Abstract
Objectives
Frailty is a risk factor for poor cognitive performance in older adults. However, few studies have evaluated the association of cognitive performance with frailty in a low- to middle-income country (LMIC). This study aimed to investigate an association between cognitive performance and frailty in older adults with memory complaints in Brazil. Secondarily, we aim to assess an association of cognitive performance with gait speed and grip strength.
Design
Cross-sectional study.
Setting
Outpatient service from a LMIC
Participants
Older adults with memory complaints reported by the participants, their proxies, or their physicians.
Measurements
Frailty was evaluated using the Cardiovascular Health Study criteria. A neuropsychological battery evaluated memory, attention, language, visuospatial function, executive function. Linear regression analysis with adjustment for age, sex, and education was used. We also evaluated the interaction of education with frailty, grip strength, and gait speed.
Results
Prefrailty was associated with poor performance in the memory domain, as well as slower gait speed was associated with worse performance in memory, attention, language, and executive function. Frailty and grip strength were not associated with cognitive performance. Interactions of education with gait speed were significant for global performance, as well as for attention and visuospatial ability.
Conclusion
In elderly patients with memory complaints, prefrailty was associated with poor memory performance. Slowness was associated with poorer performance in some cognitive domains, mainly in participants with low education.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty syndrome and neurocognitive disorder are of cardinal importance in the current context of a rapidly aging world (1). The prevalence of dementia among community-dwelling older people is estimated to be 4.7% worldwide, and it is estimated to be even higher in Latin America (8.5%) (2). Frailty has a prevalence of 10.7% worldwide (3) and 13.5% in Brazil (4). Both conditions are known for enhancing adverse health outcomes, like hospitalization, disability, and mortality (5,6). Therefore, in low- and middle-income countries (LMIC), the burden of population aging can be even more challenging. Both conditions share mutual physiopathology, like inflammation, insulin resistance, endocrine distress (7), and age is the most important risk factor for both (8,9). The coexistence of frailty syndrome and cognitive impairment has been described, although the association mechanisms are not clear (10). Frailty syndrome is a risk factor for cognitive impairment, and poor cognitive performance can predict frailty (11∓13).
One of the most common complaints among older adults is forgetfulness (14). Evaluating individuals with memory complaints is usually the trigger to assess cognitive function in individuals at risk for cognitive impairment. Early evaluation of cognitive complaints is important to manage lifestyle, risk factors, and to start cognitive rehabilitation (15). Cognition evaluation is frequently done using screening tests, like the Mini-Mental State Examination (16). They show good reliability and are useful for general clinical and geriatric practice (17), but they lack detailed information in cognitive domains that helps to provide a more precise diagnosis of the etiology of the cognitive impairment (18). A neuropsychological battery can help to provide detailed information about several cognitive domains (19).
Cognitive domain patterns associated with frailty syndrome have been studied in some countries (20∓22), but as far as we know, not in LMIC, where low education and more impoverished socioeconomic conditions negatively influence general health conditions and increase the risks for both frailty and cognitive impairment. Therefore, we aimed to investigate the association of performance on cognitive domains with frailty in a sample from a LMIC. Additionally, we aimed to evaluate the association of cognitive performance with walking speed and strength.
Methods
Design, setting, and participants
Patients with memory complaints were recruited from an outpatient geriatric service at a tertiary hospital located in the city of São Paulo, Brazil. Trained geriatricians and neuropsychologists evaluated participants from June 2016 to August 2018. Patients of 60 years or more were included if memory complaints were reported by the participants, their proxies, or their physicians. Exclusion criteria were a previous diagnosis of dementia, presence of delirium, psychosis, drug or alcohol abuse, low visual acuity (Snellen test equal 20/200 or worst), incapacitating aphasia or dysarthria, inability to walk without proxy help, clinical instability (e.g. hypoxemia, hypotension, decompensated heart failure, chemotherapy, Child Pugg class C liver failure, hemodialysis, and end-life clinical conditions), or absence of fluency in Portuguese. Informed consent was applied and the local ethical committee in research approved this study.
