2010 Early Breast Cancer Trialists’ Cooperative Group meta-analysis again found anthracycline-containing regimens to improve outcomes, while the ABC Trials have shown the superiority of regimens including doxorubicin versus regimens with docetaxel and cyclophosphamide alone in early-stage breast cancer. New risk stratification tools—such as Oncotype DX®—are helping oncologists decide which patients may be able to avoid chemotherapy.
Summary
Sequential doxorubicin/cyclophosphamide therapy, followed by treatment with docetaxel, improves outcomes in nearly all early-stage breast cancer, with the notable exception of Her2+ disease. Newer risk stratification tools allow better risk/reward calculations in which patients may be able to avoid anthracycline-based chemotherapy and its significant side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is still the most common cancer diagnosed in the USA, with an estimated 252,710 cases (15% of all cancer cases) and an estimated 40,610 deaths (6.8% of total cancer deaths) projected by the National Institute of Health’s (NIH) National Cancer Institute for 2017 [1]. The incidence of breast cancer is stably high at 130.6 per 100,000 in 2014 [1], with a distribution of 61% localized disease (stage I and some stage II), 32% regionally advanced disease (generally stage II and III, depending on size and nodal involvement), 6% advanced disease stage (some stage IIIc and all stage IV), and 2% unstaged [2]. The stark difference between incidence and mortality rate (declining by 1.8% each year from 2005 to 2014) [2] has been attributed to multiple interventions, including improved screening and systemic treatment modalities. The problem in treatment, however, lies in the selection of treatment modalities.
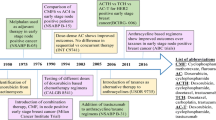
Treatment of breast cancer going back centuries involved surgery of various degrees, supplemented by radiation as time went on; however, with the publication of Sidney Farber’s 1948 report on the benefit of aminopterin and amethopterin in childhood acute lymphoblastic leukemia, interest in chemotherapy began to grow [3]. The first successful demonstration of chemotherapy in breast cancer occurred in 1951, with three out of four patients with metastatic disease showing subjective and objective signs of response to amethopterin (an additional six breast cancer patients did not respond to other folate acid antagonists) [4]. The first randomized trial showing benefit of adjuvant chemotherapy in breast cancer was the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-01 trial, reporting the benefit of thiotepa (an alkylating agent) decreasing recurrence rate in premenopausal patients with ≥ 4 positive axillary nodes in 1968 [5].
Daunorubicin—the first of the antitumor anthracycline class of antibiotics isolated from Streptomyces peucetius—was discovered in 1963 independently by Italian [6] and French [7] researchers; by 1966, it already showed significant antitumor activity in acute lymphoblastic leukemia [8], as well as the potential to produce fatal cardiotoxicity [9]. Doxorubicin—created by mutating S. peucetius with N-nitroso-N-methyl urethane—showed better antitumor activity and a higher therapeutic index, but still had significant cardiotoxicity [10]. The search for less cardiotoxic compounds eventually led to epirubicin, an epimer with a 4′-hydroxyl group on doxorubicin’s sugar molecule [11]. Early studies seemed to indicate similar efficacy between the two with lower rates of cardiotoxicity in epirubicin. For example, one clinical trial looking at fluorouracil, cyclophosphamide, and either doxorubicin (N available for analysis = 113) or epirubicin (N available for analysis = 117) showed no statistical difference in overall response rate (ORR), time to response, duration of response, or response rate according to tumor site, while toxicity analysis showed three episodes of heart failure in the doxorubicin group (N available for analysis = 120) and none in the epirubicin group (N available for analysis = 124) [12]. However, such differences were not always consistent (some authors wondered if the two were really being dosed equally for comparison), and epirubicin’s patent protection up until 2007 (costing 22 times doxorubicin by reports) likely precluded use in both trials and private practice [13]. This unclear cardiotoxicity benefit with real cost and use experience differential is likely why epirubicin is not used so often.
The first demonstration of the benefit of combination adjuvant chemotherapy in breast cancer came with the cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) regimen in 1976 [14]. A flurry of new combination therapies were then formulated and tested against one another, including doxorubicin/vincristine shown to be non-inferior to CMF in 1978 [15]. Eventually, in 1990, NSABP B-15 showed equivalency between 6 months of CMF and four cycles of a combination doxorubicin and cyclophosphamide (AC) given every 3 weeks; due to faster completion time, fewer health care provider visits (a third compared to conventional CMF), and less need for nausea-control medication (12 on AC versus 84 on CMF), the NSABP group recommended AC [16].
