Abstract
Triple negative breast cancers (TNBC) are often described as biologically aggressive tumors, with poorer survival compared to other breast cancer subtypes. The fact that TNBC lacks an obvious target like estrogen receptor and HER2 represents a major challenge in the management of these patients. Genomic analyses have revealed that TNBC comprises a diverse group of cancers, which have distinct molecular profiles and different prognosis. These studies also highlighted molecular aberrations that could serve as potential treatment targets. On the other hand, a high percentage of TNBCs express some important surface receptors that have been already exploited in the development of promising targeted therapies, which are currently tested in clinical trials. In this review, we will provide an overview on the molecular diversity of TNBC with special emphasis on the evolving role of some potential biomarkers that may be utilized in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 15 % of newly diagnosed breast cancers do not express estrogen receptor (ER), progesterone receptor (PR), and HER-2, and are thus defined as triple negative breast cancer (TNBC) [1]. Compared to other breast cancer subtypes, patients with TNBC have a worse clinical outcome, in terms of shorter disease free and overall survival [2]. The natural history of the disease entails an early peak of recurrence within the first 2–3 years from diagnosis, with a higher prevalence of visceral metastasis, particularly in the lungs and brain [3, 4]. This is followed by a definite sharp decline in the relapse rate during subsequent years, with relapses being seldom reported after 8 years from diagnosis.
It has been unequivocally acknowledged that TNBC is highly responsive to chemotherapy [5]. This is based on the results of clinical trials in the neo-adjuvant setting in which triple negative tumors were consistently shown to have a very high rate of pathological complete response (pCR) [6]. In general around 30 % of patients with TNBC are expected to achieve pCR following a standard anthracycline/taxane-based chemotherapy [6]. These patients have a favorable prognosis, with less than 20 % developing distant recurrence at 5 years, which is similar to the outcome of patients attaining pCR with other breast cancer subtypes [2, 7]. However, patients with TNBC who do not experience pCR have a significantly higher risk of recurrence and death compared to other subtypes [2, 6, 7].
It is clear that the major challenge in managing patients with TNBC is the lack of an effective targeted therapy that can prolong survival. Thus, several attempts have been made to dissect the key molecular events encountered in TNBC tumors, in order to find potential therapeutic targets that may improve the treatment outcome of this disease.
TNBC Is Not a Single Disease
Earlier work using gene expression profiling has allowed the classification of breast cancer into four main molecular subtypes [8, 9]. It was shown that the majority of TNBC phenotype clustered within the basal-like (BL) tumors. Many studies have subsequently demonstrated that TNBC and BL have a lot of biological similarities, including a high frequency of poorly differentiated tumors, expression of basal cytokeratins, epidermal growth factor receptor (EGFR) expression, BRCA1 germline mutations, and p53 mutations [1, 10]. Nevertheless, TNBC and BL are not synonymous terms, where 25–30 % of TNBC defined by immunohistochemistry do not have the genomic characteristics of basal like breast cancers [1, 10].
The molecular landscape of TNBC was further refined by the pivotal work of Lehmann et al. who analyzed publically available data on gene expression profiles from 587 TNBC cases [11]. This study identified six different TNBC subtypes, each displaying a unique gene expression pattern [two basal-like (BL1, BL2 which are characterized by high levels of proliferation-related genes), an immunomodulatory, a mesenchymal (M), a mesenchymal stem-like (MSL), and a luminal androgen receptor subtype]. In a subsequent similar work, Burstein et al. performed RNA and DNA profiling analyses on 198 TNBC tumors at Baylor College of Medicine and identified four stable TNBC subtypes, characterized by expression of distinct molecular profiles that have distinct prognosis [12].
Both classifications show good, yet incomplete overlap between the different genomic subtypes of TNBCs, which for simplicity may be separated into three main categories: BL, a mesenchymal stem-like (MSL), and luminal androgen receptor (LAR) subtypes. Indeed, the LAR forms the most distinct subgroup within two classifications that shares features similar to ER-positive breast cancer [11]. In addition, it appears to be a subtype that can be relatively easier to identify in routine practice, by virtue of evaluating the expression of androgen receptor (AR) by immunohistochemistry (IHC), which has been reported by several studies [13–16] and discussed later in detail. Importantly, these studies and others were able to confirm the heterogeneity of TNBC subtypes in terms of incidence, response to chemotherapy, and prognosis [11, 12, 17].
