Abstract
Background
Recent studies have shown that hyperlipidemia is closely related to the progression of kidney disease and glomerulosclerosis has similar pathophysiological mechanisms with atherosclerosis. Atherosclerosis is essentially a chronic inflammatory process and various kidney diseases are characterized by a micro-inflammatory state. Hyperlipidemia levels are not parallel to the degree of glomerulosclerosis, inflammatory factors together with lipids may contribute to the pathogenesis of glomerulosclerosis. Therefore, it is key to clarify lipid-mediated renal injury through studying the mechanism by which inflammation affects cholesterol homeostasis at the cellular level. Intracellular lipid homeostasis involves both lipid uptake and excretion, therefore in this study, we aimed to explore whether interleukin-1β (IL-1β) promotes the uptake of oxidized low-density lipoprotein (Ox-LDL) to increase in intracellular lipid levels, and to clarify the effect of IL-1β on the expression of lectin-like oxidized LDL receptor 1 (LOX-1) and ATP-binding cassette transporter A1 (ABCA1), which may regulate cholesterol homeostasis in human mesangial cells (HMCs).
Methods
The effect of IL-1β on uptake of Ox-LDL labeled with fluorescent Dil (Dil-Ox-LDL) by HMCs was observed using laser confocal microscopy. The effect of IL-1β on LOX-1 and ABCA1 expression in HMCs was detected by polymerase chain reaction and western blotting.
Results
Laser confocal microscopy revealed that HMCs took up Dil-Ox-LDL. Treatment of HMCs with 5 ng/ml IL-1β for 24 h significantly increased uptake of Dil-Ox-LDL. IL-1β also promoted LOX-1 mRNA and protein expression in a dose-dependent manner. Moreover, ABCA1 mRNA and protein expression were reduced by IL-1β in lipid-loaded HMCs in a dose-dependent manner.
Conclusions
IL-1β promotes the uptake of Ox-LDL and expression of LOX-1 in HMCs, whereas it inhibits expression of ABCA1 under lipid load. The imbalance in intracellular cholesterol resulted by IL-1β can in turn transform HMCs into foam cells and aggravate glomerulosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Clinical studies have shown that lipid metabolism abnormalities are observed in many kidney diseases, additionally, hyperlipidemia itself participates in the progression of kidney diseases (1). Accumulating evidence has demonstrated that glomerulosclerosis and atherosclerosis have similar pathophysiological mechanisms (2). Atherosclerosis is chronic inflammation process of the vascular wall essentially (3), inflammatory factors may participate in the formation of atherosclerosis together with lipids. Hyperlipidemia levels are not parallel to the degree of glomerulosclerosis and various kidney diseases are characterized by a micro-inflammatory state (4), analogously, inflammatory factors may contribute to the pathogenesis of glomerulosclerosis along with lipids. Therefore, it is crucial to clarify lipid-mediated atherosclerosis and renal injury through studying the mechanism by which inflammation affects cholesterol homeostasis at the cellular level (5).
Previous studies have revealed that oxidized low-density lipoprotein (Ox-LDL) is an important risk factor in cardiovascular events and plays a critical role in atherosclerosis. The lectin-like oxidized low-density lipoprotein receptor (LOX-1) is a novel and major Ox-LDL receptor. Recent studies have demonstrated that LOX-1 plays a key role in the development and progression of atherosclerosis (6).
Intracellular lipid homeostasis involves both lipid uptake and excretion, the ATP-binding cassette transporter A1 (ABCA1) is a member of the ATP-binding box transporter superfamily and plays an important role in the initial steps of cholesterol transport and high-density lipoprotein (HDL) production. ABCA1 performs an anti-early atherosclerosis function and inhibits the formation of atherosclerotic plaques (7).
Only few relevant reports exist regarding the mechanism by which inflammatory factors affect the cholesterol balance in HMCs. This study explored the effects of IL-1β on LOX-1 and ABCA1 expression in HMCs homeostasis of intracellular cholesterol, and possible interventions that might affect receptor expression and delay the progression of glomerulosclerosis.
