Abstract
Tumor stroma is constituted by blood vessels, fibroblasts, leukocytes, and intercellular fibers. As opposed to the stroma of normal tissue, tumor stroma has an aberrant physiology. The tumor cells can secrete growth factors that can reprogram the stroma so that it exerts several tumor progression roles and supports a positive feedback loop that preserve a microenvironment favorable for the tumor and hostile for the patient’s interests (limiting the delivery of chemotherapy, blocking immune response, and keeping hypoxic conditions). In this review, we discuss the recent advances in understanding this tumor-stromal cross-talk and how the signaling systems implicated on it can be useful for targeted therapeutics. We will discuss the available results from trials completed with targeted agents with antistromal effects and also retrospective studies conducted with patient cohorts taking other non-cancer drugs to which recently several antistromal properties have been described, such as antihypertensives. Finally, we will show our preclinical results regarding hypoxia tracking with 18F-fluoromisonidazole, a PET tracer, as a potential tool to specifically monitor the positive or negative effects of antistromal agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most frequent malignant disease in women in the Western world, and its incidence has been steadily increasing for the last 30 years. As the incidence is related to industrial development (because of exposure to chemicals with estrogen-like activity), the worldwide incidence can only be expected to increase as well.
International consortia efforts utilizing several high-throughput platforms (next-generation sequencing, gene-expression profiling, proteomic chips, and comparative-genomic hybridization among others) are clustering the disease in an increasing number of subtypes [1–4]. Each subtype may be driven by one or more unique genetic alterations [1–4]. This situation complicates the panorama of precision medicine. However, several common hallmarks remain stable across different tumor subtypes, namely cancer metabolism and abnormal tumor stroma [5–7, 8•]. In addition, these two alterations (abnormal metabolism and abnormal stroma) show a unique physiology that does not occur anywhere else in the organism—in other words, these unique features of cancer could be cancer weaknesses as they would offer therapeutic index. In the case of tumor stroma, the therapeutic potential is even more appealing, as it is not as variant from one tumor to one another as the genetic landscape is [5–7, 8•]. Rather, tumor stroma is constituted by “healthy” tissue reprogrammed by the tumor but genetically stable and thus with limited capacity of change, and subsequently, limited possibilities of acquired resistance to therapies [9].
Tumor Stroma: Homeostatic Stroma
The stroma acts as the supporting tissue of an organ, grants a structural framework, and contributes to its consistency, strength, and elasticity. It is formed by both cellular and non-cellular components. A key component of the stroma is fibroblasts, which play a major role in stromal homeostasis. They are responsible of wound healing and secreting extracellular matrix, thus keeping stromal integrity. This extracellular matrix (ECM) is a three-dimensional network of proteoglycans (heparan sulfate, chondroitin sulfate, keratan sulfate,…), other saccharides, like hyaluronic acid, and protein fibers (collagens, elastin, fibronectin…) that support cell adhesion and migration, contribute to cell-cell communication, and provide the tissue with structure and elasticity. ECM proteoglycans also act as an extracellular storage of growth factors that are exposed or release when the ECM is damaged. Thin specialized layers of ECM form the basement membrane, which separates the epithelial and stromal compartments and supports epithelial cells anchorage, adhesion, and growth. In addition, blood capillaries provide oxygen and nutrients; in a non-tumor stroma, endothelial cells have an uninterrupted basement membrane, normal intercellular gaps, and permeability and are wrapped by pericytes, which are critical for endothelial differentiation, survival, and angiogenesis.
Another key component of cellular stroma is resident leucocytes, such as macrophages and granulocytes. They have an important role in clearing debris and are critical in response to infection and inflammation resolution.
Abnormal Stroma: Rationale for Stromal Targeting
Normal components of the stroma can be “re-educated” to serve as tumor progression factors [9].
Abnormal Blood Vessels
Probably the stromal compartment more implicated in tumor progression is the tumor vascular bed. Tumor blood vessels are tortuous, saccular, and chaotic in their organization [8•, 10•]. Tumor blood vessels can harbor large fenestrations to allow cell clumps to enter the bloodstream and favor metastasis; inefficient blood flow (secondary to spatially and temporally heterogeneous blood perfusion) can perpetuate tumor hypoxia, which is a factor of chemoresistance [8•, 10•]. The heterogeneous blood flow is caused by solid stress forces, generated by tumor growth, and by leakiness (which in certain areas can hemoconcentrate the blood and lead to stasis). In any case, it limits the access of chemotherapeutic drugs and immune cells to random tumor regions [8•, 10•].