Clinical and sociodemographic evaluation
Patients or proxies were interviewed for sociodemographic data, and electronic medical records were assessed to evaluate the Charlson comorbidity index. We also measured de body mass index (BMI), using the weight and height squared.
Frailty evaluation
Frailty was evaluated using the Phenotypic Criteria of the Cardiovascular Health Study (23), composed of five dichotomous items: (A) Unintended weight loss of 5% or more in the last 12 months (B); Weakness measured with a handgrip dynamometer, using the best of three measures from the dominant hand (Supplementary Table 1) (24); (C) Slowness was evaluated by the time taken to walk 4 meters, using the best of two measures (Supplementary Table 2) (24); (D) Exhaustion was defined by answering “a moderate amount of the time” or “most of the time” during the last week for the two questions “I could not get going” or “I felt that everything I did was an effort”; (E) Physical inactivity was evaluated using the International Physical Activity Questionnaire (25). Participants were classified as robust if their score was 0, prefrail if their score was 1 or 2, or frail if their score was 3 or higher.
Neuropsychological Evaluation
Thirteen cognitive tests were applied in a single session, in a quiet and bright room by a single researcher for an average of two hours. These tests were grouped into five cognitive domains. A z-score was calculated for each test by subtracting the participant’s test score from the sample mean score and dividing the difference by the sample standard deviation. We then calculated the domain z-score by averaging the z-scores of the tests for each domain:
-
(A)
Memory: Hopkins Verbal Learning Test-Revised (HVLT-R) Total Recall and Logical Memory I from the Wechsler Memory Scale-Revised (WMS-R) — Paragraph A;(26) HVLT-R Delayed Recall, HVLT-R Discrimination Index and Logical Memory II WMS-R — Paragraph A; (26, 27)
-
(B)
Language: Boston Naming test (30 items) and Category Fluency (Animals);(28, 29)
-
(C)
Visuospatial Functions: Line Orientation of Repeatable Battery for Assessment of Neuropsychological Status (RBANS) (30) and clock drawing task CLOX 1;(31)
-
(D)
Executive Functions: Wechsler Abbreviated Scale of Intelligence (WASI) Color Trails 2 and Matrix Reasoning Subtest from Wechsler Abbreviated Scale of Intelligence (WASI);(32, 33)
-
(E)
Attention: Digit Span Subtest from Wechsler Adult Intelligence Scale-III (WAIS-III) and Color Trails 1.(32, 34)
A global composite z-score was calculated by averaging all tests and then generating a z-score from this average. We added a negative sign for tests Color Trails 1 and Color Trails 2 tests because the lower the time spent, the better the result, as opposed to the other tests.
Statistical Analysis
Characterization of the sample was done using means and standard deviation for interval variables, and frequencies for categorical ones. For descriptive analyses, we used chi-square for categorical variables, and one-way ANOVA and Kruskal-Wallis tests for interval variables according to their distribution. The dependent variables were the global composite score and the z-scores for each cognitive domain. The independent variables were the frailty status with robust category as the reference. We evaluate the association of frailty with cognitive performance using linear regression models adjusted for age, sex, and education. We also investigated the association of cognitive performance with muscle strength and gait speed (continuous variables), using linear models adjusted for the same demographic variables. Additionally, we evaluate the interaction of education with frailty, gait speed, and grip strength on cognitive performance using linear regression analysis models adjusted for age and sex. We used Stata 15 (StataCorp 2017, College Station, TX) for statistical analyses. The alpha level was set at the 0.05 level.
Results
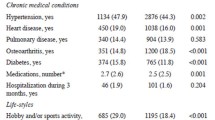
The flowchart of the study participants is presented in Figure 1. In 160 individuals, the mean age was 80.3±6.5 years old, 73.1% were women, mean education was 5.6±5.2 years, and 63.7% were white. Regarding the frailty evaluation, 16.3% were robust, 61.3% prefrail, and 22.4% frail (Table 1). Prefrailty was associated with poor performance in the memory domain (β=−0.37, 95%CI= −0.74 to −0.01, p=0.046) in adjusted models (Table 2). Slowness was associated with poor global composite z-scores and all cognitive domains except visuospatial ability. (Table 3). Frailty and grip strength were not associated with worse cognitive performance.