Methods
Materials
Fetal calf serum (FCS) and RPMI-1640 culture solution (Gibco, Gaithersburg, MD, USA), Dil-Ox-LDL (Biomed Technologies, Mt. Arlington, NJ, USA), Ox-LDL and HMCs (Basic Institute of Peking Union Medical College, Beijing, China), IL-1β and LOX-1 antibody (R&D, Abingdon, UK), and ABCA1 antibody (Novus biologicals, Fullerton, CA, U.S.A) were used. PCR system, reverse transcriptase buffer and dNTPs (Promega, Madison, WI, USA), Oligo(dT) primers (Sangon Biotech, Shanghai, China) were used. The primer sequences were as follows: LOX 1 forward, 5′-ACAGAGGCCATTCCGAAATCA-3′; reverse, 5′-GGTAGAGTCTGGAGATGGACCACA-3′;ABCA1 forward, 5′-ATTCGCTCTGAGATGAGCACCA-3′; reverse, 5′-TTTCAAGCGGGCATAGAACCA-3′;GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′; reverse, 5′-ATGGTGGTGAAGACGCCAGT-3′.
Culture and identification of HMCs
HMCs were cultured in RPMI 1640 cell culture medium with 10% FCS in an incubator at 37°C and 5% CO2. HMCs were washed with 0.01 M PBS, digested with 0.25% trypsin/0.025% EDTA for 1 min, and subcultured by suspension in RPMI 1640 culture medium with 10% FCS. HMCs were identified using an indirect immunofluorescence assay in which cytoplasmic actin and collagen IV staining were positive and cytokeratin and Factor VIII staining were negative.
Confocal laser scanning microscopy
HMCs (5×104/mL) were inoculated into 8-well plates and were cultured to quiescent state, then 5 ng/mL IL-1β+0.2% RPMI 1640 was added for continuous culture for 24 hours. After 10 µg/mL Dil-Ox-LDL added for continuous incubation for 5 hours, culture plates were washed with PBS. Slides were fixed with 5% formalin for 1 hour and sealed with 90% glycerol. Under a confocal laser scanning microscope, 5–8 fields were randomly selected per well, and the average fluorescence value per unit area of 30 cells was calculated.
Reverse transcription quantitative polymerase chain reaction (RT qPCR) analysis
After culturing to the quiescent state, HMCs were randomly divided into four groups and were harvested at 12 hours after culturing in media containing 0, 2.5, 5, and 10 ng/mL IL-1β. Total RNA was extracted and cDNA was synthesized. PCR amplification was performed in a 25-µL reaction system using the respective LOX-1 primers, for 50 cycles at 95°C for 10 seconds, 95°C for 15 seconds, and 60°C for 30 seconds.
After 40µg/mL Ox-LDL pre-incubates HMCs for 24 hours to form lipid loaded, quiescent state cells were randomly divided into four groups and were harvested at 24 hours after culturing in media containing 0, 2.5, 5, and 10 ng/mL IL-1β, Total RNA was extracted and cDNA was synthesized, similarly, PCR amplification was performed using the respective ABCA1 primers for 60 cycles.
Western blotting
Quiescent state HMCs were randomly divided into four groups and were harvested at 24 hours after culturing in media containing 0, 2.5, 5, and 10 ng/mL IL-1β. Each lane of a gel was loaded with 250 µg of the sample protein for electrophoresis, then protein was transferred to a membrane for 1 hour. The membrane was blocked with tris-buffered saline with Tween 20 (TBST)-bovine serum albumin (BSA) buffer overnight, incubated with 0.2 ng/mL LOX-1 primary antibody by slowly shaking for 2 hours at 25°C, washed, incubated with 1:6000 secondary antibody by slowly shaking for 1 hour at 25°C, washed, and subjected to ECL development.
