Abstract
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that is the most common form of dementia. There are currently FDA-approved symptomatic therapies for AD and a recently approved, potentially disease-modifying drug, Aducanumab; however, there are no curative or preventative therapies. Research suggests that diet may play a role in AD, but it is inconclusive relative to which dietary approach provides the most neuroprotective effects. There are other life-style approaches that have been found to possibly play a role in AD prevention/treatment. These include exercise, brain training, and social interaction. A combined approach may be more effective than any one modality alone. The ketogenic diet (KD) is one specific diet that has been studied vis a vis neurodegenerative diseases. Similar benefits to those of a KD can also be achieved through consuming a normal diet and supplementing with ketogenic agents. The purpose of this review is to compare the methods of inducing hyperketonemia and their impact on AD prevention/treatment, as well as to explore the possible benefits of a combined approach.
Methods
The PubMed database was searched for clinical trials and randomized, controlled trials involving the KD or exogenous ketone administration and AD. Key search terms used included “ketogenic diet and Alzheimer’s disease,” “ketosis and Alzheimer’s disease,” “MCT and Alzheimer’s disease,” and “exercise and diet and Alzheimer’s disease.” Only studies involving patients diagnosed with AD were included in this paper, but for the combined approach section, studies included patients diagnosed with MCI due to a paucity of combined approach studies involving AD patients alone.
Results
There is evidence that the KD and exogenous ketone supplementation may provide treatment benefits in AD patients. It is unclear whether one method is better than the other. The specific food composition of the KD should be considered, because certain types of fat sources are healthier than others. Many forms of the KD require strict monitoring of carbohydrate intake, which would often fall under the responsibility of the caregiver. Future studies may be more feasible in an institutional setting, where it would be easier to administer and to monitor a dietary protocol. Exogenous supplementation may be more likely to be adhered to as a long-term treatment, because the dietary changes are not as drastic. A multidomain approach may be the most effective in possibly preventing/delaying AD and in improving/stabilizing and possibly slowing disease progression in those with AD.
Conclusion
Most current studies are small, often uncontrolled, and only look at the short-term effects of ketosis on cognition. Large, long-term, randomized, controlled trials relative to the impact of the KD in patients with cognitive impairment and AD are lacking and thus needed. Combined approaches may prove to be more beneficial in possibly preventing/delaying AD and in improving/stabilizing and possibly slowing disease progression in those with MCI or AD. Future research should investigate the effect of additional combined approaches relative to neurocognitive decline in AD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that is the most common form of dementia. It is currently the sixth leading cause of death in the United States, but it may be as high as the third leading cause among older adults (1). In 2020, an estimated 5.8 million Americans had AD. This number is predicted to increase to 14 million by 2060 (2). Currently, there are FDA-approved symptomatic therapies for AD and a recently approved, potentially disease-modifying treatment, aducanumab; however, there are no curative or preventative therapies (3). Other interventional methods may play a role in the management and/or prevention of AD, such as diet (4). Research supports the notion that diet plays a role in AD, but there are non-specific and conflicting conclusions relative to which dietary approach would provide the most neuroprotective effects (5). The ketogenic diet (KD) is one specific diet that has been studied vis a vis neurodegenerative diseases. Historically, the KD has been used in the treatment of epilepsy, but recent studies are suggesting that it may play a role in the treatment/prevention of AD as well (6). Similar benefits to those of a KD can also be achieved through consuming a normal diet and supplementing with ketogenic agents, such as medium chain triglycerides (MCT). There are other life-style approaches, in addition to diet, that have been found to possibly play a role in AD treatment/prevention. These include good control of cardiovascular risk-factors such as smoking and hypertension, exercise, brain training, and social interaction. A combined approach involving one or more of these domains may be more effective than any one modality alone (7). The purpose of this review is to compare the methods of inducing hyperketonemia and their impact on AD treatment, as well as to explore the possible benefits of a multidomain approach.
Ketogenic diet
The “classic” KD consists of either a 4:1 or 3:1 ratio of fat to both carbohydrate and protein by weight. The 4:1 ratio provides 90% of energy from fat, 2–4% from carbohydrate, and 6–8% from protein, while the 3:1 ratio provides 85–90% of energy from fat, 2–5% from carbohydrate, and 8–12% from protein (8). For reference, the typical American diet derives 35% of energy from fat, 49% from carbohydrates, and 16% from protein (9). There are four types of dietary fat: saturated, monounsaturated, polyunsaturated, and trans-fat. Saturated fats are a type of fat that increase low-density lipoprotein (LDL) levels and risk for cardiovascular disease. They are found in foods such as full-fat milk, cheese, butter, fatty meat, and coconut oil. Monounsaturated and polyunsaturated fat consumption has a lower risk of cardiovascular disease and helps to lower LDL levels. These fats are found in nuts, avocado, olive oil, canola oil, vegetable oils, and fish (10).