After decades of research showing improved outcomes in the treatment of all varieties of breast cancer—node-positive [5] or node-negative [17], hormone receptor-positive [18] or hormone receptor-negative, pre- [5] or postmenopausal—the NIH issued a statement in 2001 stating “[b]ecause adjuvant polychemotherapy improves survival, it should be recommended to the majority of women with localized breast cancer regardless of lymph node, menopausal, or hormone receptor status,” and further stated that “[t]he inclusion of anthracyclines in adjuvant chemotherapy regimens produces a small but statistically significant improvement in survival over nonanthracycline-containing regimens” [19]. This latter addition was included due to the meta-analysis of 18,000 women in 47 trials by the Early Breast Cancer Trialists Cooperative Group (EBCTCG), which stated a 4.2% relative and 3% absolute 5-year mortality benefit for anthracycline-containing regimens versus CMF in women 50–69 [20]. However, this benefit may have been influenced by the increasing usage of taxanes, which initially emerged as a highly effective therapy in the metastatic setting [21], but began finding increasing use as an alternate standard adjuvant therapy regimen. Indeed, by 2005, there was a sharp increase in taxane-based therapy at the expense of anthracycline-based, and by 2008, some Medicare cohorts were > 50% taxane-based in response to concerns about anthracycline-associated toxicities [22].
Toxicities
The fear of nondiscriminatory use of anthracyclines stems from very real and valid concerns of heart failure and therapy-related myelodysplastic syndrome (t-MDS)/therapy-related acute myeloid leukemia (t-AML) development. The main mechanism by which the antitumor effect of anthracyclines is exerted is direct topoisomerase II inhibition after DNA insertion [23]; multiple other effects of anthracyclines are known to exist, including generation of oxygen radicals [24], direct DNA damage, and depletion of cardiac cytochrome c leading to apoptosis [25]. When these latter mechanisms lead to the development of cardiac or hematologic side effects, treatment options are generally limited and with worse outcomes than non-anthracycline causes of the same.
Cardiotoxicity
The exact reason why anthracyclines preferentially affect cardiac tissue is still unclear, though the leading hypothesis is some element of sensitivity to oxidant stress/damage caused by the relative enrichment of mitochondria in cardiac tissue [25]. Regardless, cardiotoxicity—in the form of arrhythmias (persistent sinus tachycardia or transient rhythm abnormalities), peri-/myocarditis, cardiomyopathies/scarring, myocardial infarction, and congestive heart failure (CHF) [26]—is the main feared side effect of anthracycline use. Although the former elements of the prior list can occur any time, the medication class is administered (mostly secondary to type I cardiotoxicity from cardiomyocyte death) [27], CHF frequently occurs in a dose-dependent manner. Early studies indicated a sharp increase in prevalence at doxorubicin doses > 550 mg/m2, but subsequent studies have shown heart failure at even “low risk” doses [28]; in fact, one retrospective analysis of three trials (N = 630 patients, 32 events) showed a cumulative prevalence of CHF at 1.7% for doxorubicin at 300 mg/m2 (CHF cumulative prevalence of 15.7% at 500 mg/m2 and 32.4% at 600 mg/m2) [29].
There are a number of risk factors associated with anthracycline-induced cardiotoxicity, including age > 65 (or < 4), female gender, hypertension, pre-existing cardiac disease, cumulative dose, high individual dose, and treatment with cyclophosphamide, paclitaxel, or trastuzumab [27]. Of these, the ones more critical to CHF development are increasing age (with one retrospective analysis showing 11% in > 75, versus 6% in 65 to 74 and 1–2% in younger women) [30], cumulative dose, and concurrent cardiotoxic medications. Though there may be an element of type II cardiotoxicity (that is, cardiomyocyte dysfunction) in anthracycline-induced heart failure and the possibility for rehabilitation, most of the damage in this case stems from cardiomyocyte apoptosis and decrease in left ventricular ejection fraction (type I cardiotoxicity) [31]. This fact likely explains why rates of CHF continue to increase in such patients over 10 years from therapy [32]. It may also explain the worse prognosis of CHF due to anthracycline-based cardiomyopathy: in a review of outcomes of 1230 patients with cardiomyopathy, the hazard ratio for death in the 15 patients who were treated with doxorubicin was 2.64 (95% confidence interval 1.35–5.17), with over half studied dead well before 5 years [33].