Although the genomic characterization of TNBC appears to be far from routine clinical application, they could help to shape the genomic landscape of TNBCs and identify some key subtype-specific molecular events that derive tumor progression. This can serve as the basis for biologically rational targeted therapies in TNBC. Genome sequencing has shown that the most common genomic events of TNBCs are TP53 mutation and Myc amplification that occur in around 80 and 40 % of all TNBC tumors, respectively [18]. Unfortunately at the present time, there are no apparent approaches to target either aberrations. Other less frequent molecular events include germline and sporadic BRCA1/2 mutations (≅15 %), PIK3CA mutation (≅9 %), EGFR amplification (≅5 %), and FGFR2 amplification (≅4 %). The later events are potentially targetable and are currently being explored in clinical trials [19].
Exploiting Genomics to Discover TNBC Therapeutic Strategies
In this review, we will focus on three molecular biomarkers that hold promise to be incorporated in routine clinical practice over the next decade. These biomarkers are BRCA1/2 mutations, AR expression, and tumor-infiltrating lymphocytes (TILs).
BRCA1/2 Mutations
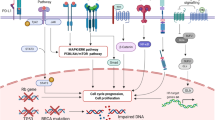
BRCA1 and BRCA2 are among the DNA repair genes, which are involved in repair of double-stranded DNA breaks (DSB) [20, 21]. Both genes are considered key components of DNA homologous recombination repair (HR) pathway, which is an error-free process essential for maintaining genomic stability [22].
In BRCA-mutated cancers, DSB repair through HR is impaired, leading to an increased dependence on the proficiency of base-excision repair (BER), which is the primary pathway to repair single-strand DNA breaks (SSB). The latter is dominantly mediated via the poly ADP ribose polymerase (PARP)1 enzyme [23]. In these tumors, the PARP pathway is upregulated as a compensatory mechanism in order to repair DNA damage via BER. In preclinical models, inhibition of the PARP pathway in BRCA-mutated tumor cells results in cell death, as these cells are unable to repair either SSB or DSB because of deficiency in HR repair mechanisms in combination with PARP inhibition [20].
Based on these data, PARP inhibitors have emerged as an exciting treatment option in BRCA-mutated TNBC [24]. List of recent and ongoing studies were recently summarized by Sonnenblick et al. [25]. Current evidence clearly indicates that these agents have weak activity in unselected TNBC [25]. The initial study with iniparib in combination with gemcitabine and carboplatin showed improvement in overall survival over chemotherapy alone, yet these results were not confirmed in phase III setting [26, 27]. Accordingly, and with the increasing appreciation of TNBC heterogeneity, all subsequent trials on PARP inhibitors have focused on patients with either a germline or somatic BRCA mutation. Fong et al. showed an impressive response rate of 47 % in BRCA-mutated breast cancer in a phase I trial using olaparib [28]. A later phase II showed a 41 % response rate using the 400-mg dose of olaparib [29]. A more recent analysis including nearly 300 patients confirmed the activity of olaparib across different tumor types (breast, ovarian, prostate, and pancreatic), provided the presence of BRCA mutation [30]. Of note, compared to chemotherapy, PARP inhibitors are relatively well tolerated, with fatigue, nausea, and vomiting (mostly grade I–II) being the most common adverse events encountered with these agents [25, 28, 29].
Recently, olaparib received regulatory approval in managing patients with advanced ovarian cancer harboring germline BRCA1/2 mutation [31]. In breast cancer, several PARP inhibitors are in advanced clinical development not only in the advanced setting but also in the adjuvant setting like the OLYMPIA trial, which evaluated the role of olaparib as a maintenance therapy in patients with BRCA-mutated TNBC [25].