Ketogenic diets normally consist of meat, fatty fish, eggs, butter, cream, cheese, nuts and seeds, oils, avocado, and non-starchy vegetables (11). Those on a KD are also advised to avoid alcohol and all grains such as rice, pasta, and cereal. The strict carbohydrate restrictions often lead to little intake of fiber-rich foods such as fruits, starchy vegetables, legumes, and whole grains, which may be detrimental to overall health (12). There are currently no recommendations on which types of fat or how many total calories should be consumed in this diet. The general KD that is most often used in scientific studies limits carbohydrate intake to normally less than 30–50 grams per day (8, 13). This KD tends to result in ketosis; however, some individuals may need to consume even fewer carbohydrates to achieve this state. Ketosis is a state in which the metabolic fuel source switches from carbohydrates to ketone bodies. This can be induced by a low-carbohydrate diet. Ketosis may also be defined as a blood level of β-hydroxybutyrate of 0.3 mmol/L (12). One of the effects that this can have on the brain is an increase in brain mitochondrial metabolism via an increase in hippocampal phosphocreatine/creatine ratio. The increase in ketone bodies may also lead to decreased brain reactive oxygen species (ROS) production. This is beneficial because neuroinflammation from ROS is often found in neurodegenerative diseases (13).
Critics of the KD express concern that individuals on this diet consume high levels of saturated fat and processed meats. Consumption of these foods has been found to increase the risk of not only cardiovascular disease, but also cancer, diabetes, and even AD (8). Even though a KD may contain high levels of saturated fats, there is research to suggest that the KD does not have negative effects on metabolic health. For example, one study that is expanded on later in this paper showed that participants consuming a KD have decreased body weight, decreased HbA1C, and increased HDL (14). This discrepancy may be due to the difference in nature of fatty acids between types of KDs. The types of fatty acids consumed in a KD is determined by what foods the dieter is consuming. An example is that a KD can supply more essential fatty acids than a normal, carbohydrate-rich diet if the dieter chooses foods that are rich in them. Foods that are rich in essential fatty acids include cold-water fatty fish, walnuts, and flaxseed. Higher consumption of these fatty acids has been associated with a lower AD risk (15). A possible explanation for this is these fatty acids may decrease inflammation in the brain and may play a role in nerve cell regeneration (16). Critics also point out that the KD is also known to cause several unwanted side effects that have collectively been termed the “keto flu.” This consists of nausea, vomiting, constipation, headache, and dizziness (17). These side-effects limit the utility of the KD because they can be highly detrimental depending on the age and health of the patient.
In summary, a KD can be followed by consuming a majority of foods that increase risk for cardiovascular disease. On the contrary, a KD can be cardioprotective if most foods consumed are rich in monounsaturated and polyunsaturated fats. More studies are needed to investigate how the specific foods consumed as part of various KDs affect overall health and impact AD.
Pathogenesis of AD and neuroprotective effects of ketones
The pathogenesis of AD involves brain anatomic changes, impaired glucose metabolism, ROS accumulation, and mitochondrial dysfunction. The AD brain is characterized by accumulations of amyloid β (Aβ) plaques and neurofibrillary tangles (18). The Aβ plaques are derived from amyloid precursor protein (APP) (18, 19). Neurofibrillary tangles are caused by hyperphosphorylation of the microtubule associated τ protein (18). Another aspect of AD is impaired glucose metabolism in the brain, or glucose hypometabolism (8, 20). Ketone bodies can serve as an alternative fuel source to glucose for the brain during this state. A randomized, controlled trial found that a high-glycemic index diet correlated with a higher cerebral amyloid burden; therefore, a KD may serve to help prevent insulin resistance (20, 21). The trial included data from 128 cognitively normal older adults to assess the relationship between dietary glycemic measures and cerebral amyloid accumulation. The relationship between dietary glycemic measures and cognitive performance was also investigated. The participants completed a battery of neuropsychological tests consisting of the Mini-Mental State Exam (MMSE), Wechsler Adult Intelligence Scale-Revised (WAIS-R), the Digit Symbol Substitution Test, Trail Making Tests A and B, The Category Fluency Test (animals and vegetables), the Stroop Color-Word Interference Test, the WAIS-R Block Design, and the total free recall score from the Free and Cued Selective Reminding Test. Participants provided their dietary data through the Web-based National Cancer Institute Diet History Questionnaire (DHQ) II, which involved providing the amount and frequency of consumption of 134 foods over the past year. Cerebral amyloid accumulation was assessed with positron emission tomography (PET) scans with an amyloid-specific ligand. From the DHQ II, dietary glycemic measures were based off daily intake of sugar and carbohydrates, glycemic load, and adherence to a high-glycemic-load diet (HGLDiet) pattern. The participants with greater amyloid levels were found to have a higher intake of sugar and carbohydrate and higher adherence to a HGLDiet. Sugar intake was associated with lower cognitive performance on the MMSE, Trail Making Test B, WAIS-R, Digit Symbol Test, and Block Design (21).
An increase in ROS is another pathologic feature of AD (22). Research suggests that low levels of carbohydrates can activate stress proteins, specifically GRP78 and HSP70, that decrease ROS and support mitochondrial activity (15). Mitochondrial dysfunction is another hallmark in the pathogenesis of AD. Protein analyses have shown that there is downregulation of oxidative phosphorylation in AD brains (23). Ketone bodies, β-hydroxybutyrate in particular, have been shown to decrease production of ROS by mitochondrial complex I. In addition, metabolism of ketone bodies increases expression of uncoupling proteins, which improves the efficiency of the electron transport chain in oxidative phosphorylation. Uncoupling proteins inhibit ROS production and reduce mitochondrial membrane potential. Ketogenic diets have also been shown to increase the NAD+/NADH ratio, which is important for oxidative phosphorylation and protects against ROS. During glycolysis, glucose reduces 4 molecules of NAD+, while oxidation of β-hydroxybutyrate reduces zero molecules of NAD+ (24). Overall, the KD has been found to play a role in several aspects of AD pathogenesis.
