Abstract
Objectives
Previous studies have reported a relationship between low protein intake and cognitive decline and have suggested that this association may be related to specific amino acid intake. However, the effects of amino acid intake on the maintenance of cognitive function have yet to be clarified. We examined the longitudinal association between dietary amino acid intake and cognitive function in community-dwelling older adults.
Design
Longitudinal epidemiological study.
Setting
Community-based setting.
Participants
This study comprised 427 study participants aged 60–82 years with no cognitive decline, defined as a Mini-Mental State Examination (MMSE) score of >27 at baseline, who also participated in a follow-up. The average and standard deviation of the follow-up period was 8.2 ± 0.3 years.
Measurements
Dietary intake was assessed using three-day dietary records at baseline. Participants were classified into quartiles (Q1–Q4) based on the intake of 19 amino acids for males and females. Next, we classified participants into Q1 and Q2–Q4 groups. Cognitive function was assessed using the MMSE both at baseline and at follow-up. Multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the associations between the Q1 group and cognitive decline (MMSE ≤;27), using the Q2–Q4 group as a reference group. Covariates were age, sex, body mass index, years of education, severity of depressive symptoms, history of lifestyle diseases (hypertension, dyslipidemia, diabetes mellitus, stroke, and ischemic heart disease), energy intake (kcal/d), protein intake (g/d), and MMSE score at baseline.
Results
Cognitive decline was present in 133 (31.1%) participants. After adjustment for covariates, including total protein intake, the ORs (95% CIs) for cognitive decline were 2.40 (1.21–4.75) for lysine, 2.05 (1.02–4.09) for phenylalanine, 2.18 (1.09–4.34) for threonine, and 2.10 (1.06–4.15) for alanine.
Conclusion
The results suggest that lysine, phenylalanine, threonine, and alanine intake is important for the maintenance of cognitive function in older people, independent of total protein intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been reported that there are 47 million cases of Alzheimer’s disease (AD) and related dementia worldwide and, with an aging population, this number is expected to increase to >131 million by 2050 (1). AD has a long preclinical phase, as neuronal and neurobiological degeneration can occur for several years before noticeable symptoms of cognitive decline are apparent (2). Therefore, precautions against cognitive decline should be taken at an earlier stage.
Lifestyle factors may have an effect on this long-term neurobiological degeneration that occurs prior to the onset of noticeable AD symptoms, and patients with lifestyle diseases such as diabetes mellitus and cardiovascular disease have been reported to have a high incidence of dementia (3). A person’s dietary habits can be a major preventive factor in relation to lifestyle diseases and dementia and, among dietary factors, daily protein intake has been shown to be important for maintaining cognitive function in older adults (4-6). A recent study reported that older people with high protein intake were found to have had low amyloid-β accumulation in their brains (7). This positive association may be due to specific amino acid intake required for neurotransmitter synthesis (8, 9). This is because adequate quality dietary protein, evaluated by a balance in essential amino acids including amino acids synthesizing neurotransmitters, involves higher utilization of amino acids in the body, while inadequate quality dietary protein involves lower utilization of amino acids in the body, and therefore, more protein must be consumed (10). Considering these previous findings, a lack of specific dietary amino acids may be related to cognitive decline. However, the effects of specific amino acid intake or protein quality on cognitive function have yet to be clarified. Therefore, we aimed to explore the longitudinal association between dietary amino acid intake and cognitive decline in community-dwelling older adults.
Participants and Methods
Study participants
We used data derived from the National Institute for Longevity Sciences — Longitudinal Study of Aging (NILS-LSA), a community-based study involving community-dwelling older adults (11). NILS-LSA participants were recruited from a population aged >40 years in Obu City and Higashiura Town in Aichi Prefecture, Japan, using stratified random sampling according to age and sex. Participants were followed up every two years from the first wave (between November 1997 and April 2000) to the seventh wave (between July 2010 and July 2012).