After 40µg/mL Ox-LDL pre-incubates HMCs for 24h to form lipid loaded, quiescent state cells were randomly divided into four groups and were harvested at 24 hours after culturing in media containing 0, 2.5, 5, and 10 ng/mL IL-1β, after electrophoresis, the protein was transferred to a membrane for 4 hours and incubated with l: 600 ABCA1 primary antibody after blocking, similarly, washed, incubated with 1:6000 secondary antibody, washed, and subjected to ECL development.
Statistical Methods
SPSS17.0 was used for data analyses. Measurement data are expressed as means ± standard deviation. The t test was used for pair-wise comparisons of mean values. One-way analysis of variance (ANOVA) was used for comparisons among multiple groups. P < 0.05 was considered statistical significance.
Results
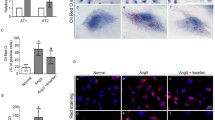
Effect of IL-1β on Dil-Ox-LDL uptake by HMCs as determined by confocal laser scanning microscopy
HMCs in the control group took up a small amount of Dil-Ox-LDL. IL-1β (5 ng/mL) promoted Dil-Ox-LDL uptake by HMCs; the intracellular fluorescence intensity after 24h was 5.03 times that of the control group (Fig. 1 magnification ×400).
Effect of IL-1β on LOX-1 mRNA expression
HMCs expressed LOX-1 mRNA in the basal state, and IL-1β promoted LOX-1 mRNA expression in a dose-dependent manner. IL-1β at 2.5, 5, and 10 ng/mL was used to stimulate the cells for 12 h. LOX-1 mRNA in the 10 ng/mL group rose to a peak that was 6.57 times higher than of the control group. (Fig. 2A)
Effect of IL-1β on LOX-1 protein expression
IL-1β (2.5, 5, and 10 ng/mL) significantly increased LOX-1 protein expression in a dose-dependent manner in HMCs over 24 h. LOX-1 level in the 10 ng/mL group reached a peak 2.57 times higher than that of the control group (Fig. 3A).
Effect of IL-1β on ABCA1 mRNA expression
In lipid loaded HMCs (40µg/mL Ox-LDL pre-incubated cells for 24 hours), IL-1β reduced ABCA1 mRNA expression in a dose-dependent manner. IL-1β at 2.5, 5, and 10 ng/mL was used to stimulate the cells for 24 h, ABCA1 mRNA in the 10ng/ml group decreased most significantly to 14.16% of the baseline (Fig. 2B).
Effect of IL-1β on ABCA1 protein expression
IL-1β (2.5, 5, and 10 ng/mL) reduced ABCA1 protein expression of HMCs after lipid loaded in a dose-dependent manner over 24 h, ABCA1 protein in the 10ng/ml group decreased most significantly to 47.97 % to those of control group (Fig. 3B).
Discussion
Clinical studies have shown that hyperlipidemia is closely related to the progression of kidney disease. Hyperlipidemia is not only a common clinical manifestation of kidney disease, but also an important risk factor for cardiovascular disease in patients with all stages of kidney disease. In addition, abnormal lipid metabolism participates in the progression of kidney disease, and hyperlipidemia itself is also an independent pathogenic factor for the development and progression of glomerulosclerosis.
Hyperlipidemia levels are not parallel to the degree of glomerulosclerosis and increasing evidence has shown that various kidney diseases, such as lupus nephritis, often exhibit a micro-inflammatory state[8]. Therefore, inflammatory factors together with lipids may contribute to the pathogenesis of glomerulosclerosis[9], making it crucial to clarify lipid-mediated renal injury through studying mechanism by which inflammation affects cholesterol homeostasis at the cellular level.