An adequate vascular network requires a tight equilibrium between pro- and antiangiogenic factors [8•]. The balance between pro- and antiangiogenic factors can be altered by an excess or deficiency on either of them. In any of the four scenarios, the vascular network would likely be abnormal [8•]. The number of endogenous pro- and anti- angiogenic systems is enormous: vasculo-endothelial growth factors (VEGF) A/B/C/D, platelet-derived growth factors (PDGF) alpha and beta, angiopoietin (ANG) 1 and 2, placental growth factor (PIGF), hepatocyte growth factor (HGF), fibroblastic growth factors (FGF) 1 to 23, and their receptors (VEGFR1–4, PDGFRA/B, TIE, FLT1, MET, and FGFR1-3) are among the pro-angiogenic. The pro-angiogenic factors can homo- and hetero-dimerize; the affinity for each receptor and downstream signal varies depending on the dimers and the relative abundance of other factors. Different receptors can be present in different cell types (endothelial cells, pericytes, fibroblasts), what gives an idea of the complexity of the system. Antiangiogenic endogenous molecules are neuropilin 1, soluble VEGFR1, thrombospondin 1 and 2, angiostatin, endostatin, calreticulin, platelet factor-4, and vasostatin. In addition, other molecules implicated in other physiological process may have indirect pro- (nitric oxide, inflammation mediators, members of the coagulation cascade) or antiangiogenic (interferon alpha and beta, prothrombin, prolactin, SPARC, osteopontin) properties. Thus, the frailty of the equilibrium to maintain an adequate vascular network can only be underestimated. Theoretically, the rationale of using antiangiogenic agents is that adding antiangiogenic exogenous factors would restore a balance prone to endogenous pro-angiogenic equilibrium, inherently abnormal; once this balance is exposed to antiangiogenics, blood vessels could function normally again [8•]. This re-acquired “normality” would represent a disadvantage for tumor progression. Back in 2006, it was proposed that antiangiogenic drugs could exert their effect by facilitating the arrival of chemotherapeutic drugs to the tumor bed, eliciting chemosensitivity as well through improved oxygenation [8•]. The clinical experience shows that a fraction of patients experiences benefit from this theoretical “normalization” effect, whereas others do not. A recent study in lung cancer patients with radioactive-labeled docetaxel showed that a number of patients actually experienced a decrease in the amount of docetaxel that arrived to the tumor after a single dose of bevacizumab [11•]. Although the hypothesis of vascular normalization is appealing, and it is proven in certain animal models, the fact is that given the complexity of the angiogenesis factors balance, the chance of a drug to restore the balance to “normal” is almost random. Taken into account the fact that there are dozens of pro- and antiangiogenic endogenous factors and receptors, it is likely that two breast cancer patients would have a different “starting-point” equilibrium. There are as well almost 20 different antiangiogenic drugs at various stages of development. Each of them targets different angiogenic systems and with different Kms. Thus, given the fact that the angiogenesis equilibrium for each given patient is unknown prior to the start of therapy, the scenario of restoring the balance to normal when exposing that equilibrium to an antiangiogenic agent in the general cancer patient population is quite optimistic, no matter how appealing is the theory of vascular normalization. As predicting the effects of an antiangiogenic drug in the angiogenic equilibrium seems challenging, below, we propose a system to trace vascular normalization with a noninvasive system.
Abnormal Lymphatic Vessels
Endothelial cells, and subsequently the vascular compartment, are not the only cells in the tumor stroma functioning abnormally. Lymph vessels show abnormal function in the tumor as well [12]. Although lymph vessels in the tumor margin are competent, and actually hyperplastic, functional lymph vessels are absent inside tumors [13, 14]. Experimental evidence shows that lymph vessels open up following the depletion of cancer cells, fibroblast, and collagen, suggesting that compression forces play a role in the abnormal function [15]. In addition, the lack of proficient lymph vessel contractions and incomplete lymph vessel valve leaflet closure has been observed [15]. However, the molecular basis for these defects are unknown; nitric oxide gradient alterations have been implicated, but so far, there are no drugs available with which we can finely tune nitric oxide production temporally and spatially [10•]. The lack of molecular understanding of the causes of lymph vessels abnormality challenges potential targeted therapeutic approaches, currently focused in blocking lymphatic metastatic spread, which is one of the main pathways of breast cancer systemic dissemination.