In this study, we selected participants who were aged ≥60 years at the time of the third wave (between May 2002 and May 2004, defined as the baseline study) and who had also participated in the seventh wave (defined as the follow-up study), because the Mini-Mental State Examination (MMSE) scores (explained below) were available for those aged ≥60 years in the NILS-LSA database. There were two reasons for setting the third wave as the baseline. First, few cognitive function measurements were taken in the first wave. Second, there was a reduction of meat consumption due to the British bovine spongiform encephalopathy (BSE) outbreak in some countries, including Japan, during the second wave (between April 2000 and May 2002) (12). Meat is the main source of amino acids, and Japanese meat consumption had been affected due to the BSE outbreak; therefore, the second wave was considered to be unsuitable to evaluate the usual dietary intake.
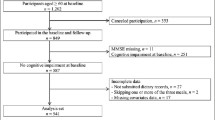
In total, 1202 participants met the age requirements for the baseline study and, of these, 626 participants partook in the follow-up study. Participants who exhibited cognitive decline at baseline (defined as having an MMSE score of ≤27 (13–15), [n = 165]) or those who had not had their cognitive function assessed (n = 8) were excluded. Participants who had not submitted dietary records (n = 15) or those with missing data for covariates (n = 11) were also excluded. Finally, 427 participants (mean ± standard deviation age, 67.2 ± 5.4 years; males, n = 205; females, n = 222) were analyzed in this study (Figure 1).
Participant flowchart
Participants who met the age requirements for the baseline study (n = 1202) and who also participated in the follow-up study (n = 626) were included. Those with cognitive decline at baseline (n = 165) or those who had not had their cognitive function assessed (n = 8) were excluded. We also excluded participants that had not submitted dietary records (n = 15) or had missing data for covariates (n = 11). Finally, data from 427 participants were analyzed in this study.
Ethical considerations
All participants provided written informed consent before study participation. The study was conducted in accordance with the Declaration of Helsinki, and the Ethics Committee of Human Research at the National Center for Geriatrics and Gerontology, Japan (No. 1115-3), and the Ethics Committee of Ajinomoto Co., Inc. (No. 2017-032) approved the study protocol.
Dietary assessment
Dietary intake was assessed using a three-day dietary record after participation in the baseline study. Dietary records were recorded for three continuous days (two weekdays and one weekend day), and participants completed the records at home and returned them within a month (16). For the dietary record, each food item was weighed using kitchen scales (1-kg kitchen scales; Sekisui Jushi, Tokyo, Japan) before being cooked. Simultaneously, a disposable camera (27 shots; Fuji Film, Tokyo, Japan) was used to take photos of meals before and after eating. These photos were used by dietitians to help complete food consumption estimates for data missing from the dietary records of participants. Dietitians telephoned participants to resolve any discrepancies or to obtain further information when necessary. Daily mean nutrients, including amino acids, and energy intake were calculated from the average intake derived from the three-day dietary records according to “The Standard Tables of Foods Composition in Japan” (17). Participants were classified into quartiles (Q1–Q4) based on their intake of 19 amino acids at baseline, and this was determined separately for males and females.
Assessment of cognitive function
Cognitive function was assessed using the Japanese version of the MMSE (18), which was implemented by a trained psychologist or by psychology graduate students both at baseline and at follow-up. The MMSE was developed in 1975 (19) and has been used worldwide in clinical practice and research (20). The MMSE score ranges from 0 to 30, with higher scores indicating better cognitive function. An MMSE score of ≤27 was used as the cut-off of cognitive decline in this study. Recent studies have shown that an MMSE score of ≤27 is indicative of mild cognitive impairment (MCI), with a sensitivity of 45%–60% and a specificity of 65%–90% (13–15). Given developing preventive measures for cognitive decline from an early stage is desirable, we chose a cut-off MMSE score of 27.