Accumulating studies on the pathogenesis of atherosclerosis have revealed that under physiological conditions, many cells, such as endothelial cells and macrophages, can modulate the expression of receptors of lipid uptake and excretion to maintain intracellular cholesterol homeostasis to avoid excessive accumulation of lipids and prevent the formation of foam cells. Inflammatory factors can impair negative feedback regulation mediated by intracellular cholesterol and increase the uptake of lipids by cells, eventually causing cells to exceed their scavenging capacity and turn into foam cells. The presence of foam cells is a characteristic manifestation of atherosclerosis, which plays an important role in promoting development of atherosclerosis. As glomerulosclerosis and atherosclerosis have similar pathophysiological mechanisms, presence of lipid-loaded cells is also a crucial feature at the early stage of glomerulosclerosis. HMCs can also take up lipid under physiological conditions, thus, presence of foam cells derived from HMCs is analogously an important feature of glomerulosclerosis. Therefore, it is believed that HMCs are closely related to lipid-mediated renal damage. By regulating the expression of receptors, inflammatory factors affect HMCs uptake and lipid degradation, which plays a crucial role in formation of foam cells.
The results of this study prove that HMCs express LOX-1 receptor and take up Ox-LDL in physiological state, and inflammatory factor IL-1β promotes Ox-LDL uptake by HMCs.A variety of Ox-LDL scavenger receptors are expressed on the surface of HMCs; six such receptors have been identified, including scavenger receptor type A[9]. Neverthless, the rate of Ox-LDL uptake by receptors and the role of LOX-1 in lipid uptake by HMCs are unclear.
Ox-LDL is an important risk factor for atherosclerosis and its uptake can be elevated in endothelial cells, vascular smooth muscle cells, and macrophages in arteriosclerosis. LOX-1 is a novel and major Ox-LDL receptor that was first identified in bovine aortic endothelial cells. In arteriosclerosis, LOX-1 can specifically bind to Ox-LDL, leading to activation and damage of endothelial cells, increasing expression adhesion and inflammatory factors, promoting mononuclear macrophages to accumulate, infiltrate, and participate in the formation of atherosclerotic plaques (11). Therefore, LOX-1 is a key factor in the occurrence and development of atherosclerosis. Under inflammatory condition, HMCs can also take up a large amount of Ox-LDL. After intake of Ox-LDL, LOX-1 can induce production of reactive oxygen species, subsequently activating nuclear factory-ϰB and other cytokine to trigger inflammatory response, which plays an important role in promoting development of glomerulosclerosis (12). Consequently in combination with Ox-LDL, LOX-1 not only promotes uptake of lipids by HMCs but also induces expression of inflammatory factors such as transforming growth factor-β1, which then stimulate the proliferation of HMCs and accelerate deterioration of renal disease, suggesting that LOX-1 is closely related to glomerulosclerosis and renal fibrosis (13).
LOX-1 also plays an important role in the progression of chronic kidney disease. Studies about rat model of renal failure with 5/6 nephrectomy found that LOX-1 was significantly increased in the residual kidneys, accompanied by decreased renal function, and ATII receptor antagonists reduced LOX-1 expression while improving renal function (13). Anti-LOX-1 therapy can revert renal enlargement, oxidative stress, and leukocyte infiltration caused by diabetes (14). Additionally, LOX-1 expression is increased in renal tubules of type 2 diabetic rats and those with aggravated renal interstitial damage, suggesting that LOX-1 plays an important role in the development and progression of diabetic nephropathy (15).
The results of this study confirm that in vitro cultured HMCs express LOX-1 and that inflammatory factor IL-1β dose-dependently promotes the expression of LOX-1 mRNA and protein, thereby promoting the uptake of Ox-LDL by HMCs. Therefore, in the presence of IL-1β, the negative feedback regulation mediated by intracellular cholesterol is broken, Ox-LDL is taken up by HMCs even when intracellular cholesterol content is high, possibly leading to the formation of foam cells. This negative feedback loop, once broken, can lead to glomerulosclerosis and deterioration of kidney function, suggesting that inflammatory responses constitute a risk for atherosclerosis and progression of renal disease.