Abnormal Intercellular Matrix
The intercellular matrix, mainly constituted by collagen, plays an important pathogenic role in breast cancer. The mechanism is double: in one hand, the tumor cells secrete FGF, VEGF, PDGF, EGFR ligands, interleukins, colony-stimulating factors, transforming growth factors, and others. These growth factors disrupt normal tissue homeostasis, similar to the process of wound healing, stimulating stromal reaction, angiogenesis, and inflammatory responses [9]. These factors activate stromal cell types such as fibroblasts, smooth muscle cells, and adipocytes, which secrete collagen, additional factors, and proteases. The proteolysis of extracellular structural molecules exposes cryptic protein domains and generates new molecule fragments that have pro-migratory, pro-invasive, and pro-angiogenic functions; finally, they can also activate tumor cell surface receptors [9]. On the other hand, dense intercellular matrix can slowdown the penetration and diffusion of large molecules [16, 17]; fibrillar collagen is able to restrict the movement of particles larger than 50 nm [16, 17]. This is of key importance for novel chemotherapeutic agents of high efficacy, such as liposomal doxorubicin or nab-bound paclitaxel. This diffusion restriction is higher as the desmoplastic reaction of the tumor increases [10•]. For the first cascade of events triggered by the abnormal stroma, there is a plethora of signal-transduction inhibitors available non-specifically designed for this purpose but for oncogenic signaling. Thus, their effects as antistromal therapies have not been properly addressed. However, the second issue, related with “excessive stroma,” opens specific therapeutic opportunities. Collagen is a structural molecule, and thus, systemic collagenase cannot be administered in humans; however, preclinical experimental evidence supports the increased volume of distribution after collagenase injection together with liposomal doxorubicin or nab-bound paclitaxel [10•]. Relaxin, an antifibrotic agent, is able to increase the penetration of large molecules [18, 19]. Finally, drugs largely prescribed for hypertension, such as angiotensin receptor blockers or angiotensin-converting enzyme inhibitors, are able to reduce collagen production by blocking the activation of transforming growth factor beta [20].
Abnormal Fibroblasts and Immune Infiltrate
Inflammatory cytokines also play a role in reprogramming the stroma [9]. Tumor cells are known to secrete IL-1β, which activates NF-κB in fibroblasts [21]. This pathway triggers a gene-expression program that turns fibroblast into what has been termed “CAFs,” or cancer-associated fibroblasts. CAFs secrete pro-inflammatory cytokines, which drive primarily M2 macrophages into the tumor, that elicit tumor growth and vascularization [21]. Transforming growth factor beta, on top of its effects in collagen production, can reprogram intratumor neutrophils [22]. Naive tumor-associated neutrophils (“N1”) are highly cytotoxic to tumor cells; however, this cytokine reprograms them into N2 neutrophils, which exhibit a protumor phenotype [22]. The former are therapeutic targets, at least in theory; however, the most important immunosuppressive axes that elicit tumor immune surveillance escape are two: PD-1/PD-L1 and CTL-4 [23]. Programmed death-ligand (PD-L1) is a transmembrane protein that regulates immune response during pregnancy and several conditions such as hepatitis or autoimmune diseases [23, 24]. It activates a receptor (PD-1) in the T8 cells surface that transmits an inhibitory signal which reduces their proliferation. PD-L1 has been observed aberrantly overexpressed in many epithelial tumor types, and it contributes to immune escape [23, 24]. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is an immune checkpoint that downregulates pathways of normal T4 cells activation [23, 24]. Molecules aberrantly produced inside the tumor, such as kynurenine, from aberrant tryptophan metabolism, can enhance the activity of this negative feedback loop [25, 26]. Targeting these two aberrantly functioning regulators of the immune infiltrate of the tumor stroma can yield positive responses; however, most of the results available regard other malignancies such as lung cancer or melanoma.