The participants were classified into the cognitive decline group if the MMSE score was ≤27 at follow-up, despite an MMSE score of >27 at baseline.
Other measurements
The body mass index (BMI) was calculated as weight (kg) divided by height (m) squared using anthropometric data. The participants’ medical history in terms of hypertension, ischemic heart disease, dyslipidemia, diabetes mellitus, and stroke and years of education were assessed using self-reported questionnaires. The severity of depressive symptoms was assessed using the self-reported Center for Epidemiologic Studies Depression Scale (CES-D, range: 0–60, with higher scores indicating more severe depression) (21). These variables were extracted at baseline and used as covariates.
Statistical analysis
The baseline characteristics of the non-cognitive decline group and the cognitive decline group at follow-up (i.e., after 8 years) were compared using χ2 tests or t-tests. Participants were classified into the following two groups based on their amino acid intake: quartile 1 (Q1) and quartiles 2 to 4 (Q2–Q4). The baseline amino acid intake concerning groups Q1 and Q2–Q4 are described using descriptive statistics for males and females. Multivariable logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for associations between amino acid intake and cognitive decline using the Q2–Q4 group as the reference group. Model 1 was adjusted for age, sex, BMI, years of education, CES-D score (as a continuous variable), history of lifestyle diseases (hypertension, dyslipidemia, diabetes mellitus, stroke, and ischemic heart disease), and MMSE score (as a continuous variable) at baseline; Model 2 was adjusted for baseline total energy intake (kcal/d) in addition to the variables in Model 1; and, Model 3 was adjusted for baseline total protein intake (g/d) in addition to the variables in Model 1. The covariates added were factors previously reported to affect cognitive function (3). After these statistical analyses, we additionally analyzed the association between amino acids and food by Pearson correlation analysis, to investigate what food intake was related to amino acid intake.
Statistical analyses were performed using Statistical Analysis System version 9.3 (SAS Institute, Cary, NC, USA). Statistical significance was indicated using two-sided P-values set at <0.05. The multiplicity was not adjusted. The effects of the amino acids were assessed using both the OR estimates and the statistical results; because the sample size was not based on a verification undertaken in this epidemiological study.
Results
Of 427 participants, the mean ± standard deviation (SD) follow-up period was 8.2 ± 0.3 years, and 48.0% of participants were male. The mean ± SD for age at baseline was 67.1 ± 5.2 years for males and 67.3 ± 5.6 years for females. The mean ± SD of the MMSE score at baseline was 29.0 ± 0.8.
Table 1 shows the baseline characteristics of the non-cognitive decline and cognitive decline groups after 8 years. Of 427 participants, 133 (31.1%) showed a cognitive decline at follow-up study. There was a significantly lower MMSE score at baseline in the cognitive decline group (28.9 ± 0.8) than in the non-cognitive decline group (29.0 ± 0.8; P =0.043). Supplemental Table 1 shows the baseline amino acid intakes of the non-cognitive decline and cognitive decline groups after 8 years.
Table 2 shows the protein intake (g/d) and amino acid intake (mg/d) of the Q1 and Q2–Q4 groups at baseline. The median, maximum, and minimum values in each group were used to examine the range of amino acid intake in the two groups. Of the 19 amino acids, glutamic acid was the most consumed, and hydroxyproline was the least consumed. The intake of all amino acids was higher in males than in females.
Table 3 shows the multivariable-adjusted associations between the intake of the 19 amino acids and the risk of cognitive decline. There were significant associations between the intakes of four amino acids and cognitive decline after 8 years. After adjustment for potential covariates and total protein intake, the adjusted ORs (95% CIs) for cognitive decline were 2.40 (1.21–1.75) for lysine, 2.05 (1.02–4.09) for phenylalanine, 2.18 (1.09–4.34) for threonine, and 2.10 (1.06–4.15) for alanine when comparing the Q1 and Q2–Q4 groups.