To address the issue of monitoring and preventing cardiac dysfunction, the American Society of Clinical Oncology (ASCO) convened a multidisciplinary group that used a systematic review to identify 104 meta-analyses, randomized trials, observational studies, and clinical experience gathered from 1996 to 2016. The recommendations, put out in 2016 and endorsed by both ASCO and the American Heart Association, recommend avoiding the use of cardiotoxic medications when available, prescreening for cardiac risk factors (as noted above), and performing an echocardiogram (or cardiac MRI/MUGA if echocardiography not feasible) and checking biomarkers (IE—troponin, natriuretic peptide) before starting potentially cardiotoxic therapy. For patients at high risk or who have evidence of cardiac disease, referral to Cardiology is appropriate. For low-risk patients, screening recommendations after therapy completion include an echocardiogram and biomarkers at 6 to 12 months; due to a paucity of evidence, no screening guidelines after 12 months exist [34].
MDS/AML
Although first recognized in long-term survivors of Hodgkin disease [35], the risk of developing t-AML/t-MDS is present after treatment of all types of cancer. The exact classification is difficult, but the two are thought to represent a single disease entity most closely resembling acute myeloid leukemia with multilineage dysplasia, and best distinguished by the percentage of blasts in the periphery and bone marrow (≥ 20% representing t-AML) [36]. Incidence, as reported by the NSABP experience, is 0.21% at 5 years for doxorubicin given at 60 mg/m2 every 21 days for four cycles (cyclophosphamide doses in these trials varied from 600 to 2400 mg/m2 every 21 days for two to four cycles) [37]; increasing doses, however, leads to higher incidence (0.37 versus 4.97% at 8 years for standard versus greater than standard epirubicin dosing) [38]. Median latency is about 5 years [39], peaking at 9 years and usually decreasing after [35]. Unlike de novo AML, t-AML/t-MDS is generally associated with poor-risk cytogenetics (15–25 versus 50–70%, respectively), resistance to therapy, and a higher risk of organ failure; median survival is about 6–8 months, with allogenic stem cell transplantation the only option for cure in most patients [40].
Anthracyclines are not a unique, nor even excessively potent, risk factor for t-AML/t-MDS development. One case control study of 182 stage I–III breast cancer patients and 534 controls in France found radiotherapy and granulocyte colony-stimulating factor (G-CSF) administration to increase t-AML/t-MDS development after controlling for chemotherapy (odds ratio (OR) 3.9 and 6.3, respectively). The same study showed mitoxantrone to be the worst risk factor for t-AML/t-MDS (OR 16.35), with anthracyclines having some effect (OR 2.84); epidophyllotoxins also had a positive effect (though not enough to rigorously analyze in their model), with alkylating agents, antimetabolites, and spindle inhibitors all crossing the line of no effect [41]. The lack of effect from alkylating agents is curious, as multiple other studies (including the above NSABP retroactive analysis of six trials, varying cyclophosphamide but keeping doxorubicin the same) [33] implicate alkylating agents. While this could be explained by a low effect size leading to type II error, the more relevant issue is that, in the said study’s listed agents, only anthracyclines are widely used and implicated first-line agents for early-stage breast cancer.
Selection
The controversy over the use of anthracyclines lies in the difficulty of patient risk stratification. Initially, breast cancers were risk-stratified based off stage, as embodied by the classic TNM system [42]. Within the last two decades, as hormone and molecular targets were recognized, gene expression assays have been used to further subtype breast cancers into one of five research categories: Her2/neu (represented by the ERBB2 gene); luminal A (estrogen receptor (ER)+, Her2−); luminal B (ER+, Her2+, or Her2−, and characterized by more aggressive tumors than luminal A); normal-like (none of the prior markers, usually good prognosis); and basal (or triple-negative disease, with poorer prognosis than any of the preceding) [43, 44]. In clinical practice, however, patients are classified as hormone receptor (ER or PR)-positive, Her2-positive, or triple-negative. Although more genes being discovered and greater tumor complexity allows for ever-more complex sub-classifications [45], these categories have not yielded any clinically meaningful treatment difference compared to the ER/PR/Her2 paradigm.