Drugs Targeting the Abnormally Functioning Stromal Compartment: Current Results in Breast Cancer
Targeting the Abnormal Blood Vessels—Old Antiangiogenics
Most available agents against the stroma in breast cancer target the vascular compartment. Hundreds of articles reviewing the concept of antiangiogenics and summarizing the results of clinical trials have been published, and there is little to add at this moment if it is not a proposal to move forward in improving therapeutic index, developing biomarkers, or predictive factors. As a summary, most of the results concern the paradigm agent, bevacizumab, a monoclonal antibody targeting VEGF; several trials have been conducted with multikinase inhibitors targeting other systems involved in aberrant signaling loops of the stroma (sunitinib or sorafenib, which blocks several members of the VEGFR and PDGFR families). The trials can be divided in two: clinical trials in the metastatic setting and in the neoadjuvant setting. The results in the metastatic setting with the single-system targeting agent, bevacizumab, showed improved response rate and progression-free survival in combination with different chemotherapeutic agents and across different treatment lines (suggesting that the hypothesis of vascular normalization is, at least in some cases, true); however, these improvements were not translated into better overall survival [27–29]. Several design and statistical issues possibly account for the impossibility to demonstrate such increase in the long term and have been reviewed elsewhere [30]. Regarding the multikinase inhibitors, the results have been more modest. The combination with chemotherapy has been proved to be too toxic for sunitinib and sorafenib; in these cases, not even an improvement in initial disease control was observed [31, 32]. Probably the decrease in chemotherapy dose density/intensity secondary to cumulative toxicity partially accounted for these results. In the neoadjuvant setting, there is consistent evidence available across several randomized trials showing how the addition of bevacizumab to standard chemotherapy-based regimens improves the standard parameter of efficacy, pathologic complete response [33, 34]. There are insufficient data to draw conclusions regarding small multikinase inhibitors.
Targeting The Abnormal Blood Vessels—Novel Antiangiogenics
A second generation of multikinase inhibitors with antistromal properties is under development. Agents such as pazopanib, dovitinib, or nintedanib have activity against other kinases regulating aberrantly functioning tumor stroma, such as the FGFR family or CSF-1R. In addition, they are, at least in theory, less toxic due to their lower Km against pro-angiogenic kinases. However, the available trials have not been strictly conducted to address their theoretical antistromal effect but to take advantage of their therapeutic potential inhibiting kinases driving oncogenic addiction in the tumor epithelial compartment such as FGFR or KIT. Other preclinical studies have highlighted the pleiotropic potential of these drugs; for example, it has been reported its role as a tool to prevent brain metastasis through its RAF inhibitory properties [35]. Overall, the results of the trials involving these new agents in breast cancer have been disappointing [36–39]. Below, we propose an approach to assess the specific effects over the stroma in a phase 0 trial with nintedanib, based on our preclinical results; based on the pharmacodynamic readouts, it would be possible to select those patients where the stromal-normalization effects are actually occurring and increase the likelihood of benefit from antistromal therapies + chemotherapy combos.
Targeting the Abnormal Intercellular Matrix
Not all the abnormal stromal compartments are currently targetable, as mentioned above regarding abnormal lymph vessels. Fortunately, abnormal extracellular matrix can be targeted, at least theoretically. There is strong experimental evidence linking the use of angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEi) and extracellular matrix relaxation and density decrease [40, 41]. These features are followed by decreased solid stress forces, increased vascular perfusion, hypoxia normalization, and increased drug diffusion [40, 41]. In the clinical setting, there are no randomized clinical trials available formally testing the benefit of adding ARBs/ACEi to standard chemotherapy. However, several retrospective studies are available. In all of them, long cohorts of patients are reviewed, and the breast cancer outcomes of patients taking ARBs/ACEi are compared with those under no hypertension treatment. These retrospective trials, necessary for generating clinical hypothesis and for justifying a prospective clinical trials, are in this case inherently biased: in all cases, the patients taking hypertension medication are older and have higher incidence of diabetes, body mass index, heart conditions, and obviously hypertension than the remainder. It is not surprising that such subgroups with older age and general worse health conditions show, overall, higher mortality than the patients not taking ARBs/ACEi. The relative risk of death (all causes) for the breast cancer patients taking ARBs/ACEi compared to the untreated patients ranges from 1.22 [42] to 1.94 [43]. Some studies report on shorter outcomes probably less influenced by the confounding effects of such long-term survival-affecting conditions. It is reasonable that if we want to estimate the “interstitium-normalizing” effects of ARBs/ACEi, we should focus on outcomes such as response rate or progression-free survival, more directly related to response to chemotherapy and, probably, to the amount of chemotherapy that is finally delivered to the tumor, a parameter that could be influenced by ARBs/ACEi. Two studies reported a higher risk of recurrence for ARBs/ACEi intakers [42, 43], although recurrence is a composite endpoint that is influenced as well by surgery, radiation therapy, and time; yet, a third study reported an almost 70 % decrease in recurrence [44]. One of these studies reports the parameter probably most directly influenced by chemotherapy delivered among all the reported outcome pathologic complete response: in the study by Chae and Chavez-Macgregor, no differences in pathologic complete response were observed between the 160 patients taking ARBs/ACEi and the almost 1300 controls [44]. However, as mentioned, retrospective case-control studies are inherently biased. Given the preclinical evidence, we believe that only a prospective randomized trial, where in addition to response rate or pathological complete response is compared serial biopsies are gathered to study the benefits stratifying by whether the stromal-normalization effects have occurred or not in the patients allocated to the experimental group, could definitively address the question. The main problem is that at the moment, not even the “ratio” of tumors where the delivery of chemotherapy is impaired versus those in where chemotherapy experiences adequate diffusion is known. In fact, there is not a parameter available to measure such a thing in the clinics. But, at least, serial biopsies to determine some sort of collagen density decrease would shed some lights in which percentage of patients ARBs/ACEi are actually exerting a meaningful pharmacodynamic effect—only in this patient subgroup, the increased chemotherapy efficacy should be sought.