Discussion
To our knowledge, this longitudinal study is the first epidemiological study to show that a low intake of lysine, phenylalanine, threonine, and alanine lead to cognitive decline, independent of total protein intake, in community-dwelling older adults. In this study, an MMSE score of ≤27 was used as the cut-off to indicate cognitive decline. An MMSE cut-off score of ≤23 is usually used to detect suspected dementia (20). One systematic review reported that the annual progression rates from MCI to AD ranged from 5.4% to 11.5% per person/year in community settings (22). Given that people with MCI have a high risk of dementia (23), preventive measures for cognitive decline should ideally be implemented from an early stage. Considering that the NILS-LSA is a longitudinal nutrition epidemiological study for local residents, a cut-off MMSE score of 27/28 was considered reasonable.
Our results observed that a lower proportion of lysine, phenylalanine, threonine, and alanine contained in the total protein was associated with cognitive decline, even when an individual’s protein intake is the same. These findings suggest that people who had lower intake of four amino acids are at risk of future cognitive function decline, even if their daily protein intake was sufficient. Neurobiological changes have a long latent phase prior to cognitive symptoms becoming noticeable (2). The intake of proteins that are abundant in these four amino acids may affect cognitive decline, especially if this is started as early as possible.
The adjusted ORs of cognitive decline were ≥2.0 for the low intakes of lysine, phenylalanine, threonine, and alanine. One recent review showed that the relative risk of potentially modifiable risk factors for dementia, such as hypertension and diabetes mellitus, were 1.4–1.9 (3), and our results were comparable to these risk ratios.
The association between these specific amino acids and cognitive function could be explained in several ways. Lysine is an essential amino acid and is transported through the blood-brain barrier (24). Lysine deficiency suppresses growth hormone secretion (25), and a growth hormone decrease leads to cognitive decline (26). Our findings suggest that low lysine intake was associated with cognitive decline after 8 years in older adults. In a sub-analysis of the present study (data not shown), a moderate correlation was observed between lysine intake and seafood consumption (r = 0.575) and meat consumption (r = 0.357). One previous study suggested that a dietary pattern of low rice intake is associated with a reduced risk of dementia in the general Japanese population (27). Lysine is a first-limiting amino acid in some cereal grains, such as wheat and rice (28), and lysine insufficiency is likely to occur when protein sources are biased towards such cereal grains.
Phenylalanine is a precursor for tyrosine and the neurotransmitters, dopamine, norepinephrine, and epinephrine (29). Phenylalanine can pass through the blood-brain barrier into the brain (30), and cannot be synthesized in the body; therefore, people need to consume it in their daily diet. In patients with AD, plasma phenylalanine levels have been found to be decreased (31) and increased in the cerebrospinal fluid (32, 33). Our findings suggest that low phenylalanine intake was associated with cognitive decline after 8 years in older adults. We found a moderate correlation between phenylalanine intake and the consumption of pulses including soybeans (r = 0.393), seafood (r = 0.458), and dairy products (r = 0.363). A previous study reported that a high intake of soybean, soy products, and dairy products reduced the risk of dementia (27). Therefore, a higher phenylalanine intake from these foods may be important for maintaining cognitive function in older adults.
Threonine is an essential amino acid that also crosses the blood-brain barrier (34). In the present study, low threonine intake was associated with cognitive decline after 8 years in older adults. A moderate correlation was observed between threonine intake and seafood consumption (r = 0.550). However, no studies have clarified the relationship between threonine and cognitive function; therefore, further research is needed.
Alanine is one of the glucogenic amino acids that is required for gluconeogenesis. In the present study, low alanine intake was associated with cognitive decline after 8 years in older adults. There was a moderate correlation between alanine intake and consumption of seafood (r = 0.603) and meat (r = 0.373). However, there is a lack of research investigating the effect of alanine intake on cognitive function, and future studies are therefore needed.