Methods
The PubMed and ScienceDirect databases were searched for clinical trials and randomized, controlled trials involving the KD or exogenous ketone administration and AD. Key search terms used included “ketogenic diet and Alzheimer’s disease,” “ketosis and Alzheimer’s disease,” “MCT and Alzheimer’s disease,” and “exercise and diet and Alzheimer’s disease.” Only studies involving patients diagnosed with AD were included in the next two sections of this paper, but for the combined approach section, studies included patients diagnosed with MCI due to AD, secondary to a paucity of combined approaches studies involving AD patients alone.
Ketogenic diet and AD
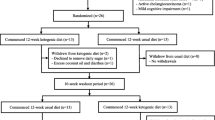
A recent, small, randomized, cross-over trial in New Zealand found that the KD improved daily function and quality of life in AD patients. The study included 26 patients who were between the ages of 50 and 90 and met the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD. Patients were randomized to either consume a modified KD for 12 weeks or a usual diet, with 13 patients in each group. The modified KD consisted primarily of eggs, meats, nuts, seeds, creams, natural oils, and green vegetables. The participants were provided with ketogenic recipes and guidelines. Participants were instructed to consume at least one serving of a breakfast, lunch, dinner, and side dish recipe each day. Guidelines also included consumption of at least 5 cups of water and adequate salt in order to avoid the dehydration and dizziness that can be caused by a KD. For the usual diet, patients were instructed to consume their regular diet and were provided with low-fat healthy eating guidelines and optional recipes. After completing the first treatment period, the patients underwent a 10-week washout period and then completed a second 12-week treatment period. The primary outcomes were mean, within-individual, changes in cognition, daily function, and quality of life from baseline. These outcomes were measured by the Addenbrooke’s Cognitive Examination - III (ACE-III) scale, Activities of Daily Living (ADCS-ADL) inventory, and Quality of Life in AD (QOL-AD) questionnaire, respectively. The secondary outcomes were mean within-individual changes in cardiovascular risk factors, including weight, body mass index (BMI), HbA1c, triglycerides, HDL, LDL, and total cholesterol from baseline. Measurements of all outcomes were taken at baseline, week 6 of treatment, and week 12 of each treatment period. The patients consuming the KD had a non-significant change from baseline in cognition as measured by the ACE-III but exhibited significant improvement in activities of daily living and quality of life, as measured by the ADCS-ADL and QOL-AD. In addition, the KD group had statistically significant decreases in weight, BMI, and HbA1c levels compared to baseline. They did not show a change in triglycerides, and they had statistically significant increased levels of HDL from baseline, which is favorable for cardiovascular health. On the contrary, their LDL and total cholesterol levels also significantly increased from baseline, which is not favorable for cardiovascular health. It is of note that half of the patients planned to continue consuming a KD after the study (14). This suggests that a KD can be palatable to AD patients and may provide improvements in cognition and quality of life.
A small pilot trial that was a single-arm study found that a KD supplemented with MCTs led to improvements in the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) scores in AD patients with mild dementia. The study involved 15 patients, each with a trial partner, who had a clinical dementia rating (CDR) of very mild AD (CDR 0.5), mild AD (CDR 1), or moderate AD (CDR 2). The mean age of the patients was 73.1 ± 9.0 years. There were 7 patients with very mild AD, 4 patients with mild AD, and 4 with moderate AD. The patients consumed a MCT oil supplemented KD for three months and then returned to consuming their normal diet for one month as a washout period. The goal ketogenic ratio for the diet was 1:1 lipid to nonlipid consumption and the goal macronutrient ratio was 70% of energy from fat, 20% from protein, and less than 10% from carbohydrates. Total energy intake and daily MCT dosage was determined using the Mifflin-St. Jeor equation. The MCT supplement consisted of a combination of C8:0 and C10:0 fatty acids. During the first week, the MCT supplement contributed to about 10% of total caloric intake. This was increased by 10% each week thereafter until 40% of total energy intake was reached. Participants could choose between consuming the MCT oil by itself, or by mixing it with food or drink (25). The MCT oil was recommended to be consumed in coffee, along with another long-chain fat source like heavy cream, because that has been found to be most tolerable method of consumption (26). The participants who completed the study consumed 1.5–3 tablespoons of MCT oil per day, which was about 60–80% of the goal MCT volume. None of the patients reached the total goal MCT volume intake. Cognition was measured at baseline, at the end of the three-month treatment period, and after the washout period. Results showed a statistically significant 4.1-point increase in ADAS-Cog scores after the treatment period compared to baseline. After the washout period, the patient’s ADAS-Cog scores reverted back to their baseline values. The participants were also administered the MMSE at the beginning of the study. MMSE scores also significantly increased from baseline to the end of the 3-month treatment period. The average MMSE score among the protocol adherent patients was 25.2 at baseline, 26.3 at month 3, and 25.4 at the end of the washout period. It is of significance that five of the patients dropped out early in the study. This was due to the burden on the caregiver that the maintenance of a KD caused. Future studies should investigate methods to improve this burden. MCTs are not known to be palatable, and most of the patients in the study opted to mix their MCT oil with either food or beverage. This can serve to improve adherence to an MCT-supplemented KD in future studies and in potential practice. Furthermore, none of the patients reported having gastrointestinal symptoms from the diet, which is a common complaint of a KD (25). Thus, this pilot trial indicates that an MCT-supplemented KD may improve cognition in AD patients. The results of this study and the previous two studies are summarized in Table 1.