Of note, it has been shown that some sporadic TNBC tumors (BRCA non-mutant) may also exhibit a variable degree of HR repair dysfunction (so-called BRCAness). This occurs via many somatic events, such as loss of heterozygosity, methylation silencing, or inactivating mutation, in the wild-type allele, which would render the tumor cells homozygous deficient for either BRCA1 or BRCA2, with the resulting profound defect in HR [32]. Development of a robust score capable of identifying tumors with defects in homologous recombination DNA repair should provide another potential biomarker of sensitivity to PARP inhibition in the sporadic TNBC population in the clinical setting. To this end, a homologous recombination deficiency (HRD) score, which reflects loss of heterozygosity of genomic regions of intermediate size, has been identified as a biomarker that correlates with BRCAness in ovarian and breast cancer [33, 34].
Given the high prevalence of DNA repair dysfunction in TNBC, the use of chemotherapeutic agents operating through inducing DNA damage seem to be an attractive strategy for these tumors. During the last few years, platinum salts (known to induce DNA cross-breaks) have been strongly suggested as an effective treatment in patients with TNBC [35], particularly those with BRCA mutation (Table 1) [36–38].
A subgroup analysis of the GeparSixto study indicated that the magnitude of benefit of adding platinum salts was highest among BRCA mutation carriers [38]. Similar findings come from the TNT trial, which is a phase III trial comparing first-line carboplatin to docetaxel in patients with TNBC [37]. Both arms showed comparable response rate, 31.4 vs. 35.6 %; however, on restricting the analysis to patients with known BRCA1/2 mutations, carboplatin was clearly superior to docetaxel: 68 vs. 33 %, p = 0.03. Of note, there was a positive interaction between the randomization arm and BRCA1/2 status indicating that BRCA status is a predictive marker of platinum benefit rather than being a marker of chemo-sensitivity.
Recently, two other studies have investigated the association between benefit to platinum salts and HRD scores in the neoadjuvant setting [39, 40]. In the GeparSixto trial, patients with HRD-positive tumors had superior benefit on adding carboplatin to anthracyclines/taxane chemotherapy (pCR: 63.5 vs. 33.9 %, p < 0.001), while those with HRD negative did not appear to benefit much of adding carboplatin (29.6 vs. 20 %, p = 0.054) [40]. Another study (PrECOG 0105) showed higher pCR rates in patients with positive HRD status who received the combination of gemcitabine, iniparib, and carboplatin [39]. The HRD assays reported in these two studies now join several other assays that will help discover how best to incorporate markers of DNA repair impairment as part of our therapeutic tools across various tumor phenotypes and not just in TNBC. Clinical use of the HRD test has the potential to identify TNBC patients likely to respond beyond those currently identified by germline BRCA1/2 mutation screening. These data combined indicate that there is a reasonable rational to consider the use of platinum salts in patients with demonstrated DNA repair deficiency. It does not appear that they be particularly active treatment options in other TNBC patients and thus the notion of the preferred use platinum salts in all TNBC is not supported by evidence and, in our opinion, should be discouraged.
In current clinical practice, DNA repair deficiency could be exploited by means of evaluation of the germline BRCA mutation status, in which we have a standardized and validated assays to use. Diagnosis at young age (<45) or <50 in case of bilateral disease or have one close relative with breast cancer, history of ovarian cancer, or personal history of male breast cancer are all reasons to screen for BRCA mutation [41]. In triple negative breast cancer, the NCCN guidelines indicate that any patient with TNBC and younger than 60 years at diagnosis should be tested while the European Society for Medical Oncology Guidelines recommended testing if less than 50 years at diagnosis [41].
Androgen Receptor
One of the constant findings of TNBC genomic studies is the presence of a LAR subtype (10–15 % of all TNBCs) [11, 12]. The findings that LAR cells are in part dependent on AR signaling and that the pharmacological inhibition of AR by the anti-androgen—bicalutamide—greatly reduces tumor growth [11] suggest a potential role of anti-androgens in the treatment of this TNBC subset.
A large meta-analysis including 19 studies and 7693 patients was recently conducted looking into the prevalence and prognostic effect of AR according to ER status [42]. In ER-negative tumors, AR was expressed in around 30 % of patients as compared to more than 70 % in ER-positive disease. Importantly, AR expression was associated with favorable disease-free and overall survival, with a 5-year reduced risk of death of more 50 % independent of ER status. This may suggest that tumors with a triple negative phenotype, yet showing high AR expression could possibly benefit of less intensive therapy that may include anti-androgen therapy. However, it was clear from this meta-analysis that a consensus is yet to be reached regarding the optimal way to evaluate AR as different methodologies were used in the different included studies.