Risk stratification of breast cancer patients drives two questions: giving chemotherapy or not; and, in patients receiving chemotherapy, giving anthracycline-containing regimens or not. Ideally, there would be tests or tools that would allow medical oncologists to identify which breast cancers are responsive to non-chemotherapy/non-anthracycline treatments (such as CDK-4/6 inhibitors or PARP inhibitors), identify patients at high-risk of recurrence, and identify patients at high risk for chemotherapy-related (specifically anthracycline) toxicity. Of these three, the latter task is the least well developed. Multiple methods have been studied for predicting anthracycline toxicity (particularly cardiotoxicity), including gene analysis [46], clinically relevant factors (e.g., cumulative dose, extremes of age or body weight, severity of co-morbidities) [47], and even imaging predictors (e.g., strain pattern or speckle tracking in echocardiography) [48, 49]. However, while all this work can inform individualization of care, no standardized implementation of any, or a combination, of these measures has become standard of care besides the use of echocardiography.
In one relatively low-risk group, answering the other questions is more straightforward. For node-negative ER+ tumors, the Oncotype DX® [50] test has been shown in retrospective data from NSABP-20 to predict women unlikely to derive benefit from chemotherapy (score < 18) [51], a result prospectively validated (score ≤ 10) in ER+ node-negative tumors in the TAILORx study [52•]. At appropriate cut-offs, it can also predict benefit in giving chemotherapy (though not advising which chemotherapies to give). In Her2+ and ER and node+ tumors, decision-making is more difficult. ASCO recently released biomarker guidelines by an expert panel looking at relevant studies from 2006 to 2014. Among other findings, these guidelines state that the 50-gene PAM50® assay (shown to help determine 5-year risk of recurrence) [53] should be restricted to ER+ node-negative tumors and that there are no useful biomarkers for ER+ node+ tumors, Her2+ tumors, or triple-negative tumors [54]. The guidelines initially stated that there was no early-stage breast cancer group suitable for the 70-gene MammaPrint® profile (contradicting the 2013 RASTER study, which promoted its use in patients with stage I or II disease) [55]; however, an update to these guidelines published in July 2017 allows for the assay in patients with ER+, Her2−, node− tumors and high clinical risk [56].
As exact cut-off points for treatment are shifting and controversial, answering the three questions above for other subtypes of early-stage breast cancer is beyond the scope of this review. Of note, however, is the 8th edition of the American Joint Committee on Cancer Staging Manual, taking effect January 1, 2018; this will include the above biomarkers, multigene assays, and other factors known to affect outcomes to yield a prognostic stage, separate from the anatomic stage [57]. Potentially, such staging could guide anthracycline versus non-anthracycline therapy.
Regimens
Data continues to accumulate that anthracyclines are beneficial to breast cancer patients. The EBCTCG’s 2000 meta-analysis update—this time with 145,000 women from 194 trials—showed an absolute, clinically significant mortality risk reduction of 3% at 5 years and 4% at 10 years between anthracycline-based and CMF chemotherapies [58]. The EBCTCG’s 2010 meta-analysis update—focusing only on 100,000 women in 123 trials to determine differences between chemotherapy regimens—showed a breast cancer mortality relative risk of 0.78 for anthracycline-based compared with CMF therapy; this study further stated the risk reduction was not affected by age, nodal status, tumor diameter or differentiation, ER status, and tamoxifen usage (further underlining the difficulty of risk-stratifying patients) [59]. Given the finer segmentation of data offered by the 2010 analysis alongside existing concerns of anthracycline toxicities, the following question arises: if a patient is committed to an anthracycline-based therapy, how does the literature recommend administering it?
Although daunorubicin, epirubicin, and idarubicin are all anthracyclines, recent large-scale trials tend to focus on doxorubicin (A); although this review will reference doxorubicin frequently below, the recommendations are likely similar as for epirubcin (E). The first issue to consider is cumulative dose. Initial therapies using four cycles of A (60 mg/m2) combined with cyclophosphamide (C) (600 mg/m2) given every 3 weeks showed superiority over no treatment (8% risk reduction and 6.5% breast cancer mortality reduction at 10 years by the EBCTCG 2010 review); however, standard AC was not shown to be superior against standard CMF therapy in NSABP B-15 [16], confirmed in the 2010 EBCTCG meta-analysis. Although six cycles of AC were not shown to be superior to four cycles in the CALGB 40101 trial [60], the 2010 EBCTCG meta-analysis reversed this finding: high-dose (cumulative A > 240 mg/m2, E > 360 mg/m2) is superior to standard-dose 4AC (cumulative A = 240 mg/m2), with an absolute decrease in mortality of 3.9% compared to CMF at 10 years (standard 4AC had a non-significant 1.2% mortality decrease).