Some critics of the KD argue that it is not healthy due to being high in saturated fat and low in fiber; however, Taylor et al. demonstrated in an additional study that the KD has the potential to be a nutritionally dense diet. The purpose of this study was to assess the changes in food quality from a baseline diet to an MCT-supplemented diet among the participants discussed in the previous study. The participants’ study partners completed 3-day food records at baseline, at month 1, and at month 2. At each study visit, a registered dietician reviewed the records with the participant and study partner. The food intake data was inputted into the Nutrition Data System for Research in order to compile food and nutrient consumption. Every week, a registered dietician called the participants and provided nutrition counseling based off the Ketogenic Diet Retention and Feasibility Trial (KDRAFT) guidelines. These guidelines included principles such as consuming eggs, dark meat poultry, fatty fish and unprocessed red meat for protein sources, and limiting processed meat. Regarding fat intake, extra virgin olive oil, avocado, olives, and whole nuts and seeds were recommended over consumption of nut and seed butters, bacon, and butter. The participants were advised to limit unsweetened, full-fat milk or yogurt to no more than ½ cup per day, but 1–2 servings of full-fat cheese was allowed. Furthermore, participants could consume unlimited amounts of nonstarchy vegetables, but were instructed to eliminate starchy vegetables from their diet. Patients were advised to decrease their fruit intake to only ½ cup of berries per day. The participants were also advised to eliminate refined and whole grains, and to replace these with almond or coconut flours. The guidelines also included elimination of sugar intake. Participants were also given a daily multivitamin, vitamin D, calcium, and phosphorous supplementation to ensure avoidance of nutrient deficiency. The results showed that the KD lead to greater consumption of olive oil, avocados, nuts and seeds, non-starchy vegetables, fresh fish, and seafood. The increased consumption of olive oil, avocados, and nuts and seeds led to an increased intake of monounsaturated fatty acids on the KD, which are one of the cardioprotective fats that were mentioned previously. There was no change in intake of polyunsaturated fatty acids between the two diets. An increase in this type of fat could be achieved in future studies by encouraging the consumption of walnuts, chia seeds, and fatty fish. The non-starchy vegetables that were found to be increased in the diet compared to baseline diet included kale, spinach, brussels sprouts, broccoli, and bell pepper. These vegetables are rich in micronutrients while being low in carbohydrates and calories. The non-starchy vegetable along with the avocado intake allowed for the high micronutrient content of the KD. In addition, the KD led to the removal of red meat, desserts, fried foods, and sugar-sweetened beverages from the diet, because the participants followed the KDRAFT guidelines. The reason for the elimination of red meat from the diet was unknown, because this was not eliminated in the diet guidelines. Because the goal of a KD is to produce ketones, the nutritional quality of the KD has not been a major emphasis of the diet guidelines in the past (26). A KD can be achieved by consuming less healthy foods such as processed fatty meat and full-fat dairy products; however, this provides less micronutrients and can be detrimental to cardiovascular health. This study illustrates that a KD can be a nutrient-dense diet.
Exogenous ketone administration and AD
Another way to induce ketonemia is to supplement the normal diet with a ketogenic agent. This allows patients to consume their regular diet. This approach may have higher levels of adherence than the traditional KD, due to the restrictive nature and potential negative side effects of the traditional KD (17). However, similar negative gastrointestinal-related side effects have also been reported from exogenous ketone supplementation (27). The clinical studies involving exogenous ketone supplementation in AD patients are summarized in Table 2.
One way to induce hyperketonemia is with a ketone monoester. A ketone monoester is a dietary source of ketones, while MCTs are metabolized into ketones. Newport et al. reported the effects of the use of this agent in a 63-year-old male patient with younger-onset sporadic AD. Before the study, the patient was experiencing severe memory loss and was unable to complete activities of daily living. The patient took the ketone monoester three times a day for eight weeks and showed significant improvements in memory and was able to complete tasks that he was unable to before the therapy was started. His memory improvement consisted of increased memory retrieval and spontaneous discussion of events that had occurred more than a week earlier. The task improvements included showering, dressing self, brushing teeth, washing dishes by hand, and choosing and ordering food from a menu. Ketone monoester administration can increase ketone levels to an amount similar to levels induced by fasting, and research suggests that this may be more beneficial than the lower levels that are caused by MCT administration (28).
MCT ingestion has been shown to be a safe way to induce ketonemia without requiring consumption of a KD (29). A study conducted by Henderson et al. investigated the effect of normal diet with daily MCT supplementation on cognitive performance in 152 patients with mild to moderate AD. The randomized, double-blind, placebo-controlled, parallel-group 90-day study utilized the MCT agent AC-1202, which is unique from other triglycerides in that it can induce ketonemia in the presence of a normal diet. AC-1202 is an MCT comprised of glycerin and caprylic acid, an 8-carbon saturated fatty acid. It was administered in a powder formula that was 64% gum acacia, 33% AC-1202, and 2.6% syloid. The isocaloric placebo consisted of 51% gum acacia, 37% dextrose, 10% safflower oil, and 2% syloid. The treatment powder and placebo powder were packaged into 30-gram sachets. Each sachet was mixed into 8 ounces of either water, milk, or juice before administration each day. It was found that the Ensure meal replacement drink helped improve tolerability of the agent, so instructions were modified to consume it with that. The participants consumed one sachet per day for the first seven days, and then consumed two per day after for the rest of the study. Administration of the AC-1202 agent resulted in a statistically significant decrease in ADAS-Cog scores compared to baseline in patients who were ApoE4(−)., but not in those who were APOE-4(+). (A decrease in ADAS-Cog score correlates with an improvement in cognitive performance). In these participants, the average baseline ADAS-Cog score was 21.9, and there was a 6.26 decrease in score on Day 45 and a 5.33 decrease on Day 90 (30). In 2009, AC-1202, or caprylidene, a proprietary formulation of caprylic triglycerides was approved by the Food and Drug Administration as the medical food Axona® (31).