Gucalp et al. reported the first trial on the use of the antiandrogen, bicalutamide at 150 mg daily, in patients with TNBC [43]. In this trial, patients with ER, PR-negative but AR-positive defined as >10 % AR nuclear staining by IHC were included. This study included 28 patients of whom the clinical benefit rate (CBR) was 19 % and a median progression-free survival (PFS) of 12 weeks. In this study, all except one patients had AR expression >50 % with a duration of response ranging from 25 to 231 weeks. More recently, another trial was reported but this time using the more potent AR antagonist: enzalutamide [44]. In this open label single arm two-stage study, 75 patients with advanced TNBC were evaluable, of whom around 50 % received single agent enzalutamide beyond second line of therapy. The primary endpoint was CBR at 16 weeks. In the intent to treat analysis (irrespective of AR expression), the clinical benefit rate at 16 weeks was 25 % with a median progression-free survival of 12.6 weeks. However, in a predefined analysis of this study using a novel genomic assay to quantitate AR expression, patients with positive AR expression had nearly 40 % clinical benefit rate at 16 weeks with median progression-free survival of 40.4 weeks compared to only 11 % and 8.9 weeks in those with negative AR expression. These studies provide proof of concept indicating that targeting AR could emerge as a very relevant target in managing subsets of TNBC harboring high AR expression.
While these studies met their objectives in demonstrating the efficacy of AR inhibitors, it should be noted that none of them clearly define whether AR is truly a predictive marker for AR targeting agents. In both studies, all patients were treated with the AR inhibitor; thus, it is plausible that high AR expression is just identifying those patients with better prognosis and not necessarily deriving superior benefit of these agents compared to standard chemotherapy. Hence, the next step should be evaluating the interaction between AR expression and benefit of AR inhibitors in the context of a randomized trial that incorporates standard chemotherapy.
TILs
Over the last few years, extensive researches have focused on the prognostic and predictive relevance of TILs in breast cancer. Several neoadjuvant studies have indicated that high TILs are independently associated with a higher chance of achieving pCR [45–47]. Summary of these studies were recently published [48, 49]. In one study by the German group, patients with lymphocyte-predominant breast cancer defined as at least 60 % of TILs had a pCR of 41.7 % compared to only 2 % in patients without any TILs detected [45]. On the other hand, several adjuvant trials have shown that high TILs identify patients with favorable prognosis yet this appears to be variable according to breast cancer subtype [50–52]. Loi et al. have evaluated more than 2000 samples for TILs within the BIG 2-98 trial. In this study, TILs was more prevalent in patients with TNBC and importantly it was only prognostic in this subtype and not in the ER-positive or HER2-positive subtypes [52]. The same results were observed in the FINHER trial [50]. These findings suggest the importance of host antitumor immune response in modifying the natural history of TNBC.
On the other hand, The Cancer Genome Atlas (TCGA) RNA sequencing data showed a higher expression of the immune check point PD-L1 mRNA in TNBC (n = 120) compared with non-TNBC (n = 716; p < 0.001). Using IHC, PD-L1 expression is present in around 20 % of TNBC specimens and is associated with greater CD8+ T cell infiltrate than PD-L1-negative tumors (p < 0.0001).