Newer chemotherapies, such as the taxane docetaxel (T), complicate this picture. US Oncology 9735 compared four cycles of TC versus AC; instead of the usual finding of non-inferiority, TC was found to have superior disease-free survival (6%) and overall survival (5%) at 7 years independent of age, Her2 status, and hormone status [61]. The comparison of TAC versus TC was the next question to answer. Both US Oncology Research (USOR) 06-090 and NSABP B-46-I/USOR 07132 attempted to answer this independently, comparing six cycles TC (TC6) versus six cycles TAC (TAC6) but did not meet prespecified patient accrual levels; meeting with their data boards, the trial investigators decided to combine efforts. Although the combination of the above two trials might address superiority of TAC6, it would not address non-inferiority of TC6. For this reason, NSABP B-49 was created to supplement these numbers (the data steering committee asked investigators to allow choice of taxane plus AC regimen). This conglomeration of trials—christened the ABC trials—analyzed 4242 patients split about evenly between TAC6 (in a variety of treatment protocols) and TC6. Interim analysis at 4 years showed an invasive disease-free survival (IDFS) of 90.7% for TAC6 versus 88.2% for TC6, with an unplanned but significant test of interaction between positive hormone receptor status and nodal status only. As the study has not yet been completed, the current report of < 1% cardiac events and no hematologic malignancies in both TAC6 and TC6 (with otherwise similar general adverse events) is likely to change, worsening for the former (TAC6 already has reports of some heart failure events). Still, these results thus demonstrate superiority of TAC6 versus TC6 in early-stage breast cancers that are hormone receptor-negative (regardless of nodal status) or are both hormone receptor- and node-positive (hormone receptor-positive but node-negative cancers did not have improved IDFS) [62••].
After considering cumulative dose and the addition of taxanes as part of standard therapy for high-risk patients, the next question regards administration protocol. The ABC trial performed a test of interaction by protocol (concurrent docetaxel administration, sequential paclitaxel administration, dense dose AC with sequential paclitaxel 80 mg/m2, and dense dose AC with sequential paclitaxel 175 mg/m2), not finding a significant effect how T is given. The literature is conflicted on this point, however. The final 10-year analysis of BCIRG-005 (comparing a total of four cycles every 3 weeks of AC followed by T versus the same for TAC) did not show superiority in DFS or overall survival (OS) in early node-positive breast cancer; of note, the concurrent arm had greater cumulative (but lower per dose administration) of T compared to the sequential arm [63•]. These findings contradict earlier findings reported by the Breast International Group’s 02-98 trial (looking at sequential versus concurrent AT followed by CMF, rather than TaxAC regimens, however), which showed a 4% improvement in 5-year survival in the sequential treatment arm, somewhat complicated by the higher dosing of both doxorubicin and docetaxel in the sequential arm [64]. NSABP B-30 also looked at sequential versus concurrent docetaxel in AC regimens in early-stage node-positive breast cancer, finding a 14% non-significant (p = 0.09) reduction in mortality and a 5% improvement in DFS at a median of 73 months; however, again, this trial had lower dosing (cumulative and dose per cycle) administration of T and lower cumulative dose of A (240 versus 200 mg/m2) in the concurrent arm than the sequential arm [65]. NSABP B-30 made reference to these specific findings, postulating that total dose of docetaxel may matter more than dose per cycle. Although the matter may not be clear until such time as a trial directly compares sequential AC-T versus concurrent ACT with equal doses of docetaxel, sequential AC-T appears to be the preferred regimen.