A randomized, double-blind, placebo-controlled study by Torosyan et al. found that supplementation with caprylidene for 45 days led to increases in cerebral blood flow in 14 APOE4(−) AD patients. The study involved 16 subjects who had mild-to-moderate AD based on NINCDS-ADRDA criteria. Caprylidene was given to 14 of the subjects and a placebo was given to 2 subjects. Out of the 14 subjects who received caprylidene, 8 were APOE4(+) and six were APOE4(−). The average age of the APOE4(+) subgroup was 82.2 years ± 4.4 years, and the average was 79.4 ± 5.3 years for the APOE4(−) subgroup. The participants had two 15O-water PET scans administered on the first day of the intervention period and two scans after the 45 days of supplementation. One scan before either caprylidene or placebo consumption and one scan 90-minutes after consumption. The effects of caprylidene were found to be different in APOE4(−) patients compared to APOE4(+) patients. Significantly increased cerebral blood flow to the superior lateral temporal cortex was present in the APOE4(−) patients but not the APOE4(+) patients after 45 days of treatment. One proposed reason for this is that APOE4(−) patients are able to achieve higher ketone levels because APOE4(+) patients have decreased mitochondrial enzyme function (32).
A study in China found that MCT supplementation three times a day for 30 days led to statistically significant improvements in the Chinese ADAS-Cog (ADAS-Cog-C). This MCT formula is different than caprylidene in that it is comprised of C8:0/C10:0 at a ratio of about 1:3. This study was a double-blind, randomized, placebo-controlled crossover study involving 53 mild to moderate APOE4(−) AD participants between the ages of 55 and 90 years old. There were 27 patients assigned to the MCT jelly group and 26 to the placebo jelly group. The placebo was comprised of canola oil. The NINCDS-ADRDA criteria were used to diagnose AD and mild to moderate AD was defined by a MMSE score between 14 and 24 and a CDR score between 0.5 and 2. The baseline ADAS-Cog-C score for the intervention group was 21.5 and it was 23.2 for the placebo group. The ADAS-Cog-C score decreased by 2.62 in the intervention group and increased by 2.57 in the placebo group (33).
Ota et al. suggested in a double-blind, placebo-controlled trial followed by a 12 week, open-label extension that there may be a difference between single and continuous administration of MCT supplementation relative to cognition in AD patients. The protocol consisted of a single administration trial and a chronic (12-week) administration trial involving the ketogenic agent Ketonformula®. This formula consists of emulsified MCTs. There were 20 participants who were diagnosed with AD based on the NINCDS-ADRDA criteria. In the first trial, each patient came to the study center two times and received either the ketogenic formula or a placebo in a randomized sequence. The placebo consisted of emulsified long-chain triglycerides. Cognitive testing was performed before and 2 hours after administration of both the ketogenic agent and the placebo. The tests used were the immediate logical memory test, delayed logical memory, digit span, and visual memory span from the Wechsler Memory Scale revised (WMS-R), the block-design and digit-symbol coding tests from the Weschler Adult Intelligence Scale (WAIS-III), the Trail Making Test (TMT), and the Stroop test. The results of the first trial did not show a statistically significant difference between cognitive scores after consumption of the ketogenic agent compared to consumption of the placebo. For the second trial, which was a longitudinal, open-label trial, 19 of the patients consumed the ketogenic agent daily as a supplement to their normal diet. The same cognitive tests were performed at baseline and weeks 4, 8, and 12. It is of note that 3 patients dropped out before the end of this trial due to complaints of diarrhea. This is the most common negative side effect of MCT consumption. Results for the second trial included statistically significant increases in immediate and delayed logical memory tests at 8 weeks compared to baseline, as well as statistically significant increases in the digit-symbol coding test and immediate logical memory tests at 12 weeks compared to baseline (34). This small study supports the notion that a normal diet supplemented with a ketogenic agent may provide cognitive benefits in AD patients.
