Abstract
Background
The loss of muscle mass, strength and function associated with increasing age has various health ramifications, including the elevated risk for falls, fractures, frailty, poor quality of life, and mortality. Several studies have confirmed the effects of protein supplementation and RT (resistance training) for this age-related change independently, but whether a combination of the two produces a stronger effect remains controversial.
Objective
This study aims to explore whether a combination of protein supplementation and RT leads to reduction of muscle mass, strength and function in the elderly.
Methods
We retrieved RCTs (randomized controlled trials) reporting the effects of protein supplementation combined with RT on muscle mass, strength and function in the elderly, published before May 2018 through PubMed, MEDLINE, Embase, and manual searches.
Results
Twenty-one RCTs were included, involving 1,249 participants. The results showed that protein supplementation combine with RT significantly enhances the muscle mass and strength of the older adults, where FFM (fat-free mass) increased by 0.23 kg (95% CI: 0.09, 0.38; P=0.002), ASMM (appendicular skeletal muscle mass) by 0.39 kg (95% CI: 0.14, 0.64; P=0.002), handgrip strength by 0.29 kg (95% CI: 0.08, 0.50; P=0.008), knee extension strength by 0.27 kg (95% CI: 0.06, 0.47; P=0.013), leg press strength by 0.33 kg (95% CI: 0.01, 0.64; P=0.04), but no significant effects were seen on muscle function.
Conclusion
Compared to simple RT, protein supplementation combine with RT is more effective in enhancing the muscle mass and strength in the elderly, and the findings do not support the benefit of combination treatment for muscle function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 12% (800 millions) of the world’s population is over the age of 60 and it is expected that by 2050, the number of aging will arrive at 2 billion. This sharp increase has led the health of the elderly to become a major social problem (1). Due to the natural losing of organ function and the accumulation of chronic diseases, single disease has been unable to fully explain the symptoms in the elderly. Therefore, gerontologist raised the term « Geriatric syndrome « to characterize the multifactorial clinical conditions among older adults. Dementia, dizziness, osteoporosis and frailty are the major components of this syndrome, and more than 50% elderly aged over 65 had suffered from the syndrome (2).
The musculoskeletal system is a complicated and multifunctional body composition, involving protection of human organs, maintaining posture and homeostasis (3). Several studies have found that human skeletal muscle mass decreases after 30 years of age, at an average rate of 15% per decade after age 50, and 30% per decade after age 70 (4). It has been argued that as a result of wasting in muscle mass, a significant proportion of older adults will experience decreased muscle strength and muscle function, leading to the development of sarcopenia (5). It is a multifactorial geriatric syndrome, the underlying etiology of it may include but not restricted to: genetic factors, age-related hormonal changes, malnutrition, neuromuscular dysfunction, and chronic low-grade inflammation (6). In elderly over 80 years, the occurrence of sarcopenia comes up to fifty percent or more, as a cornerstone of other geriatric syndrome, it represents an rising incidence of fall, fractures, frailty, poor quality of life, disability and overall mortality (7).
Currently there are no effective therapies in preventing this age-related disease. Pharmacological inventions including vitamin D and androgen supplementations have not led to consistently beneficial effects (8). Clinical trials have confirmed that amino acids have a role in stimulating MPS(muscle protein synthesis), especially leucine, it has been shown as the great MPS benefits among the older and younger adults, through the activation of mTORC1 signaling (9, 10). RCTs have shown a positive relationship between dietary protein supplementation with muscle mass, strength and function in the elderly (11-13). In addition, in the elderly, RT has been shown to foster the production of muscle protein, and reduce the attenuation of muscle strength and muscle mass (14, 15), at present, it has been regarded as the cornerstone to improve muscle mass and strength (16). Therefore, a combination of protein supplementation and RT is considered to produce an amplification effect, as several RCTs have suggested(17-19). But due to the diversity existence in study protocols, populations or methodologies, there also exists inconsistency (20, 21). Indeed, Four meta-analysis have been performed previously (22-25), all of them have shown that protein supplementation during RT promote greater increase in FFM than RT alone (22-24). But based on the existing literatures, it has not been well-defined whether protein supplementation during RT can produce a better effect on the gain in muscle strength and/or muscle function, since Cermak and Morton have demonstrated a favorable effect of protein supplementation on leg-press strength as well as onerepetition- maximum strength separately (23), more strength and functional tests analysis are necessary.
Here, we conduct a meta-analysis to explore whether a combination of protein supplementation with RT is effectively in enhancing muscle mass, strength and function in the elderly in a new perspective, and analyze the changes in the body composition as a supplementary for the first time.