The above regimens give the preferred anthracycline regimen and administration for early-stage breast cancer, regardless of hormone receptor status, nodal status, and (in the case of NSABP B-30) menopausal status. In terms of Her2 status, the evidence is not as strongly in favor of anthracycline usage. BCIRG-006—whose intent was to compare two trastuzumab-containing regimens to a standard anthracycline-containing regimen—looked at 3222 with early-stage Her2+ breast cancer patients at a median follow-up of 65 months and found DFS to be 75% for AC-T, 84% for AC-T with added trastuzumab (AC-TH), and 81% when receiving TCH. Although the study concluded there was no statistically significant difference between the trastuzumab-containing regimens, the study was not powered to compare the latter two regimens. Furthermore, the 3% improvement for the AC-TH regimen versus non-anthracycline came at a statistically significant five-fold increase in CHF and cardiac dysfunction in AC-TH (2%) versus TCH (0.4%) [66]; these findings were confirmed in the final 10-year analysis of BCIRG-006 presented in 2016 [67]. Given the cardiotoxicity of trastuzumab, this may be the one high-risk early breast cancer group where the current emphasis on sequential AC-T is not recommended.
Future Directions
The future of anthracycline therapy in early-stage breast cancer is rooted in risk stratification: determining which patients have the best chance of response to anthracycline therapy, which patients have the highest risk of recurrence, and which patients have the highest risk of developing toxicities related to therapy. A more concrete answer on sequential versus concurrent T in AC would also be useful. It is very possible that, over time, newer targeted therapies will gain a foothold after proving efficacy in the metastatic field. Meanwhile, for nearly every high-risk patient group (except perhaps patients with Her2+ disease), sequential AC-T should be given.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Cancer Society. Breast Cancer Facts & Figures 2015–2016. Atlanta: American Cancer Society Inc. 2015.
Cancer of the Breast (Female) - Cancer Stat Facts. NIH - National Cancer Institute. Available at https://seer.cancer.gov/statfacts/html/breast.html. Accessed 7/1/17.
Farber S, Diamond LK, Mercer RD, Sylvester RF, Wolff JA. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin). NEJM. 1948;238(23):787–93.
Wright JC, Prigot A, Wright BP, Weintraub S, Wright LT. An evaluation of folic acid antagonists in adults with neoplastic diseases. J Natl Med Assoc. 1951;43(4):211–40.
Fisher B, Ravdin RG, Ausman RK, Slack NH, Moore GE, Noer RJ. Surgical adjuvant chemotherapy in cancer of the breast: results of a decade of cooperative investigation. Ann Surg. 1968;168(3):337–56.
Grelin A, Spella C, Di Marco A, Canevazzi G. Descrizione e classificazione di un attionamicette (Streptomyces Peucetius sp nova) produltore di un sostanza ad attivite antitumorale; La daunomicina. Giorn Microbio. 1963;11:109–18.
Dubost M, Gauter P, Maral R, et al. Un novel antibiotique a proprietes cytostatiques; la rubidomycine. CR Acad Sci Paris. 1963;257:1813–5.
Jacquillat C, Boiron M, Weil M, Tanzer J, Najean Y, Bernard J. Rubidomycin: a new agent active in the treatment of acute lymphoblastic leukaemia. Lancet. 1966;2(7453):27–8.
Lefrak EA, Pitha J, Rosenheim S, et al. A clinicopathological analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–14.
Arcamone F, Cassinelli G, Fantini G, et al. Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from S. Peucetius var. caesius. Biotechn Bioengin. 1969;11(6):1101–10.
Arcamone F, Penco S, Vigevani A, et al. Synthesis and antitumor properties of new glycosides of daunomycinone and adriamycinone. J Med Chem. 1975;18:703–7.
French Epirubicin Study Group [no authors listed]. A prospective randomized phase III trial comparing combination chemotherapy with cyclophosphamide, fluorouracil, and either doxorubicin or epirubicin. French Epirubicin Study Group. J Clin Oncol 1988;6(4):679–688.
Ventura GJ. Cardiotoxicity of epirubicin versus doxorubicin: cost and clinical results. J Clin Oncol. 2005;23(12):2873–3.
Bonadonna G, Brusamolino E, Valagussa P, Rossi A, Brugnatelli L, Brambilla C, et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405–10.
Brambilla C, Valagussa P, Bonadonna G. Sequential combination chemotherapy in advanced breast cancer. Cancer Chemother Pharmacol. 1978;1(1):35–9.
Fisher B, Brown AM, Dimitrov NV, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol. 1990;8(9):1483–96.
Mansour EG, Gray R, Shatila AH, Tormey DC, Cooper MR, Osborne CK, et al. Survival advantage of adjuvant chemotherapy in high-risk node-negative breast cancer: ten-year analysis—an intergroup study. J Clin Oncol. 1998;16:3486–92.
Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–82.