Combined approaches
Evidence suggests that a combined approach to AD therapy may elicit stronger results, rather than diet alone. For example, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was a 2-year multidomain double-blind randomized controlled trial to assess changes in cognition in at-risk older adults. The domains included were a diet, a physical exercise program, a cognitive training regimen, and a vascular risk monitoring schedule. There were 1260 participants between the ages of 60–77 years old who had a Cardiovascular Risk Factors, Aging, and Dementia (CAIDE) dementia Risk Score of 6 points or higher and whose cognition was screened at baseline using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychological test battery (NTB). The CAIDE score ranges from 0–15 points and is based on age, sex, education level, systolic blood pressure, BMI, total cholesterol, and physical activity. The CAIDE Dementia Risk Score is a validated tool that uses midlife vascular risk factors to predict late-life dementia risk. A score of 6 or more indicates the presence of modifiable risk factors (35). The primary outcome measure was a change in cognition, indicated by the NTB score. The participants were randomly assigned to either a treatment or control group, with 631 and 629 participants in each, respectively. The treatment group underwent a diet, exercise, cognitive training, and vascular risk monitoring, and the control group received general health advice. Per protocol, both groups met the study nurse at screening, baseline, and at months 6, 12, and 24 to have blood pressure, blood work, BMI, and body circumference measured. For the intervention group, the diet aspect of the protocol was based on the Finnish Nutrition Recommendations. Members of this group attended individual and group sessions, where the participant’s personal diet and approaches to implementing lifestyle changes were discussed, respectively. The composition of the diet provided 25–35% of daily calories from fat, 45–55% from carbohydrates, 10–20% from protein, and 23–35 g/day of fiber. Specific fat recommendations included consuming less than 10% of total fat intake from saturated and trans fats, consuming 10–20% from monounsaturated fatty acids, 5–10% from polyunsaturated fatty acids, and 2.5–3 grams per day of omega-3 fatty acids. The intervention participants were also advised to consume less than 5 grams of salt per day and less than 5% of their total calorie intake from alcohol. Exercise regime for the intervention group consisted of muscle strength training 1–3 times per week and aerobic training 2–5 times per week and was adapted from the Dose Responses to Exercise Training (DR’S EXTRA) study protocol. For cognitive training, participants completed group sessions on age-related cognitive changes and individual sessions on the computer. The computer sessions were divided into two, 6-month periods, and each period consisted of 10–15 minutes of training a day, 3 times a week. The training was designed to improve executive processes, working memory, and mental speed. At the end of the 24 months, all patients completed the NTB again. The participants in the intervention group were found to have a statistically significant increase in NTB scores from baseline compared to the control group. Executive function scores were also found to be 83% higher in the intervention group than the control group and processing speed was 150% higher. There were not a lot of reported side effects, but the most common one was mild musculoskeletal pain from the exercise. This multidomain study suggests that a multifaceted approach to dementia can lead to robust results (7). A table summarizing this study is found in Table 3. It is also of note that the U.S. Alzheimer’s Association is currently funding a study similar to the FINGER study, the U.S. Pointer study, and the results are pending.
High intensity interval training, or HIIT, combined with the KD may serve as an effective method to decrease cognitive decline. Research suggests that HIIT may improve insulin resistance when compared to continuous exercise training (36). A case study of a 57-year-old woman with comorbid mild cognitive impairment (MCI) and metabolic syndrome (MetS) involving a 12-week intervention of KD, HIIT, and memory training resulted in significant improvements in memory and metabolic markers. The diet was calorically restricted, low in carbohydrates, and high in fat. Every other week, the participant completed a 20-minute HIIT session, for a total of 6 sessions. At the end of the intervention, the participant showed improvements in triglycerides, HDL, and VLDL levels. Memory training was conducted through PEAK brain training games and the participant was assigned to play them on a mobile device five days per week. The patient exhibited improvements in the measured areas of problem solving, focus, memory, mental agility, and language (37). Improvement in insulin resistance is a desirable goal in AD patients because research suggests a link between diabetes and AD (38). Research suggests that diabetic patients have a 56% greater risk of AD (39). A KD along with physical exercise, specifically HIIT, may be an effective way to improve insulin resistance and decrease cognitive decline in patients with MCI.
Morrill et al. reported a case of a 71-year-old female who was heterozygous for apolipoprotein E (ApoE4) with a dual diagnosis of mild AD/metabolic syndrome (MetS). ApoE4 is a gene that is involved in cholesterol transport in the brain, and the E4 allele is associated with defects in the receptor which causes amyloid plaque to accumulate in the brain (40). The patient was placed on a 10-week nutrition regimen, which included physical exercise and cognition training. Nutrition protocol consisted of a low carbohydrate/high fat diet and time restricted eating. For the exercise regimen, the patient walked on a treadmill while holding light hand weights for 3–5 days per week for 30 minutes. Cognitive training involved daily use of the PEAK Brain Training Application. This is a mobile device application that includes games and puzzles. The training domains of this application include language, problem solving, mental agility, memory, and focus with the goal of strengthening frontal, parietal, occipital, prefrontal cortical, temporal, and hippocampal mediated functions. Her cognition was measured before and after the intervention period with the Montreal Cognitive Assessment (MoCA). Results showed a statistically significant improvement in the score from baseline, from 21/30 points to 28/30 points. The patient’s metabolic markers were also monitored before, halfway, and after the treatment. She had statistically significant decreases in homoeostatic model assessment of insulin resistance (HOMA-IR), triglycerides, VLDL, and HgA1c (41). This case report serves as an example of the possible benefits of a combined approach in an AD patient.