The question is how can we exploit these findings to boost an immune-mediated anti-tumor response in TNBC? It is well acknowledged that the underlying basis of cancer immunotherapy is to activate a patient’s own T cells to specifically recognize and kill cancer cells, a therapeutic goal that is potentially achievable by recently developed immune check-point inhibitors [48]. The recent practice changing success reported by immune checkpoint inhibitors in melanoma [53] has opened the door for incorporating these agents in other diseases as well. Recently, the anti-PD-1 monoclonal antibody, nivolumab, has been approved for the treatment of advanced squamous NSCLC that has failed chemotherapy indicating that these agents have the potential to change the treatment landscape of tumors previously considered as non-immunogenic cancers [54]. Currently, several anti PD1 and PD-L1 inhibitors are being tested in TNBC as well. Emens et al. have shown an overall response rate of 33 % in nine patients with metastatic TNBC including one complete response using the PD-L1 inhibitor: MPDL3280A [55]. Another study using the PD-1, Pembrolizumab, has shown an 18.5 % response rate in 32 patients with TNBC including one complete response [56]. Other studies are also underway and hold a lot of promise in refining the treatment paradigm of this disease. Of note, to date, all studies are conducted in unselected TNBC patients. As for TILs, all data is extrapolated from the early setting and hence their relevance in the advanced setting is not very clear. Thus, whether TILs, or PD-1/PD-L1 expression, or other markers can be used to define subsets that would benefit the most, this represents an important question that would need to be addressed while developing these agents.
Exploring Other Targets Outside the Genomic Landscape of TNBC
Apart from the potential therapeutic targets emerging from the genomic studies, a high percentage of TNBCs may express some important surface receptors that can be exploited in the development of promising target therapies. Two surface biomarkers are already implicated in drug development of two agents that carry promise to be potentially incorporated in the treatment paradigms of TNBC in the years to come.
Luteinizing Hormone-Releasing Hormone Receptor (LHRH-R)
LHRH-R is a member of the seven-transmembrane, G-protein coupled receptor family, which binds luteinizing hormone release hormone (LHRH). These receptors are normally expressed in the gonadotropes in the pituitary gland and reproductive organs but have been also detected on many human tumors, such as breast, ovarian, endometrial, and prostate cancers [57, 58]. Cloning of the mRNA for GNRHR in these cancers demonstrated that their nucleotide sequence is identical to that of the pituitary receptor [58].
In clinical practice, LHRH analogues are used in managing prostate cancer and premenopausal ER-positive breast cancers and prostate carcinoma. Although their mechanism of action is primarily attributed to sex hormones suppression, in vitro studies on LHRH-R expressing human breast cancer cell lines have suggested a direct anti-proliferative action of these agents mediated by tumoral LHRH receptors [59].
In TNBC, expression of the LHRH-receptor is reported in around 50 % of patients with no—as yet—identified impact on the prognosis [60]. In vitro studies have shown that treatment of LHRH receptor-positive TNBC cell lines MDA-MB-231 and HCC 1806, with LHRH analogues, is associated with growth inhibition, and apoptotic cell death with synergistic anti-cancer effects occurred with co-administration of cisplatin [61]. The proliferation of both TNBC cell lines was also significantly inhibited in vitro by the LHRH antagonist cetrorelix [62]. Given the known safety of LHRH agonists/antagonists, these data would suggest that targeting LHRH receptors in combination with chemotherapy could be ready for clinical testing.
Of note, the recent POEMS trial that tested the role of LHRH agonist, goserelin, as a means to preserve fertility in young breast cancer patients has shown a rather unexpected finding of improved rates of disease-free (adjusted hazard ratio, 0.49; 95 % CI, 0.24 to 0.97; p = 0.04) and overall survival (adjusted hazard ratio, 0.43; 95 % CI, 0.18 to 1.00; p = 0.05) in the goserelin group [63]. In this trial, all patients in this study were ER-negative (85 % TNBC phenotype). The potential favorable survival outcome with LHRH agonist in this study may suggest a possible therapeutic effect of targeting LHRH receptors in patients with TNBC especially when co-administrated with chemotherapy as suggested by the previously discussed preclinical studies. This hypothesis requires further validation.
More intriguingly, it is well known that the LHRH receptor will react by receptor internalization following its binding to LHRH analogues. Thus, several conjugates consisting of an LHRH analogue linked to an antitumor agent with the aim to deliver the cytotoxic drug directly to cancerous cells [64].