Nab-bound paclitaxel is increasingly raising interest as a stroma-targeting strategy. It is a formulation of paclitaxel where the drug is bound to nano-albumin particles. Most of the studies have been performed in pancreas cancer models, characterized by a stiff and dense stroma. The proposed mechanism of action is as follows: SPARC is a protein that is overexpressed in the stroma; this protein is implicated in stroma formation and trafficking [45]. SPARC binds albumin, and thus, the delivery of nab-bound paclitaxel might be facilitated. Preclinical studies with patient-derived pancreas xenografts showed how treatment with nab-bound paclitaxel collapsed the tumor stroma; when gemcitabine was co-administered, the intratumor concentrations were threefold higher than when gemcitabine was administered alone [46]. Finally, a clinical trial in pancreas cancer in the neoadjuvant setting, specifically designed to address the pharmacodynamic effects of nab-bound paclitaxel in the pancreas cancer stroma, demonstrated that this treatment led to a significant decrease in cancer-associated fibroblast and collagen fibers, compared with tumors treated with other chemotherapy agents [47]. Well-designed studies like this one, specifically assessing the pharmacodynamic endpoint over the stroma, are lacking in the literature and are extremely necessary to move on successfully in the development of antistromal drugs.
A randomized trial published in 2009 compared nab-paclitaxel with docetaxel, another first-choice drug for breast cancer in first-line metastatic disease. The results favored nab-paclitaxel for more than 5 months, what raised significant enthusiasm. However, this trial was followed by mostly phase II trials without comparator arms [48–52]; moreover, correlative studies assessing potential effects in the stroma were never included, what complicates the interpretation. Particularly, studies measuring the concentration of companion chemotherapy agents are missed. Unfortunately, the results from the first randomized trial have not been reproduced by independent groups; the recent long-term update results with overall survival data reported similar outcomes for docetaxel and nab-paclitaxel [53]. Thus, despite almost 15 trials performed with nab-paclitaxel in breast cancer, a poor biomarker-guided development leaves the agent with no clear niche and many questions unanswered.
Targeting the Aberrant Immune Infiltrate
Finally, there are compounds available that target aberrant tryptophan metabolism that leads to the production of kynurenine, related to the reprogramming of the stromal immune infiltrate, such as d- or l-methyl-tryptophan. However, they are orphan compounds, and only independent research by cooperative groups or academic institutions will likely conduct trials with such compounds. More likely is the research with the PD-L1 and PD-1 inhibitors, although so far, most of the work is directed to malignancies where strong overexpression of such molecules have been observed, such as melanoma and lung cancer. Nivolumab, a monoclonal antibody against PD-1, that has shown activity in melanoma [54], is not currently under clinical testing in breast cancer. Such is the case for another drug from the same company targeting PD-L1, BMS-936559. Pembrolizumab, another monoclonal antibody against PD-L1, is under development in kidney cancer, lung cancer, melanoma, and hematologic malignancies. The other PD-L1 blocker, MPDL3280A, is currently focused as well in lung cancer and melanoma development. However, the development of these compounds is still on its infancy, and it is probably a matter of time that breast cancer raises attention as well, as PD-L1 increased expression has already been reported in a subset of breast cancers [55].
Tracking Stromal Normalization with Noninvasive Imaging
The concept of targeted therapies is based on the fact that the antitumor effect is elicited by the action of the drug on a specific target. Although there are tumor-related factors determining drug sensitivity or resistance, the response to a given drug is heterogeneous across patients of a large cohort. It is reasonable to think that due to different reasons (drug absorption, metabolism, polymorphisms, or genetic variation in the targets among others), the effects on the targets are not going to be exactly the same in all patients and that the positive effects of drug exposure should be aggregated in those patients where the target has been actioned. Moreover, in patients where there is no proof of pharmacodynamic effect, no drug positive effect should be expected. However, and despite these assumptions, pharmacodynamic testing is rarely incorporated in the clinics.