We found interactions between education and gait speed, suggesting a worse performance in global cognitive function, attention, language visuospatial ability (Figure 2). However, interactions of education with frailty and muscle strength were not significant (Supplementary Table 3).
Discussion
In this study of older adults with memory complaints, prefrailty was associated with poor memory performance. Older adults with frailty presented worse cognitive performance in all domains, but these associations did not remain after adjusting for sociodemographic variables. Slower performance in the gait speed test was associated with a worse performance in global cognitive function, memory, attention, and language. Besides, the associations were significant in all cognitive domains when gait speed was performed with a distractor. Muscular strength was not associated with cognitive performance. We found an interaction between education and gait speed in the global cognitive function, language, attention, and visuospatial function.
Some points may explain our finding of worse memory performance in older adults with prefrailty. The first point is that the individuals with memory complaints are expected to have a high frequency of amnestic patterns, which may justify the lower performance in the memory domain in our sample. Moreover, during the trajectory of cognitive decline in neurodegenerative diseases, the decline in memory function is usually one of the earliest symptoms (35). The third point is that possibly the severity of the frailty syndrome and the cognitive impairment evolve together. The robust state coincides with normal cognitive aging. As physical status changes to prefrailty, there may be an agreement with the development of early cognitive symptoms. In this way, frailty occurs with advanced stages of dementia. Both frailty and pathological cognitive aging lead to high consumption of physiological reserve. Along with these lines, when there is high consumption of physical and cognitive physiological reserve, we have the diagnosis of cognitive frailty, which is defined as the presence of cognitive impairment in the presence of physical frailty (130), been the cognitive component not attributable to Alzheimer’s disease or some other dementia (36). The association of poorer memory performance with prefrailty was not found in another study with a small sample but agreed with other important studies (37). A cross-sectional study in Ireland evaluated the association of cognitive domains with prefrailty and frailty (20). This study found an association of all cognitive domains with prefrailty and frailty, but discrete cognitive differences between prefrailty and frailty status (21). In another large cross-sectional study in Greece, the greater the number of compromised cognitive domains, the greater the chance of frailty, with an odds ratio of 1.56 (CI 1.04∓2.36; p = 0.03) (22).
Our finding of gait speed association with lower global cognitive performance, memory, attention, and language is consistent with the literature (38). Slowness was associated with a worse performance in overall cognitive performance and language in some Brazilian studies (39) (40). Gait speed is a significant predictor of global health and cognitive dysfunction; mostly if associated with a memory complaint, configuring the motor cognitive risk syndrome, which has a high predictive value for conversion to dementia, especially vascular dementia (41). This phenomenon occurs mainly when vascular dementia is caused by microvascular disease in the subcortical area, an area also responsible for gait automatism (42). Indeed, gait requires more than automatic mechanisms to be performed (43). The concomitant execution of motor functions with a cognitive demand, called dual-task, is part of routine life and has been studied to evaluate motor outcomes, especially falls. However, little has been studied about its association with cognitive outcomes. When we used a cognitive task with the gait speed test, we tried to introduce a test with some similarity to the daily challenges. We hypothesized that the introduction of this dual-task would sensitize our assessment of the association of gait speed with cognitive function, not only by slowing the participant but by adding complexity to the cognitive task. The association of all cognitive functions with gait speed with a distractor suggests that this dual-task can be a useful evaluation model. Our findings agree with a study in which the execution of an isolated motor task did not show any difference between the control group of cognitively preserved older individuals compared to a group with cognitive impairment, but the addition of the double task generated a slowing in the motor response in the group of older adults with cognitive impairment (44).