Adjuvant therapy for breast cancer. NIH Consens Statement 2000; 17(4): 1–23.
Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–42.
Nabholtz JM, Gelmon K, Bontenbal M, et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996;14(6):1858–67.
Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18):2232–9.
Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226(4673):466–8.
Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261(7):3060–7.
Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53(2):105–13.
Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22(4):263–302.
Volkova M, Russell R 3rd. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7(4):214–20.
Von Hoff DD, Layard MW, Basa P, Davis HL, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–7.
Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin. Cancer. 2003;97(11):2869–79.
Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–305.
Alexander J, Dainiak N, Berger HJ, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med. 1979;300(6):278–83.
Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–15.
Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077–84.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35(8):893–911.
Foss AA, Andersen A, Nome O, et al. Long-term risk of second malignancy after treatment of Hodgkin’s disease: the influence of treatment, age and follow-up time. Ann Oncol. 2002;13(11):1786–91.
Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35(4):418–29.
Smith RE, Bryant J, DeCillis A, Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–204.
Praga C, Bergh J, Bliss J, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005;23:4179–91.
Kantarjian HM, Keating MJ, Walters RS, Smith TL, Cork A, McCredie KB, et al. Therapy-related leukemia and myelodysplastic syndrome: clinical, cytogenetic, and prognostic features. J Clin Oncol. 1986;4:1748–57.
Klimek VM. Recent advances in the management of therapy-related myelodysplastic syndromes and acute myeloid leukemia. Curr Opin Hematol. 2013;20(2):137–43.
Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25:292–300.
AJCC (American Joint Committee on Cancer). Cancer staging manual, 7th edition, Edge SB, Byrd DR, Compton CC, et al (Eds), Springer-Verlag, New York 2010.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–23.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52.
Vulsteke C, Pfeil AM, Maggen C, Schwenkglenks M, Pettengell R, Szucs TD, et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res Treat. 2015;152(1):67–76.
Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D’Ascenzo F, Malavasi V, et al. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112(12):1980–4.
Migrino RQ, Aggarwal D, Konorev E, Brahmbhatt T, Bright M, Kalyanaraman B. Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol. 2008;34(2):208–14.
Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. 2014;16(3):300–8.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34.
• Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005–14. Prospective validation of using Oncotype DX to decide in which women to withhold chemo
Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105(19):1504–11.
Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(10):1134–50.
Drukker CA, Bueno-de-Mesquita JM, Retèl VP, et al. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer. 2013;133(4):929–36.
Krop I, Ismaila N, Andre F, Bast RC, Barlow W, Collyar DE, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline focused update. J Clin Oncol. 2017;35(24):2838–47.
AJCC (American Joint Committee on Cancer). In: Amin MB, Edge SB, Greene FL, et al., editors. Cancer staging manual. 8th ed. Chicago: Springer; 2017.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44.
LN Shulman CT, Cirrincione DA, Berry BHP, Perez EA, O’Regan R, et al. Six cycles of doxorubicin and cyclophosphamide or paclitaxel are not superior to 4 cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. J Clin Oncol. 2012;30(33):4071–6.
Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27(8):1177–83.
•• Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE Jr, Jacobs SA, et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017:JCO2016714147. Compared TAC (various administration regimens) vs TC. Found superiority in regimens w/A.
• Mackey JR, Pieńkowski T, Crown J, Sadeghi S, Martin M, Chan A. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann Oncol. 2016;27(6):1041–7. Review of the study comparing concurrent TAC to sequential AC-T in Her2(-), lymph-node positive therapy. Stated no difference between the two regimens in terms of outcomes, though the actual amount of docetaxel differed in the two arms
Francis P, Crown J, Di Leo A, Buyse M, Balil A, Andersson M, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02-98 randomized trial. J Natl Cancer Inst. 2008;100(2):121–33.
Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362(22):2053–65.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83.
Slamon DJ, Eiermann W, Robert NJ, et al: Ten-year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2-positive early breast cancer patients. 2015 San Antonio Breast Cancer Symposium. Abstract S5-04. Presented December 11, 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Systemic Therapies
Rights and permissions
About this article
Cite this article
Kota, K.J., Brufsky, A.M. The Double-Edged Sword: Controversies in Anthracycline-Based Chemotherapy for Breast Cancer. Curr Breast Cancer Rep 9, 210–216 (2017). https://doi.org/10.1007/s12609-017-0254-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-017-0254-7