AEZS-108 (also known as AN-152) is among such cytotoxic hybrid molecules consisting of doxorubicin linked to an LHRH agonist [65] (Fig. 1). Xenografts TNBC models have shown interesting activity with no apparent adverse effects. In the clinical setting, AEZS-108 was tested in 44 patients with advanced LHRH receptor-positive endometrial cancers in which it was given as a 2-h infusion of 267 mg/m2 (equimolar to 76.8 mg/m2 of free doxorubicin) on day 1 of a 21-day cycle [66]. The overall response rate was 23 % (including 2 CRs), with an overall CB rate of 70 %. The median time to progression was 7 months, and the median overall survival was 15 months, with neutropenia (12 %) and leucopenia (9 %) being the most common ≥Grade 3 toxicities reported in the study. This highly appealing safety and efficacy profile along with the supporting preclinical data in TNBC could open the door for exploring this strategy in TNBC patients with LHRH receptor expression.
Glycoprotein Non-metastatic Melanoma Protein B (GPNMB)
GPNMB is a transmembrane protein that is expressed at high levels in several human cancers and was shown to promote angiogenesis, migration, invasion, and metastasis [67–69]. In TNBC, GPNMB is overexpressed in approximately 40 % of patient and is associated with poor prognosis [67]. Intriguingly, in normal cells, localization of GPNMB tends to be restricted to intracellular compartments. In contrast, GPNMB expression in tumor cells is enriched on the cell surface [70]. This preferential pattern of GPNMB expression makes it an attractive therapeutic target for an antibody-based therapy. Of note, the surface GPNMB protein is internalized on antibody binding, which is an ideal situation for an antibody-drug conjugate approach.
Glembatumumab vedotin (GV) is an antibody-drug conjugate that combines a fully human monoclonal antibody against an extracellular domain of GPNMB and a potent microtubule inhibitor mono-methyl auristatin E (MMAE) using a cathepsin cleavable linker [71] (Fig. 1). This drug was evaluated in the clinical setting in which 34 heavily pretreated breast cancer patients (median of seven prior anti-cancer regimens) were investigated [72]. Thirty-three percent met the primary endpoint, which was 12-week PFS. Two patients had confirmed partial responses; both had GPNMB-positive tumors. Importantly in the TNBC cohort, overall response rate (ORR) was 20 % and PFS at 12 weeks was 60 %. In a subsequent phase II study, 124 patients with heavily pretreated (median number of prior lines: 6) GPNMB-expressing (>5 % by IHC) breast cancer were randomly assigned at 2:1 ratio to receive either GV (n = 83) or investigator’s choice (IC) chemotherapy (n = 41) [73]. Thirty-one percent of patients had TNBC. In the intent to treat analysis, no difference in ORR was observed between both arms; however, in an unplanned analysis in patients with TNBC, ORR was 18 % in the GV. The difference was even more striking in patients with high GPNMB expression (>25 %), in which ORR was 40 versus 0 %. These results were encouraging to proceed to phase 3, which is currently underway in which 300 women with metastatic GPNMB-overexpressing TNBC (≥25 % of tumor epithelium by central IHC) are randomized to receive GV or capecitabine at a 2:1 ratio, with PFS as the primary end point.
Conclusions and Future Directions Towards Improving Drug Development in TNBC
In the last few years, the advent of next generation sequencing technologies is changing the way cancer disease is perceived. This technological revolution, together with the identification of critical pathways involved in carcinogenesis, metastasis, and drug resistance, fuels the dream of “personalized medicine,” in which the molecular landscape of an individual’s cancer will inform clinical decision making, particularly the selection of “tailored” targeted therapies. Several clinical trials in not only in breast cancer but also in other tumors have already reported results that show better outcomes for patients undergoing molecular screening [74, 75]. Now, the SAFIR02 program (NCT01414933)—a multicentric phase II randomized trial, using high throughput genome analysis as a therapeutic decision tool, comparing a targeted treatment (administered according to the identified molecular anomaly of the tumor) with a chemotherapy administered without considering the tumor genome analysis—aims at demonstrating the medical benefits of a genomic analysis based treatment.
The main challenge in applying such approach in TNBC is in its heterogeneity. Thus, conducting large-sized trials in subsets within TNBC requires novel approaches to study design and optimally international collaborations. The concept of Master-protocol trials is increasingly adopted nowadays, which allows access to different targeted agents in parallel within independent cohorts of patients defined by specific molecular aberrations that could predict sensitivity to the investigational agent under assessment [76]. This approach reduces the percentage of screening failures, as patients with different aberrations can be enrolled in one of the different molecularly defined cohorts. Applying such strategy for drug development in TNBC could have important medical, scientific, and economic implications and may lead to accelerated development of several agents in this disease in the coming years.