Intracellular lipid homeostasis involves both lipid uptake and excretion, a balance between the two processes is very important. ABCA1, a member of the ABC superfamily that uses ATP as an energy source to promote the release of free cholesterol and phospholipids in cells, plays an important role in the initial steps of reverse cholesterol transport and HDL generation. ABCA1 is expressed on the surface of vascular smooth muscle cells and endothelial cells, and plays an important role in cholesterol excretion, which is crucial for the protective pathogenesis from atherosclerosis (17). Mutation of the ABCA1 gene causes severe HDL-deficient syndrome with clinical manifestations of extensive atherosclerosis and early coronary heart disease, confirming the anti-atherosclerosis effect of ABCA1 (18). ABCA1 not only promotes cholesterol outflow from arterial walls and inhibits the formation of atherosclerotic plaques, but also participates in lipid metabolism in the kidneys. The liver X receptor (LXR-α) agonist can increase the expression of ABCA1 in rabbit mesangial cells and promote cholesterol outflow, indicating that LXR-α is involved in regulation of the balance of renal lipids via the ABCA1 pathway (19). Previous studies have demonstrated that IL-1β inhibits lipid excretion (20). In this study, ABCA1 mRNA and protein expression in lipid-loaded HMCs was reduced by IL-1β in a dose-dependent manner. IL-1β is known to reduce lipid excretion by inhibiting ABCAl expression, resulting in an imbalance of intracellular cholesterol, which leads to formation of foam cell, subsequently exacerbating glomerulosclerosis.
Conclusions
The results of this study prove that IL-1β promotes expression of LOX-1, which in turn promotes Ox-LDL uptake, meanwhile, IL-1β reduces that of ABCA1 in lipid-loaded HMCs, thereby inhibiting lipid excretion. As Intracellular lipid homeostasis involves both lipid uptake and excretion, this study has revealed under inflammatory conditions, IL-1β may change expression of receptors which can regulate the uptake and excretion of lipids, leading to imbalances in intracellular cholesterol levels, resulting in the formation of foam cells and exacerbating progression of chronic kidney diseases. While LOX-1 and ABCA1 may be a new target for the treatment of multiple diseases, our research needs to be further explored. Anti-LOX pathway may prevent inflammatory response and pathological damage induced by Ox-LDL uptake. Meanwhile, protective method of promoting expression of ABCA1 inhibited by IL-1β may help maintain cellular lipid homeostasis, and alleviate kidney damage caused by lipids under inflammatory conditions. Our findings provide a theoretical basis for exploring possible measures toward the prevention and treatment of glomerulosclerosis.
Availability of data and material: All data generated or analyzed during this study are included within the article.
Abbreviations
- IL-1β:
-
interleukin-1β
- Ox-LDL:
-
oxidized low-density lipoprotein
- LOX-1:
-
lectin-like oxidized LDL receptor 1
- ABCA1:
-
ATP-binding cassette transporter A1
- HMCs:
-
human mesangial cells
- Dil-Ox-LDL:
-
Ox-LDL labeled with 1, -dioctadecyl, 3, 3, 3, 3, tetramethylindo-carbocyanine perchlorate
References
Moorhead JF, Chan MK, EI-Nahas M, et a1. Lipid nephrotoxicity in chronic progressive glumerular and tubulointerstitial disease. Lancet, 1982, 2(8311): 1309–1311.
Moorhead JF, Brunton C, Varghese Z. Glomerular atherosclerosis. Miner Electrolyte Metab, 1997, 23: 287–290.
Nasonov EL, Popkova TV. Atherosclerosis: perspectives of anti-inflammatory therapy. Ter Arkh. 2018, 90(5): 4–12.
Wanner C, Zimmermann J, Schwedler S, et al. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl, 2002,80:S99–S102.