As discussed, the structure and function of tumor stroma are determined by many signaling pathways; in addition, antistromal drugs are often pleiotropic (i.e., they block several signaling pathways). It would be difficult to set up a test measuring the effects over different signaling systems simultaneous, but, if the role of targeting the stroma wants to be established, certain metrics need to be adopted. No matter which is the mechanism of action of the agent under test, the reported endpoint of the clinical trial studying it is usually either of the following: tumor shrinkage, progression-free survival, or overall survival. Of course, such parameters are the final goal of any cancer therapy, but the benefits of a given agent may be overlooked if no attention is paid to a parameter measuring its true activity. For the case of antistromal agents, prior to study the tumor shrinkage or progression-free survival advantage with regard to standard therapies, we have to determine in which patients the expected effects in the stroma are occurring, and then conduct the study only in those patients: clearing-out of PD-L1-expressing cells in case of a PD-L1 antibody, collagen density decrease in case of ARBs/AECi, or other ad hoc parameters to other drug candidates under evaluation.
We have conducted a preclinical study to monitor the pharmacodynamic activity of multikinase inhibitor antistromal agents. Accepting the hypothesis of vascular/stromal normalization, we assumed that each tumor would have a unique balance between growth factors with pleiotropic downstream properties, both in the tumor blood vessels, in the CAFs, and in the collagen. The exposure to a multikinase inhibitor could turn this equilibrium closer to the normal status or worsen it. A case with abnormal vessels and dense stroma would, theoretically, be hypoxic; conversely, a case with a baseline equilibrium close to normal could be normoxic. In a similar line, the exposure to a multikinase inhibitor could turn the first case normoxic but be detrimental for the second case. In the first case, the arrival of chemotherapy would be facilitated, whereas in the other, it would be impeded by the addition of the antiangiogenic.
18F-fluoromisonidazole is a PET tracer that binds to hypoxic areas. We have demonstrated in a preclinical model how the hypoxia status can be traced dynamically. We exposed a breast cancer model to two different antiangiogenics, a monoclonal antibody with activity against VEGF and a multikinase inhibitor with activity against VEGFR1-4, PDGFRA/B, and FGFR1-3. The baseline hypoxia worsened in the first case, but improved in the first case, suggesting how the same initial balance can be pushed toward opposite directions depending on the drug used. In the first case, the combination with chemotherapy was not effective whereas in the second was synergistic. The results are shown in Figs. 1 and 2.
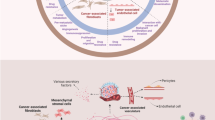
Stromal normalization: stromal components of the stroma during homeostasis, tumor progression, and stromal normalization after angiogenesis block. a Normal epithelium supported by a continuous basement membrane. A network of proteoglycans, saccharides, and proteins form the extracellular matrix (ECM), which supports other cellular components such as fibroblasts, blood capillaries, and resident granulocytes and macrophages. b As tumor progresses, epithelial cells break through the basement membrane. Stroma acquires an activated phenotype; fibroblasts become activated and differentiate into myofibroblasts; together with an increased inflammatory infiltrate, this results in growth factor and protease secretion, increased angiogenesis, and ECM degradation. Due to maintained angiogenesis signals, capillary number increases; however, these blood vessels are leaky, they have an incomplete basement membrane, and they lose their pericyte wrapping. c Angiogenesis block reverses stromal activation. This allows ECM components to grow again, and a basement membrane is re-established. Also, blood vessels recover their basement membrane and their pericytic coverage
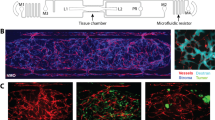
Tracking hypoxia as a pharmacodynamic marker of antistromal therapies. The murine model of breast cancer MMTV-PyMT was used for this experiment. A 18F-fluoromisonidazole PET was performed before (upper-left inset) and after 1 week of treatment with a monoclonal antibody against VEGF (right inset) or a multikinase inhibitor targeting several signaling systems of the abnormal stroma (lower inset). Representative images from the two different treatment groups are shown. It can be appreciated how the same “baseline equilibrium” between pro- and antiangiogenic factors can be pushed toward a more abnormal (hypoxic) or normoxic microenvironment. In the first case, the combination of chemotherapy (paclitaxel) plus the monoclonal antibody (“Combo”) was not better than vehicle (“vh”) or the monoclonal antibody (“Moab”) alone. In the second, the combination therapy was synergistic, suggesting that the misonidazole PET is mirroring the improvement in the vascular network and thus can be used to restrict the use of antistromal therapies exclusively to the patients where the positive effects in the stroma are occurring
Currently, we are implementing this stroma-normalizing test in a clinical trial with nintedanib in breast cancer (NCT01484080). Should this monitoring test work in the clinical setting as well, we would have available a biomarker to monitor stromal normalization in exposure to drugs targeting the vascular compartment and study the efficacy in patient subgroups experiencing the expected positive stromal effect exclusively, which would be an important advance in the field of treatment personalization.