The handgrip strength measure seems to be higher in developed countries than in developing countries (45), which suggests that the socioeconomic level positively influences this measure. Weakness was associated with lower global cognitive performance (46). However, another study found that a test called functional reach (a validated test that tested the ability to pick up small objects and transport them with a spoon repeatedly) showed a more strong association with cognitive performance compared to the strength measurement (47). Therefore, the assessment of fine motor functions, such as coordination and speed, may be better predictors of the risk of cognitive decline than the assessment of strength. Thus, in cross-sectional studies, the magnitude of the association of cognitive performance with strength was weaker than that the one found with gait speed, which was not supported by longitudinal evaluations (48).
We found an effect modification on global cognitive performance and visuospatial function when we investigated the interaction of education with gait speed These interactions were expected since the low educational level is a known risk factor for worse cognitive performance, and it is associated with a low socioeconomic level, increased comorbidities, and worse global health conditions (49). Besides, low education is a risk factor for frailty (8). Additionally, gait speed performance depends on the musculoskeletal system, which in turn depends on good nutrition and engagement in physical activity (50). On the other hand, the lower the level of education, the lower the engagement with physical activity, and the worse the quality of nutrition (51), which may interfere with gait quality. With aging, postural control is increasingly dependent on cortical control (52), therefore depending on cognition.
As far as we could ascertain, we did not find any studies to compare the results of the analysis of the interaction of education with frailty and its components.
The main strength of this study is the neuropsychological assessment in a sample from a LMIC. As far as we know, it is the only study in Latin America to apply a neuropsychological battery to assess cognitive domains and investigate their association with frailty syndrome. Also, we investigated the interaction of education with frailty and its components to identify whether this socioeconomic variable could modify the effect of frailty on cognitive performance. The concept of frailty has evolved, and it is proposed that it has an intricate network of pathophysiological connections (53). For this reason, statistical analyzes that move towards clinical complexity are essential for the evolution of knowledge of this condition.
However, we need to consider our study limitations. We excluded patients incapable of performing physical tests, unable to walk alone, and decompensated clinical diseases. This may have led to the exclusion of more frail individuals. Another limitation of this study is the discrete sample number, which may have influenced the lack of associations between cognitive performance and frailty. In this study, we did not categorize individuals by cognitive diagnosis. However, we believe that in this sample, the study of cognitive performance is more appropriate since the study of the association of frailty with cognitive categories would require a considerably larger sample. Finally, this study was performed in a single medical center, which decreases the external validity of our findings. Finally, this was a cross-sectional study in which the causal association between physical performance and cognitive function changes cannot be determined.
In conclusion, in older people with memory complaints, early assessment of frailty and slowness can contribute to the detection of cognitive dysfunction.
References
World Health Organization. World Report on Ageing and Health. Geneva [Internet]. 2015. Available from: World Health Organization. https://apps.who.int/iris/handle/10665/186463
Custodio N, Wheelock A, Thumala D, Slachevsky A. Dementia in Latin America: Epidemiological Evidence and Implications for Public Policy. Front Aging Neurosci. 2017;9:221. https://doi.org/10.3389/fnagi.2017.00221.
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012 Aug;60(8):1487–92. https://doi.org/10.1111/j.1532-5415.2012.04054.x
Andrade JM, Duarte YA de O, Alves LC, Andrade FCD, Souza Junior PRB de, Lima-Costa MF, et al. Frailty profile in Brazilian older adults: ELSI-Brazil. Rev Saude Publica. 2018 Oct;52Suppl 2(Suppl 2):17s. https://doi.org/10.11606/S1518-8787.2018052000616
Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012 Aug;60(8):1478–86. https://doi.org/10.1111/j.1532-5415.2012.04074.x.
Noale M, Maggi S, Minicuci N, Marzari C, Destro C, Farchi G, et al. Dementia and disability: impact on mortality. The Italian Longitudinal Study on Aging. Dement Geriatr Cogn Disord. 2003;16(1):7–14. https://doi.org/10.1159/000069987.
Halil M, Cemal Kizilarslanoglu M, Emin Kuyumcu M, Yesil Y, Cruz Jentoft AJ. Cognitive aspects of frailty: mechanisms behind the link between frailty and cognitive impairment. J Nutr Health Aging. 2015 Mar;19(3):276–83. https://doi.org/10.1007/s12603-014-0535-z.