Another notable combined approach is a modified Mediterranean KD (MMKD). Nagpal et al. compared the effects of a MMKD to the American Heart Association Diet (AHAD) in 17 patients with subjective memory complaints (SMC) or mild cognitive impairment (MCI). The MMKD diet was a low-carbohydrate, high fat diet consisting of lean meats, fish, and extra virgin olive oil. On the contrary, the AHAD diet was a high-carbohydrate, low fat diet that was comprised of fruits, vegetables, and fiber-rich carbohydrate sources. This study also investigated the effect of diet on gut bacteria. It is of note that the participants with MCI had a lower level of fungal diversity than the SMC group at baseline, suggesting that the gut microbiome may be involved in MCI pathogenesis. The participants were randomly assigned to one of the diets and then completed a 6-week washout period before consuming the second diet for 6 weeks. Each participant completed a lumbar puncture and gave fresh fecal specimens at baseline and at the end of each diet. The results were that the MMKD participants showed improvements in cerebral perfusion, increased cerebral ketone body uptake, improvements in CSF biomarkers, and improvements in peripheral metabolic measures. The MMKD increased CSF Aβ42 levels, decreased CSF tau, and increased the CSF Aβ42/tau ratio (42, 43). (Levels of CSF Aβ42 are typically decreased in AD). Decreases in this biomarker are thought to indicate Aβ amyloid aggregation and plaque deposition and may be associated with brain atrophy (44). The study also found that the two diets affected the gut microbiome differently. The MMKD was found to increase fungal diversity in the MCI patients more than the SMC patients. This suggests that a modified KD may hold cognitive benefits and that the gut microbiome may play a role in cognitive impairment (42).
Conclusion
There is evidence that the KD and exogenous ketone supplementation may provide treatment benefits in AD patients. It is unclear whether one method is better than the other. Exogenous supplementation may be more likely to be adhered to as a long-term treatment, because the dietary changes are not as drastic. However, some studies reported negative side effects from the exogenous supplements, so these must be considered for future trials. The cost of exogenous supplementation is another factor that can affect the feasibility of the treatment. An obstacle to administering a KD to an AD patient is that it can create a burden on the caregiver. Many forms of the KD require strict monitoring of carbohydrate intake, which would often fall under the responsibility of the caregiver. Future studies involving the KD in AD patients may be more feasible in an institutional setting, where it would be easier to administer and to monitor (27). In addition, it is difficult to conduct research involving dietary approaches because many patients struggle to adhere to protocol, thus affecting results. Furthermore, most current studies are small, often uncontrolled, and only look at the short-term effects of ketosis on cognition. Large, long-term, randomized, controlled trials relative to the impact of the KD in patients at risk for or with mild cognitive impairment and AD are lacking and thus needed (45). Combined approaches may prove to be more beneficial in possibly preventing/delaying AD and in improving/stabilizing and possibly slowing disease-progression in those with MCI or AD. Future research should investigate the effect of additional combined approaches relative to neurocognitive decline in older adults and AD patients.
References
National Institute on Aging (2021) Alzheimer’s Disease Fact Sheet. https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet. Accessed 30 August 2021.
CDC (2021) What is Alzheimer’s Disease? https://www.cdc.gov/aging/aginginfo/alzheimers.htm. Accessed 30 August 2021.
National Institute on Aging (2021) How Is Alzheimer’s Disease Treated? http://www.nia.nih.gov/health/how-alzheimers-disease-treated Accessed 1 September 2021.
Information NC for B, Pike USNL of M 8600 R, MD B, Usa 20894. Non-Drug Interventions for Alzheimer’s Disease. Institute for Quality and Efficiency in Health Care (IQWiG); 2017. Accessed September 1, 2021. https://www.ncbi.nlm.nih.gov/books/NBK279355/
Yusufov M, Weyandt LL, Piryatinsky I. Alzheimer’s disease and diet: a systematic review. International Journal of Neuroscience. 2017;127(2):161–175. doi:https://doi.org/10.3109/00207454.2016.1155572
Pavón S, Lázaro E, Martínez O, et al. Ketogenic diet and cognition in neurological diseases: a systematic review. Nutrition Reviews. 2021;79(7):802–813. doi:https://doi.org/10.1093/nutrit/nuaa113
Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi:https://doi.org/10.1016/S0140-6736(15)60461-5
Crosby L, Davis B, Joshi S, et al. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front Nutr. 2021;8:702802. doi:https://doi.org/10.3389/fnut.2021.702802
Hintze KJ, Benninghoff AD, Cho CE, Ward RE. Modeling the Western Diet for Preclinical Investigations. Adv Nutr. 2018;9(3):263–271. doi:https://doi.org/10.1093/advances/nmy002
Dietary fat - Better Health Channel. Accessed November 15, 2021. https://www.betterhealth.vic.gov.au/health/healthyliving/fats-and-oils
Mawer R (2020). The Ketogenic Diet: A Detailed Beginner’s Guide to Keto. Healthline. https://www.healthline.com/nutrition/ketogenic-diet-101#_noHeaderPrefixedContent. Accessed 22 November 2021.
Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force - ClinicalKey. Accessed November 15, 2021. https://www-clinicalkey-com.ezp.slu.edu/#!/content/playContent/1-s2.0-S1933287419302673?returnurl=null&referrer=null
Jensen NJ, Wodschow HZ, Nilsson M, Rungby J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int J Mol Sci. 2020;21(22):E8767. doi:https://doi.org/10.3390/ijms21228767
Phillips MCL, Deprez LM, Mortimer GMN, et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Research & Therapy. 2021;13(1):51. doi:https://doi.org/10.1186/s13195-021-00783-x
Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav Pharmacol. 2006;17(5–6):431–439.
Fish, meat, and risk of dementia: cohort study. Accessed January 9, 2022. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC130057/
Masood W, Annamaraju P, Uppaluri KR. Ketogenic Diet. In: StatPearls. StatPearls Publishing; 2021. Accessed 13 September 2021. http://www.ncbi.nlm.nih.gov/books/NBK499830/
Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 2019;14:5541–5554. doi:https://doi.org/10.2147/IJN.S200490
Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clin Interv Aging. 2007;2(3):347–359.