Conclusions
Tumor cells become highly proficient in turning the stroma favorable for tumor progression along their natural history. The changes in the stroma consist in an aberrant tumor vascularization that increases tumor hypoxia, a factor or chemoresistance and tumor progression, and impedes the arrival of conventional chemotherapeutic agents, an immune reprogramming toward an immunosuppressive phenotype, an increased density of the intercellular matrix that blocks the diffusion of chemotherapy, and a cross-talk with cancer-associated fibroblasts consisting in positive feedback loops related with inflammation, immunosuppression, angiogenesis, and secretion of mediators that help in degrading and invading surrounding tissues. The most explored therapeutic approach against the stroma has been targeting abnormal blood vessels. In this field, the clinical trials without the incorporation of biomarkers seem to have reached a plateau in efficacy. Tracking the positive effects in the stroma with noninvasive tests such as 18F-fluoromisonidazole PET may help to make personalized decisions in this field. Should the PD-L1-positive tumors represent a significant fraction of breast cancers, this is possibly the field of stromal targeting with a brighter future in this disease, according to the results observed with anti-PD1/PD-L1 agents in other malignancies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400–4.
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9.
Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486(7403):353–60.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. Seminal paper proposing the theory of vascular normalization in response to antiangiogenic drugs as the main mechanism of action of this drug class. Antiangiogenics would restore the equilibrium between pro- and antiangiogenic factors and lead to a fully functioning vascular network that would allow adequate chemotherapy delivery.
Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–49.
Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205–18. A comprehensive review of the different components of the tumor stroma that behave abnormally and what is the therapeutic relevance of their normalization.
Van der Veldt AA, Lubberink M, Bahce I, Walraven M, de Boer MP, Greuter HN, et al. Rapid decrease in delivery of chemotherapy to tumors after anti-VEGF therapy: implications for scheduling of anti-angiogenic drugs. Cancer Cell. 2012;21(1):82–91. An excellent clinical study that proves that vascular normalization not always occurs in response to antiangiogenics. Indeed, in this study, it is proven how the intratumoral concentrations of docetaxel decrease after exposure to bevacizumab.
Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371–80.
Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60(16):4324–7.
Leu AJ, Berk DA, Yuan F, Jain RK. Flow velocity in the superficial lymphatic network of the mouse tail. Am J Physiol. 1994;267(4 Pt 2):H1507–13.
Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, Munn LL, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A. 2011;108(46):18784–9.
Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54(13):3352–6.
McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66(5):2509–13.
Perentes JY, McKee TD, Ley CD, Mathiew H, Dawson M, Padera TP, et al. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat Methods. 2009;6(2):143–5.
Brown E, McKee T, di Tomaso E, Pluen A, Seed B, Boucher Y, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9(6):796–800.
Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108(7):2909–14.
Erez N, Truitt M, Olson P, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17(2):135–47.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10.
Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300–9.
Adams S, Braidy N, Bessede A, Brew BJ, Grant R, Teo C, et al. The kynurenine pathway in brain tumor pathogenesis. Cancer Res. 2012;72(22):5649–57.
Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72(21):5435–40.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76.
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29(32):4286–93.
Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–60.
Cortes J, Calvo E, Gonzalez-Martin A, Dawood S, Llombart-Cussac A, De Mattos-Arruda L, et al. Progress against solid tumors in danger: the metastatic breast cancer example. J Clin Oncol. 2012;30(28):3444–7.
Martin M, Roche H, Pinter T, Crown J, Kennedy MJ, Provencher L, et al. Motesanib, or open-label bevacizumab, in combination with paclitaxel, as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol. 2011;12(4):369–76.
Rugo HS, Stopeck AT, Joy AA, Chan S, Verma S, Lluch A, et al. Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol. 2011;29(18):2459–65.