Feng Z, Lugtenberg M, Franse C, Fang X, Hu S, Jin C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS One. 2017/06/16. 2017;12(6):e0178383. https://doi.org/10.1371/journal.pone.0178383.
Chen J-H, Lin K-P, Chen Y-C. Risk factors for dementia. J Formos Med Assoc. 2009 Oct;108(10):754–64. https://doi.org/10.1016/S0929-6646(09)60402-2.
Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res Rev [Internet]. 2013;12(4):840–51. https://doi.org/10.1016/j.arr.2013.06.004.
Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom Med. 2007 Jun;69(5):483–9. https://doi.org/10.1097/psy.0b013e318068de1d.
Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010 Feb;58(2):248–55. https://doi.org/10.1111/j.1532-5415.2009.02671.x.
Raji MA, Al Snih S, Ostir G V, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010 Nov;65(11):1228–34. https://doi.org/10.1093/gerona/glq121
Ponds RW, Commissaris KJ, Jolles J. Prevalence and covariates of subjective forgetfulness in a normal population in The Netherlands. Int J Aging Hum Dev. 1997;45(3):207–21. https://doi.org/10.1016/s0738-3991(98)00040-8.
Si T, Xing G, Han Y. Subjective Cognitive Decline and Related Cognitive Deficits. Front Neurol. 2020;11:247. https://doi.org/10.3389/fneur.2020.00247.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
De Roeck EE, De Deyn PP, Dierckx E, Engelborghs S. Brief cognitive screening instruments for early detection of Alzheimer’s disease: a systematic review. Alzheimers Res Ther [Internet]. 2019;11(1):21. https://doi.org/10.1186/s13195-019-0474-3
Votruba KL, Persad C, Giordani B. Cognitive Deficits in Healthy Elderly Population With “Normal” Scores on the Mini-Mental State Examination. J Geriatr Psychiatry Neurol. 2016 May;29(3):126–32. https://doi.org/10.1177/0891988716629858.
Zgaljardic DJ, Temple RO. Neuropsychological Assessment Battery (NAB): Performance in a sample of patients with moderate-to-severe traumatic brain injury. Appl Neuropsychol. 2010 Oct;17(4):283–8. https://doi.org/10.1080/09084282.2010.525118.
Robertson DA, Savva GM, Coen RF, Kenny R-A. Cognitive function in the prefrailty and frailty syndrome. J Am Geriatr Soc. 2014 Nov;62(11):2118–24. https://doi.org/10.1111/jgs.13111.
Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc [Internet]. 2008/09/22. 2008 Oct;56(10):1845–52. https://doi.org/10.1111/jgs.13111.
Margioti E, Kosmidis M-H, Yannakoulia M, Dardiotis E, Hadjigeorgiou G, Sakka P, et al. Exploring the association between subjective cognitive decline and frailty: the Hellenic Longitudinal Investigation of Aging and Diet Study (HELIAD). Aging Ment Health. 2020 Jan;24(1):137–47. https://doi.org/10.1080/13607863.2018.1525604.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–56. https://doi.org/10.1093/gerona/56.3.m146.
Nunes DP, Duarte YA de O, Santos JLF, Lebrão ML, Nunes DP, Duarte YA de O, et al. Screening for frailty in older adults using a self-reported instrument. Rev Saude Publica. 2015;49(0). https://doi.org/10.1590/s0034-8910.2015049005516.
HALLAL PC, VICTORA CG, WELLS JCK, LIMA RC. Physical Inactivity: Prevalence and Associated Variables in Brazilian Adults. Med Sci Sport Exerc. 2003 Nov;35(11):1894–900. https://doi.org/10.1249/01.MSS.0000093615.33774.0E.
Shapiro AM, Benedict RHB, Schretlen D, Brandt J. Construct and Concurrent Validity of the Hopkins Verbal Learning Test — Revised. Clin Neuropsychol. 1999 Aug;13(3):348–58. https://doi.org/10.1076/clin.13.3.348.1749.