References
Criscitiello C, Azim Jr HA, Schouten PC, et al. Understanding the biology of triple-negative breast cancer. Ann Oncol. 2012;23 Suppl 6:vi13–8.
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81.
Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34.
Metzger-Filho O, Sun Z, Viale G, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin Oncol. 2013;31:3083–90.
Andre F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol. 2012;23 Suppl 6:vi46–51.
von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Sotiriou C, Neo SY, McShane LM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–8.
Metzger-Filho O, Tutt A, de Azambuja E, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30:1879–87.
Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67.
Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–98.
Park S, Koo J, Park HS, et al. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21:488–92.
Niemeier LA, Dabbs DJ, Beriwal S, et al. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23:205–12.
Yu Q, Niu Y, Liu N, et al. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol. 2011;22:1288–94.
Gonzalez LO, Corte MD, Vazquez J, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer. 2008;8:149.
Masuda H, Baggerly KA, Wang Y, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–40.
Comprehensive molecular portraits of human breast tumours. Nature 2012; 490:61–70.
Turner NC, Reis-Filho JS. Tackling the diversity of triple-negative breast cancer. Clin Cancer Res. 2013;19:6380–8.
Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21.
Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7.
Helleday T, Petermann E, Lundin C, et al. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204.
Beck C, Robert I, Reina-San-Martin B, et al. Poly(ADP-ribose) polymerases in double-strand break repair: focus on PARP1, PARP2 and PARP3. Exp Cell Res. 2014;329:18–25.
Anders CK, Winer EP, Ford JM, et al. Poly(ADP-Ribose) polymerase inhibition: “targeted” therapy for triple-negative breast cancer. Clin Cancer Res. 2010;16:4702–10.
Sonnenblick A, de Azambuja E, Azim Jr HA, Piccart M. An update on PARP inhibitors—moving to the adjuvant setting. Nature reviews. Clin Oncol. 2015;12:27–41.
O’Shaughnessy J, Schwartzberg L, Danso MA, et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32:3840–7.
O’Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–14.
Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34.
Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44.
Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–50.
Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–9.
Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–9.
Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16:211.
Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–82.
Guan X, Ma F, Fan Y, et al. Platinum-based chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis of randomized-controlled trials. Anticancer Drugs. 2015.
Isakoff SJ, Mayer EL, He L, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902–9.
Tutt A, Ellis P, Kilburn L, et al. The TNT trial: a randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012). Proceeding San Antonio Breast Cancer Symposium 2014.
von Minckwitz G, Hahnen E, Fasching P, et al. Pathological complete response (pCR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (gBRCA) mutation and triple-negative breast cancer (TNBC): results from GeparSixto. J Clin Oncol. 2014;32.
Telli ML, Jensen KC, Vinayak S, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol. 2015;33:1895–901.
von Minckwitz G, Timms K, Untch M, et al. Prediction of pathological complete response (pCR) by homologous recombination deficiency (HRD) after carboplatin-containing neoadjuvant chemotherapy in patients with TNBC: results from GeparSixto. J Clin Oncol. 2015;33:1004.
Zagouri F, Liakou P, Bartsch R, et al. Discrepancies between ESMO and NCCN breast cancer guidelines: an appraisal. Breast. 2015;24:513–23.
Vera-Badillo FE, Templeton AJ, de Gouveia P, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:djt319.
Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505–12.
Traina TA, Miller K, Yardley DA, et al. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). J Clin Oncol. 2015;33:1003.
Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13.
Yamaguchi R, Tanaka M, Yano A, et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol. 2012;43:1688–94.
Ono M, Tsuda H, Shimizu C, et al. Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat. 2012;132:793–805.
Gingras I, Azim Jr HA, Ignatiadis M, Sotiriou C. Immunology and breast cancer: toward a new way of understanding breast cancer and developing novel therapeutic strategies. Clin Adv Hematol Oncol. 2015;13:1–11.
Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71.
Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50.
Mohammed ZM, Going JJ, Edwards J, et al. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107:864–73.
Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7.
Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015.