Our findings and those of several studies on dietary patterns (27, 35, 36) suggest that diet quality, such as a high dietary diversity, may be important to prevent cognitive decline. In this longitudinal study, a low intake of specific amino acids that was associated with cognitive decline was related to low consumption of seafood, meat, dairy products, and pulses. These foods contain high-quality protein, being rich in many kinds of amino acids including essential amino acids (28). Our previous study showed that people with higher dietary diversity consumed more seafood, dairy products, and pulses (35). Therefore, increasing the intake of lysine, phenylalanine, threonine, and alanine through the consumption of various foods, including high-quality protein foods, could help to maintain cognitive function in older people.
This study had some limitations. First, only participants who were able to participate in both the baseline study and the follow-up study were selected, and those who dropped out during the study were excluded. Therefore, the participants may be biased towards healthier people who could continue their participation in the NILS-LSA survey. However, the food intake of participants was similar to that reported by the National Health and Nutrition Survey (37), which was conducted by the Japanese government and, therefore, reflects the actual food intake among community-dwelling Japanese people. Second, amino acid intake was only assessed at baseline. Dietary habits may change frequently as a result of factors related to aging (38). Third, a definitive diagnosis of dementia could not be made because cognitive function was only assessed using the MMSE. Fourth, the association between amino acid intake and cognitive decline may have been affected by other nutrients because these amino acids are also related to several other food intakes including seafood. Particularly, seafood was moderately correlated with all four amino acids associated with cognitive decline in this study. A previous review showed that higher consumption of fish rich in omega-3 fatty acids can prevent dementia (39). However, we did not evaluate the effect of other nutrients in this study.
In conclusion, our study findings indicated that low intakes of lysine, phenylalanine, threonine, and alanine were associated with cognitive decline, following multivariate adjustments including total protein intake, after 8 years in community-dwelling older people. Specific amino acid intake could be important, independent of total protein intake, for maintaining cognitive functioning in older people.
Abbreviations
- AD:
-
Alzheimer’s disease
- BMI:
-
body mass index
- BSE:
-
bovine spongiform encephalopathy
- CES-D:
-
Center for Epidemiologic Studies Depression Scale
- CI:
-
confidence interval
- MCI:
-
mild cognitive impairment
- MMSE:
-
Mini-Mental State Examination
- NILS-LSA:
-
National Institute for Longevity Sciences — Longitudinal Study of Aging
- OR:
-
odds ratio
- Q:
-
quartiles
- SD:
-
standard deviation
References
Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future, 2016.
Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology 2005;19:520–531.
Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734.
Nes M, Sem SW, Rousseau B, Bjorneboe GE, Engedal K, Trygg K, Pedersen JI. Dietary intakes and nutritional status of old people with dementia living at home in Oslo. Eur J ClinNutr 1988;42:581–593.
Thomas DE, Chung AOKO, Dickerson JW, Tidmarsh SF, Shaw DM. Tryptophan and nutritional status of patients with senile dementia. Psychol Med 1986;16:297–305.
Roberts RO, Roberts LA, Geda YE, Cha RH, Pankratz VS, O’Connor HM, Knopman DS, Petersen RC. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimers Dis 2012;32:329–339.
Fernando W, Rainey-Smith SR, Gardener SL, Villemagne VL, Burnham SC, Macaulay SL et al. Associations of Dietary Protein and Fiber Intake with Brain and Blood Amyloid-beta. J Alzheimers Dis 2018;61:1589–1598.
van de Rest O, van der Zwaluw NL, de Groot LC. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids 2013;45:1035–1045.
Griffin JW, Bradshaw PC. Amino Acid Catabolism in Alzheimer’s Disease Brain: Friend or Foe? Oxid Med Cell Longev 2017;2017:5472792.
FAO. Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAO Expert Consultation. FAO Food Nutr Pap 2013;92:1–66.