Hoffman RG, Tremont G, Scott JG, Adams RL, Mittenberg W. Cross-Validation of Predicted Wechsler Memory Scale-Revised Scores in a Normative Sample of 25- to 34-Year-Old Patients. Arch Clin Neuropsychol. 1997 Jan;12(7):677–82.
Mansur LL, Radanovic M, Araújo G de C, Taquemori LY, Greco LL. Teste de nomeação de Boston: desempenho de uma população de São Paulo. Pró-Fono Rev Atualização Científica. 2006 Jan;18(1):13–20. https://doi.org/10.1590/S0104-56872006000100003
Brucki SMD, Malheiros SMF, Okamoto IH, Bertolucci PHF. Dados normativos para o teste de fluência verbal categoria animais em nosso meio. Arq Neuropsiquiatr. 1997;55(1):56–61. https://doi.org/10.1590/S0004-282X1997000100009
Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J Clin Exp Neuropsychol. 1998 Jun;20(3):310–9. https://doi.org/10.1076/jcen.20.3.310.823.
Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998 May;64(5):588–94. https://doi.org/10.1136/jnnp.64.5.588
Ryan JJ, Carruthers CA, Miller LJ, Souheaver GT, Gontkovsky ST, Zehr MD. The WASI matrix reasoning subtest: performance in traumatic brain injury, stroke, and dementia. Int J Neurosci. 2005;115(1):129–36. https://doi.org/10.1080/00207450490512704.
Kilbourn VMM. The Influence of Verbal Mediation on Matrix Reasoning (Doctoral dissertation, Pacific University). 2011.
Figueiredo VLM de, Nascimento E do. Desempenhos nas duas tarefas do subteste dígitos do WISC-III e do WAIS-III. Psicol Teor e Pesqui. 2007 Sep;23(3):313–8. https://doi.org/10.1590/S0102-37722007000300010
Cloutier S, Chertkow H, Kergoat M-J, Gauthier S, Belleville S. Patterns of Cognitive Decline Prior to Dementia in Persons with Mild Cognitive Impairment. J Alzheimers Dis. 2015;47(4):901–13. https://doi.org/10.3233/JAD-142910.
Kelaiditi E, Cesari M, Canevelli M, Abellan van Kan G, Ousset P-J, Gillette-Guyonnet S, et al. Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J Nutr Health Aging [Internet]. 2013;17(9):726–34. Available from: https://doi.org/10.1007/s12603-013-0367-2
Langlois F, Vu TTM, Kergoat M-J, Chassé K, Dupuis G, Bherer L. The multiple dimensions of frailty: physical capacity, cognition, and quality of life. Int psychogeriatrics. 2012 Sep;24(9):1429–36. https://doi.org/10.1017/S1041610212000634.
Grande G, Triolo F, Nuara A, Welmer A-K, Fratiglioni L, Vetrano DL. Measuring gait speed to better identify prodromal dementia. Exp Gerontol. 2019 Sep;124:110625. https://doi.org/10.1016/j.exger.2019.05.014.
Macuco CRM, Batistoni SST, Lopes A, Cachioni M, da Silva Falcão DV, Neri AL, et al. Mini-Mental State Examination performance in frail, pre-frail, and non-frail community dwelling older adults in Ermelino Matarazzo, São Paulo, Brazil. Int psychogeriatrics. 2012 Nov;24(11):1725–31. https://doi.org/10.1017/S1041610212000907.
LenardtI MH, SousaI JAV de, GrdenI CRB, BetiolliI SE, CarneiroI NHK, RibeiroI DK de MN. Gait speed and cognitive score in elderly users of the primary care service. Rev Bras Enferm. 2015;86(6). https://doi.org/10.1590/0034-7167.2015680623i.
Semba RD, Tian Q, Carlson MC, Xue Q-L, Ferrucci L. Motoric cognitive risk syndrome: Integration of two early harbingers of dementia in older adults. Ageing Res Rev. 2020 Mar;58:101022. https://doi.org/10.1016/j.arr.2020.101022.