Emens LA, Braiteh FS, Cassier P. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. In: San Antonio Breast Cancer Symposium. San Antonio. 2014; Abstract PD1-6.
Nanda R, Chow LQ, Dees EC. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. In San Antonio Breast Cancer Symposium. San Antonio. 2014. Abstract S1-09.
Baumann KH, Kiesel L, Kaufmann M, et al. Characterization of binding sites for a GnRH-agonist (buserelin) in human breast cancer biopsies and their distribution in relation to tumor parameters. Breast Cancer Res Treat. 1993;25:37–46.
Kakar SS, Grizzle WE, Neill JD. The nucleotide sequences of human GnRH receptors in breast and ovarian tumors are identical with that found in pituitary. Mol Cell Endocrinol. 1994;106:145–9.
Buchholz S, Seitz S, Engel JB, et al. Search for novel therapies for triple negative breast cancers (TNBC): analogs of luteinizing hormone-releasing hormone (LHRH) and growth hormone-releasing hormone (GHRH). Horm Mol Biol Clin Invest. 2012;9:87–94.
Seitz S, Buchholz S, Schally AV, et al. Triple negative breast cancers express receptors for LHRH and are potential therapeutic targets for cytotoxic LHRH-analogs, AEZS 108 and AEZS 125. BMC Cancer. 2014;14:847.
Kwok CW, Treeck O, Buchholz S, et al. Receptors for luteinizing hormone-releasing hormone (GnRH) as therapeutic targets in triple negative breast cancers (TNBC). Target Oncol. 2014.
Buchholz S, Seitz S, Schally AV, et al. Triple-negative breast cancers express receptors for luteinizing hormone-releasing hormone (LHRH) and respond to LHRH antagonist cetrorelix with growth inhibition. Int J Oncol. 2009;35:789–96.
Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923–32.
Engel JB. GHRH-antagonists in TNBC. Oncotarget. 2015;6:1898–9.
Engel J, Emons G, Pinski J, Schally AV. AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs. 2012;21:891–9.
Emons G, Gorchev G, Harter P, et al. Efficacy and safety of AEZS-108 (LHRH agonist linked to doxorubicin) in women with advanced or recurrent endometrial cancer expressing LHRH receptors: a multicenter phase 2 trial (AGO-GYN5). Int J Gynecol Cancer. 2014;24:260–5.
Rose AA, Grosset AA, Dong Z, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res. 2010;16:2147–56.
Rose AA, Pepin F, Russo C, et al. Osteoactivin promotes breast cancer metastasis to bone. Mol Cancer Res MCR. 2007;5:1001–14.
Rose AA, Annis MG, Dong Z, et al. ADAM10 releases a soluble form of the GPNMB/Osteoactivin extracellular domain with angiogenic properties. PLoS One. 2010;5, e12093.
Maric G, Rose AA, Annis MG, Siegel PM. Glycoprotein non-metastatic b (GPNMB): a metastatic mediator and emerging therapeutic target in cancer. Onco Targets Ther. 2013;6:839–52.
Tse KF, Jeffers M, Pollack VA, et al. CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res. 2006;12:1373–82.
Bendell J, Saleh M, Rose AA, et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2014;32:3619–25.
Yardley DA, Weaver R, Melisko ME, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-expressing breast cancer. J Clin Oncol. 2015;33:1609–19.
Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53.
Tsimberidou AM, Iskander NG, Hong DS, et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin Cancer Res. 2012;18:6373–83.
Fadoukhair Z, Zardavas D, Chad MA, et al. Evaluation of targeted therapies in advanced breast cancer: the need for large-scale molecular screening and transformative clinical trial designs. Oncogene. 2015.
Compliance with Ethics Guidelines
Conflict of Interest
Hamdy A. Azim reports personal fees from GSK, Novartis, Roche, and Merck.
Hatem A. Azim Jr reports personal fees from GSK and Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Biomarkers
Rights and permissions
About this article
Cite this article
Azim, H.A., Azim, H.A. Potential Therapeutic Targets in Triple Negative Breast Cancer. Curr Breast Cancer Rep 7, 215–223 (2015). https://doi.org/10.1007/s12609-015-0192-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-015-0192-1