Rusek M, Pluta R, Ułamek-Kozioł M, Czuczwar SJ. Ketogenic Diet in Alzheimer’s Disease. Int J Mol Sci. 2019;20(16):3892. doi:https://doi.org/10.3390/ijms20163892
Taylor MK, Sullivan DK, Swerdlow RH, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463–1470. doi:https://doi.org/10.3945/ajcn.117.162263
Tillery EE, Ellis KD, Threatt TB, Reyes HA, Plummer CS, Barney LR. The use of the ketogenic diet in the treatment of psychiatric disorders. Ment Health Clin. 2021;11(3):211–219. doi:https://doi.org/10.9740/mhc.2021.05.211
Wang W, Zhao F, Ma X, Perry G, Zhu X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Molecular Neurodegeneration. 2020;15(1):30. doi:https://doi.org/10.1186/s13024-020-00376-6
Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants. 2018;7(5):63. doi:https://doi.org/10.3390/antiox7050063
Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2018;4(1):28–36. doi:https://doi.org/10.1016/j.trci.2017.11.002
Taylor MK, Swerdlow RH, Burns JM, Sullivan DK. An Experimental Ketogenic Diet for Alzheimer Disease Was Nutritionally Dense and Rich in Vegetables and Avocado. Curr Dev Nutr. 2019;3(4):nzz003. doi:https://doi.org/10.1093/cdn/nzz003
Utility of Ketone Supplementation to Enhance Physical Performance: A Systematic Review
Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s. Alzheimers Dement. 2015;11(1):99–103. doi:https://doi.org/10.1016/j.jalz.2014.01.006
Courchesne-Loyer A, Fortier M, Tremblay-Mercier J, et al. Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition. 2013;29(4):635–640. doi:https://doi.org/10.1016/j.nut.2012.09.009
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6:31. doi:https://doi.org/10.1186/1743-7075-6-31
Sharma A, Bemis M, Desilets AR. Role of Medium Chain Triglycerides (Axona®) in the Treatment of Mild to Moderate Alzheimer’s Disease. Am J Alzheimers Dis Other Demen. 2014;29(5):409–414. doi:https://doi.org/10.1177/1533317513518650
Torosyan N, Sethanandha C, Grill JD, et al. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: Results of a randomized, double-blinded, pilot study. Exp Gerontol. 2018;111:118–121. doi:https://doi.org/10.1016/j.exger.2018.07.009
Xu Q, Zhang Y, Zhang X, et al. Medium-chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer’s disease patients with APOE4-/-: A double-blind, randomized, placebo-controlled crossover trial. Clin Nutr. 2020;39(7):2092–2105. doi:https://doi.org/10.1016/j.clnu.2019.10.017
Ota M, Matsuo J, Ishida I, et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci Lett. 2019;690:232–236. doi:https://doi.org/10.1016/j.neulet.2018.10.048
Sindi S, Calov E, Fokkens J, et al. The CAIDE Dementia Risk Score App: The development of an evidence-based mobile application to predict the risk of dementia. Alzheimers Dement (Amst). 2015;1(3):328–333. doi:https://doi.org/10.1016/j.dadm.2015.06.005
Jelleyman C, Yates T, O’Donovan G, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obesity Reviews. 2015;16(11):942–961. doi:https://doi.org/10.1111/obr.12317
Dahlgren K, Gibas KJ. Ketogenic diet, high intensity interval training (HIIT) and memory training in the treatment of mild cognitive impairment: A case study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2018;12(5):819–822. doi:https://doi.org/10.1016/j.dsx.2018.04.031
Pereira JD, Fraga VG, Santos ALM, Carvalho M das G, Caramelli P, Gomes KB. Alzheimer’s disease and type 2 diabetes mellitus: A systematic review of proteomic studies. Journal of Neurochemistry. 2021;156(6):753–776. doi:https://doi.org/10.1111/jnc.15166
Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. Journal of Diabetes Investigation. 2013;4(6):640–650. doi:https://doi.org/10.1111/jdi.12087
Apolipoprotein E: Far More Than a Lipid Transport Protein ∣ Annual Review of Genomics and Human Genetics. Accessed November 27, 2021. https://www-annualreviews-org.ezp.slu.edu/doi/10.1146/annurev.genom.1.1.507
Morrill S, Gibas K. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2019;13. doi:https://doi.org/10.1016/j.dsx.2019.01.035
Nagpal R, Neth BJ, Wang S, Mishra SP, Craft S, Yadav H. Gut mycobiome and its interaction with diet, gut bacteria and alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine. 2020;59:102950. doi:https://doi.org/10.1016/j.ebiom.2020.102950
Neth BJ, Mintz A, Whitlow C, et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: a pilot study. Neurobiol Aging. f 2020;86:54–63. doi:https://doi.org/10.1016/j.neurobiolaging.2019.09.015
Fagan AM, Head D, Shah AR, et al. Decreased CSF Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65(2):176–183. doi:https://doi.org/10.1002/ana.21559
Włodarek D. Food for thought: the emerging role of a ketogenic diet in Alzheimer’s disease management. Expert Review of Neurotherapeutics. 2021;21(7):727–730. doi:https://doi.org/10.1080/14737175.2021.1951235
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: None.
Rights and permissions
About this article
Cite this article
Hersant, H., Grossberg, G. The Ketogenic Diet and Alzheimer’s Disease. J Nutr Health Aging 26, 606–614 (2022). https://doi.org/10.1007/s12603-022-1807-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-022-1807-7