Scherder E, Eggermont L, Swaab D, van Heuvelen M, Kamsma Y, de Greef M, et al. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31(4):485–97. https://doi.org/10.1016/j.neubiorev.2006.11.007.
Wang L, Larson EB, Bowen JD, van Belle G. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006 May;166(10):1115–20. https://doi.org/10.1001/archinte.166.10.1115.
Toosizadeh N, Najafi B, Reiman EM, Mager RM, Veldhuizen JK, O’Connor K, et al. Upper-Extremity Dual-Task Function: An Innovative Method to Assess Cognitive Impairment in Older Adults. Front Aging Neurosci. 2016;8:167. https://doi.org/10.3389/fnagi.2016.00167
Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing. 2016 Mar;45(2):209–16. https://doi.org/10.1093/ageing/afv192.
Clouston SAP, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, et al. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35(1):33–50. https://doi.org/10.1093/epirev/mxs004.
Hooyman A, Malek-Ahmadi M, Fauth EB, Schaefer SY. Challenging the relationship of grip strength with cognitive status in older adults. Int J Geriatr Psychiatry. 2020 Oct; https://doi.org/10.1002/gps.5441
Zammit AR, Robitaille A, Piccinin AM, Muniz-Terrera G, Hofer SM. Associations Between Aging-Related Changes in Grip Strength and Cognitive Function in Older Adults: A Systematic Review. J Gerontol A Biol Sci Med Sci. 2019 Mar;74(4):519–27. https://doi.org/10.1093/gerona/gly046.
Kawachi I, Adler NE, Dow WH. Money, schooling, and health: Mechanisms and causal evidence. Ann N Y Acad Sci. 2010 Feb;1186:56–68. https://doi.org/10.1111/J.1749-6632.2009.05340.x.
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (London, England). 2019 Jun;393(10191):2636–46. https://doi.org/10.1016/S0140-6736(19)31138-9.
Lachat C, Otchere S, Roberfroid D, Abdulai A, Seret FMA, Milesevic J, et al. Diet and physical activity for the prevention of noncommunicable diseases in low- and middle-income countries: a systematic policy review. PLoS Med. 2013;10(6):e1001465. https://doi.org/10.1371/journal.pmed.1001465.
Lacour M, Bernard-Demanze L, Dumitrescu M. Posture control, aging, and attention resources: Models and posture-analysis methods. Neurophysiol Clin. 2008;38(6):411–21. https://doi.org/10.1016/j.neucli.2008.09.005.
Fried LP, Cohen AA, Xue Q-L, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging [Internet]. 2021;1(1):36–46. Available from: https://doi.org/10.1038/s43587-020-00017-z. https://doi.org/10.1038/s43587-020-00017-z
Acknowledgments
We thank all participants and all staff of the outpatient memory clinic for the elderly and frailty clinic at the University of Sao Paulo hospital of clinics.
Funding
Funding: None.
Author information
Authors and Affiliations
Contributions
Authors’ contributions: Sumika Mori Lin conceived the study design, collected data, performed the data analysis, interpreted the results, and drafted the manuscript. Daniel Apolinário conceived the study, collected data, reviewed the manuscript. Gisele Cristine Vieira Gomes collected data and interpreted the results. Fabiana Tosi collected the data, interpreted the results, and reviewed the manuscript. Regina Miksian Magaldi conceived the study design, collected data, interpreted the results. Ivan Aprahamian contributed to discussions of the study design and reviewed the manuscript. Wilson Jacob Filho contributed to the study design and reviewed the manuscript. Claudia Kimie Suemoto conceived the study design, performed the data analysis, interpreted the results, and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest: None to declare.
Ethical statement: This study was approved by the local ethical committee and the study protocol complied with the local and international ethical standards.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lin, S.M., Apolinário, D., Vieira Gomes, G.C. et al. Association of Cognitive Performance with Frailty in Older Individuals with Cognitive Complaints. J Nutr Health Aging 26, 89–95 (2022). https://doi.org/10.1007/s12603-021-1712-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-021-1712-5