Bear HD, Tang G, Rastogi P, Geyer CE, Jr., Robidoux A, Atkins JN, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366(4):310–20.
von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366(4):299–309.
Gril B, Palmieri D, Qian Y, Smart D, Ileva L, Liewehr DJ, et al. Pazopanib reveals a role for tumor cell B-Raf in the prevention of HER2+ breast cancer brain metastasis. Clin Cancer Res. 2011;17(1):142–53.
Johnston SR, Gomez H, Stemmer SM, Richie M, Durante M, Pandite L, et al. A randomized and open-label trial evaluating the addition of pazopanib to lapatinib as first-line therapy in patients with HER2-positive advanced breast cancer. Breast Cancer Res Treat. 2013;137(3):755–66.
Cristofanilli M, Johnston SR, Manikhas A, Gomez HL, Gladkov O, Shao Z, et al. A randomized phase II study of lapatinib + pazopanib versus lapatinib in patients with HER2+ inflammatory breast cancer. Breast Cancer Res Treat. 2013;137(2):471–82.
Taylor SK, Chia S, Dent S, Clemons M, Agulnik M, Grenci P, et al. A phase II study of pazopanib in patients with recurrent or metastatic invasive breast carcinoma: a trial of the Princess Margaret Hospital phase II consortium. Oncologist. 2010;15(8):810–8.
Andre F, Bachelot T, Campone M, Dalenc F, Perez-Garcia JM, Hurvitz SA, et al. Targeting FGFR with dovitinib (TKI258): preclinical and clinical data in breast cancer. Clin Cancer Res. 2013;19(13):3693–702.
Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516.
Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108(7):2909–14.
Holmes S, Griffith EJ, Musto G, Minuk GY. Antihypertensive medications and survival in patients with cancer: a population-based retrospective cohort study. Cancer Epidemiol. 2013;37(6):881–5.
Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat. 2011;129(2):549–56.
Chae YK, Brown EN, Lei X, Melhem-Bertrandt A, Giordano SH, Litton JK, et al. Use of ACE inhibitors and angiotensin receptor blockers and primary breast cancer outcomes. J Cancer. 2013;4(7):549–56.
Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25(3):319–25.
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–54.
Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer. 2013;109(4):926–33.
Hersh EM, O’Day SJ, Ribas A, Samlowski WE, Gordon MS, Shechter DE, et al. A phase 2 clinical trial of nab-paclitaxel in previously treated and chemotherapy-naive patients with metastatic melanoma. Cancer. 2010;116(1):155–63.
Lobo C, Lopes G, Baez O, Castrellon A, Ferrell A, Higgins C, et al. Final results of a phase II study of nab-paclitaxel, bevacizumab, and gemcitabine as first-line therapy for patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2010;123(2):427–35.
McArthur HL, Rugo H, Nulsen B, Hawks L, Grothusen J, Melisko M, et al. A feasibility study of bevacizumab plus dose-dense doxorubicin-cyclophosphamide (AC) followed by nanoparticle albumin-bound paclitaxel in early-stage breast cancer. Clin Cancer Res. 2011;17(10):3398–407.
Yardley DA, Raefsky E, Castillo R, Lahiry A, Locicero R, Thompson D, et al. Phase II study of neoadjuvant weekly nab-paclitaxel and carboplatin, with bevacizumab and trastuzumab, as treatment for women with locally advanced HER2+ breast cancer. Clin Breast Cancer. 2011; 11(5):297–305.
Hamilton E, Kimmick G, Hopkins J, Marcom PK, Rocha G, Welch R, et al. Nab-paclitaxel/bevacizumab/carboplatin chemotherapy in first-line triple negative metastatic breast cancer. Clin Breast Cancer. 2013;13(6):416–20.
Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Phase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer. 2012;12(5):313–21.
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33.
Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9(2):e88557.
Acknowledgments
MQF is a recipient of the following grants: FIS PI-2010/0288, FIS PI-2013/00430, AECC Scientific Foundation “Beca de Retorno 2010”, and the SEOM 2012—Spanish Society of Medical Oncology.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Jesus Sanchez declares that he has no conflict of interest.
Miguel Quintela-Fandino has received grants and research funds from Boehringer Ingelheim and Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Systemic Research
Rights and permissions
About this article
Cite this article
Sanchez-Ruiz, J., Quintela-Fandino, M. Targeting the Tumor Stroma in Breast Cancer. Curr Breast Cancer Rep 7, 71–79 (2015). https://doi.org/10.1007/s12609-014-0173-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-014-0173-9