Shimokata H, Ando F, Niino N. A new comprehensive study on aging—the National Institute for Longevity Sciences, Longitudinal Study of Aging (NILS-LSA). J Epidemiol 2000; 10(1 Suppl):S1–9.
Matthews D. BSE: a global update. J Appl Microbiol 2003 94 Suppl: 120s–125s.
Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry 2006;14:900–910.
Saxton J, Morrow L, Eschman A, Archer G, Luther J, Zuccolotto A. Computer assessment of mild cognitive impairment. Postgrad Med 2009;121:177–185.
Kaufer DI, Williams CS, Braaten AJ, Gill K, Zimmerman S, Sloane PD. Cognitive screening for dementia and mild cognitive impairment in assisted living: comparison of 3 tests. J Am Med Dir Assoc 2008;9:586–593.
Imai T, Sakai S, Mori K, Ando F, Niino N, Shimokata H. Nutritional assessments of 3-day dietary records in National Institute for Longevity Sciences—Longitudinal Study of Aging (NILS-LSA). J Epidemiol 2000; 10(1 Suppl): S70–76.
Ministry of education culture, sports, science and technology-Japan. Standard Tables of Food Composition Japan-2015- (Seventh Revised Version), https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm Accessed 18 May 2020
Mori E, Mitani Y, Yamadori A. Usefulness of a Japanese Version of the Mini-Mental State Test in Neurological Patients. Jpn J Neuropsychol 1985;1:82–90.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198.
Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res 2009;43:411–431.
Radioff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1977;01:385–401.
Ward A, Tardiff S, Dye C, Arrighi HM. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: a systematic review of the literature. Dement Geriatr Cogn Dis Extra 2013;3:320–332.
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308.
Hawkins RA, O’Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr 2006; 136(1 Suppl):218s–226s.
Ishida A, Nakashima K, Kyoya T, Katsumata M. Compensatory growth of C2C12 myotubes induced by the combined effect of lysine sufficiency and modulation of IGF-I and glucocorticoid levels. Biosci Biotechnol Biochem 2013;77:2302–2304.
Hersch EC, Merriam GR. Growth hormone (GH)-releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus? Clin Interv Aging 2008;3:121–129.
Ozawa M, Ninomiya T, Ohara T, Doi Y, Uchida K, Shirota T, Yonemoto K, Kitazono T, Kiyohara Y. Dietary patterns and risk of dementia in an elderly Japanese population: the Hisayama Study. Am J Clin Nutr 2013;97:1076–1082.
FAO, 2013. Dietary protein quality evaluation in human nutrition. Report of an FAQ Expert Consultation. FAO Food and Nutrition Paper No. 92. Rome, http://www.fao.org/ag/humannutrition/35978-02317b979a686a57aa4593304ffc17f06.pdf. Accessed 18 May 2020
Fernstrom JD, Fernstrom MH. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J Nutr 2007; 137(6 Suppl 1): 1539S–1547S.
Fernstrom JD. Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids 2013;45:419–430.
Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL. Metabolite profiling for the identification of altered metabolic pathways in Alzheimer’s disease. J Pharm Biomed Anal 2015;107:75–81.
Xu J, Begley P, Church SJ, Patassini S, Hollywood KA, Jullig M, Curtis MA, Waldvogel HJ, Faull RL, Unwin RD, Cooper GJ. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim Biophys Acta 2016;1862:1084–1092.
Nilsen LH, Witter MP, Sonnewald U. Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J Cereb Blood Flow Metab 2014;34:906–914.
Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr 2000;130(4S Suppl): 1016S–1022S.
Otsuka R, Nishita Y, Tange C, Tomida M, Kato Y, Nakamoto M, Imai T, Ando F, Shimokata H. Dietary diversity decreases the risk of cognitive decline among Japanese older adults. Geriatr Gerontol Int 2017;17:937–944.