Ruan XZ, Varghese Z, Powis SH, et al. Dysregulation of LDL receptor under the influence of inflammatory cytokines: a new pathway for foam cell formation. Kidney Int, 2001, 60: 1716–1725.
Oram JF, HDL apolipoproteins and ABCA1 partners in the removal of excess cellular cholesterol. Arteroscler Thromb Vasc Biol, 2003,23:720–727.
Morawietz H. LOX-1 receptor as a novel target in endothelial dysfunction and atherosclerosis. Dtsch Med Wochenschr, 2010, 135:308–312.
Fu R, Guo C, Wang S, et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol, 2017, 69: 1636–1646.
Ruan XZ, Varghese Z, Moorhead JF, et al. Inflammation modifies lipid-mediated renal injury. Nephrol Dial Transplant, 2003, 18:27–32.
Ruan XZ, Varghese Z, Powis SH, et al. Human mesangial cells express inducible macrophage scavenger receptor. Kidney Int, 1999, 56: 440–451.
Nagase M, Ando K, Nagase T, et al. Redox-sensitive regulation of LOX-1 gene expression in vascular endothelium. Biochem Biophys Res Commun, 2001, 281: 720–725.
Li P, Zhang H, Sheng Z, et al. Role of LOX-1 in oxidized low-density lipoprotein induced expression of transforming growth factor-β 1 in human glomerular mesangial cells. Journal of China Medical University, 2007, 36(1): 23–26.
Kumaz O, Akadam-Teker AB, Yilmaz-Aydogan H, et al. The LOX-1 3′UTRl88CT polymorphism and coronary artery disease in Turkish patients. Mol Biol Rep, 2012, 39(4): 4351–4358.
Ueno T, Kaname S, Takaichi K, et al. LOX-1, an oxidized low-density lipoprotein receptor, was upregulated in the kidneys of chronic renal failure rats. Hypertens Res, 2003, 26(1):117–122.
Yamamoto N, Toyoda M, Abe M, et al. Lectin-like oxidized LDL receptor-1(LOX-1) expression in the tubulointerstitial are likely plays an important role in human diabetic nephropathy. Intern Med, 2009, 48(4): 189–194.
Du Y, Qian J, Song G, et al. Expression of LOX-1 in type 2 diabetic rat tubular interstitial tissues. Chinese Journal of Pathophysiology, 2013, 29(1): 50–55.
Jiang Z, Zhou R, Xu C, et al. Genetic valiation of the ATP-binding cassette transporter A1 and susceptibility to coronary heart disease. Mol Genet Metab, 2011, 103(1): 81–88.
Attie AD, Kastelein JP, Hayden MR, Pivotal role of ABCAl in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res, 2001, 42:1717–1726.
Wu J, Zhang Y, Wang N, et a1. Liver X receptor-alpha mediates cholesterol efflux in glomerular mesangial cells. Am J Physiol Renal Physiol, 2004, 287:F886–F895.
Ruan XZ, Moorhead JF, Fernando R, et al. PPAR agonists protect mesangial cells from interleukin 1beta-induced intracellular lipid accumulation by activating the ABCA1 cholesterol efflux pathway. J Am Soc Nephrol, 2003, 14:593–600.
Acknowledgements
Not applicable.
Funding
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Authors’ contributions: Hua Liu designed the study and wrote the paper; Yinghui Deng did data analysis and cell culture; Leiyun Wu did RT-PCR; Yinping Li did Western blotting; Na Lin did confocal laser scanning; Wen Li did RT-PCR and cell culture; Xingtong Dong did Western blotting and cell culture; Lina Ma designed the study and did data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate: Not applicable.
Additional information
Consent for publication: All data published here are under the consent for publication.
Competing interests: The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Liu, H., Deng, Y., Wu, L. et al. Interleukin-1β Regulates Lipid Homeostasis in Human Glomerular Mesangial Cells. J Nutr Health Aging 24, 246–250 (2020). https://doi.org/10.1007/s12603-019-1302-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-019-1302-y