Yin Z, Fei Z, Qiu C, Brasher MS, Kraus VB, Zhao W, Shi X, Zeng Y. Dietary Diversity and Cognitive Function among Elderly People: A Population-Based Study. J Nutr Health Aging 2017;21:1089–1094.
Ministry of Health, Labour and Welfare. National Health and Nutrition Survey, https://www.nibiohn.go.jp/eiken/english/research/pdf/nhns2006_outline.pdf. Accessed 18 May 2020
Zhu K, Devine A, Suleska A, Tan CY, Toh CZ, Kerr D, Prince RL. Adequacy and change in nutrient and food intakes with aging in a seven-year cohort study in elderly women. J Nutr Health Aging 2010;14:723–729.
Cederholm T. Fish consumption and omega-3 fatty acid supplementation for prevention or treatment of cognitive decline, dementia or Alzheimer’s disease in older adults — any news? Curr Opin Clin Nutr Metab Care 2017;20:104–109.
Acknowledgments
We thank all participants and all staff in the NILS-LSA for their cooperation and contribution to this study. We would like to thank Editage (https://www.editage.com) for English language editing.
Author information
Authors and Affiliations
Contributions
Author contributions: Kaori Kinoshita conceived the study design, performed the data analysis, interpreted the results, and drafted the initial manuscript. Rei Otsuka collected the data, conceived the study design, interpreted the results, contributed to discussions, and had primary responsibility for the final content. Michihiro Takada conceived the study design, interpreted the results, and contributed to discussions. Masako Tsukamoto-Yasui conceived the study design. Yukiko Nishita, Chikako Tange, and Makiko Tomida collected the data, interpreted the results, and contributed to discussions. Hiroshi Shimokata designed the NILS-LSA, interpreted the results, contributed to discussions. Masafumi Kuzuya supervised the study, interpreted the results, and contributed to discussions. Akira Imaizumi conceived the study design and interpreted the results. Hidenori Arai supervised the study, conceived the study design, interpreted the results, and contributed to discussions. All authors critically revised the manuscript for intellectual content and approved the final manuscript..
Corresponding author
Ethics declarations
Conflicts of interest: The authors have read the journal’s policy and report the following conflicts of interest: Michihiro Takada, Akira Imaizumi, and Masako Tsukamoto-Yasui are employees of Ajinomoto Co., Inc., and Kaori Kinoshita, Rei Otsuka, and Hidenori Arai have received grants from Ajinomoto Co., Inc. Kaori Kinoshita, Rei Otsuka, Michihiro Takada, Masako Tsukamoto-Yasui, Akira Imaizumi, and Hidenori Arai have applied for patents of “Food evaluation methods for cognitive function”.
Conflicts of interest: Michihiro Takada, Akira Imaizumi, and Masako Tsukamoto-Yasui are employees of Ajinomoto Co., Inc., Kaori Kinoshita, Rei Otsuka, and Hidenori Arai received grants from Ajinomoto Co., Inc. Kaori Kinoshita, Rei Otsuka, Michihiro Takada, Masako Tsukamoto-Yasui, Akira Imaizumi, and Hidenori Arai have applied for patents for “Food evaluation methods for cognitive function”.
Additional information
Sources of Support: This study was supported in part by grants from Ajinomoto Co., Inc., and Research Funding for Longevity Sciences from the National Center for Geriatrics and Gerontology, Japan (grant number 28–40, 19–10).
Electronic Supplementary Material
SUPPLEMENTAL TABLE 1
Baseline amino acid intakes of the non-cognitive decline and cognitive decline groups after 8 years
Rights and permissions
About this article
Cite this article
Kinoshita, K., Otsuka, R., Takada, M. et al. The Association between Dietary Amino Acid Intake and Cognitive Decline 8 Years Later in Japanese Community-Dwelling Older Adults. J Nutr Health Aging 25, 165–171 (2021). https://doi.org/10.1007/s12603-020-1470-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-020-1470-9