Methods
Search strategy
We searched existing studies through different electronic databases including PubMed, MEDLINE and Embase. The search terms were composed of the following combinations: ‘older adults’ OR ‘older population’ OR ‘older people’ OR ‘elderly’ OR ‘aging’ OR ‘senior’ AND ‘nutrition’ OR ‘protein’ OR ‘amino acid’ AND ‘resistance training’ OR ‘resistance exercise’. To avoid missing any published data, we also checked the reference lists of the retrieved articles. The search was performed by two researchers independently.
Study selection criteria
Articles were included if they met the following criteria: (1) Published between January 2004 and May 2018; (2) The study type is RCT, and the language is limited to English; (3) Type of participants: adults over 50 years; (4) Interventions: we included protein supplementations containing leucine, whey protein, casein, lean meat, low-fat milk or related mixture, compared with placebo including maltodextrin, carbohydrate, lactose or nothing supplemented, both groups undergo RT 1–4 times a week. (5) Outcome measures: muscle mass (FFM, ASMM), muscle strength (hand grip strength, knee extension strength, leg press strength) and muscle function (gait speed, time up and go test, chair rise time, stair climb test, SPPB (Short Physical Performance Battery)). The articles were excluded if they met one of the following: (1) Duplicated publications; (2) Non RCTs, including comments, letters, reviews, meta-analysis, animal studies and pilot studies; (3) Documents with incomplete data; (4) Other supplements known to stimulate muscle synthesis was provide with protein supplementation (e.g., creatine).
Data extraction
Two researchers selected literatures independently according to the established screening criteria, read the full text of the literatures that comply with the inclusion standards, and extracted the following information: (1) The basic information of the study (the name, first author, published time); (2) Baseline characteristics of the study subjects (sample size, age, gender ratio); (3) The implementation sites of the study; (4) The pattern of interventions on EG (experimental group) and CG (control group); (5) The length of the study; (6) Outcome indicators.
For each study, Treatment effects were estimated after the extraction of mean differences (pre-interventions subtracted from the post-interventions) and SDs (Standard Deviations). The changes in SDs were calculated through following equation if the means and SDs of pre and post interventions were available(26): SD change = [(SDpre)2+(SDpost)2-2*cor r(pre,post)*SDpre*SDpost]1/2. We used 0.95 as the correlation coefficients between pre and post interventions. But when the means and 95% CI are shown in the documents, we calculated the SDs changes through following equation(27, 28): SD change = [(UCI-LCI)/3.92]*n1/2 (UCI: upper limit of confidence interval; LCI: lower limit of confidence interval). For the measurement of gait speed, more than one test was provided, including 6-minutes walking test, 300-m walking test. All tests describing walking capacities were transformed into gait speed, it was calculated as the walking distance divided by the time elapsed.
Methodologic quality of included trials
The potential risk of bias was evaluated according to the Cochrane collaboration’s tool, it’s a five domain-based assessment: selection, performance, detection, attrition and reporting bias (29). Two investigators make a judgement of whether the study had a “low risk”, “high risk”, “unclear risk” independently, and the divergent would be resolved by group consultation or invite a third person to assess.
Statistical analysis
Stata12.0 software was used for statistical analysis. The data included in the studies were continuous variables, all extracted data were expressed in means and SDs. We calculated SMD (standardized mean difference), generated forest plots and adopted the random effects model to estimate study-specific effect, all tests were 2-tailed and a P value less than 0.05 was denoted as statistically significant.
The statistical heterogeneity among studies was determined according to the Chi2 test and I2 statistic, with P>0.1 and I2<50% was considered as low heterogeneity, and otherwise it indicates high heterogeneity when the I2 value was higher than 50%, then sensitivity analysis was conducted to investigate the sources of it. Funnel plot was carried out for the inspection of publication bias. The Egger’s test of the included indicators was conducted to test asymmetry, P<0.1 was considered meaningful.
Results
Basic characteristics of the participants
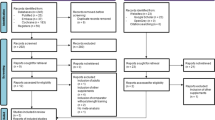
The selection process and detailed characteristics are described in Figure 1 and Table 1 respectively. We identified 1353 potential records in total, and screened the title and abstract of 1144 after duplicates were ruled out. Full-text of 62 articles were assessed for eligibility, and 21 studies (1249 subjects) from 11 countries worldwide were included in the systematic review ultimately (19-21, 30-47). Among these participants, 639 (51.16%) received protein or amino acids products and 610 (48.84%) received either placebo or nothing. All the participants experienced RT 2–4 times in inconsecutive days per week. The majority of the studies included in our research were comprised of both males and females (47.62%), 3 trials (14.29%) were exclusively males (36, 38, 43) and 8 (38.10%) constitute of females only (21, 32, 33, 35, 42, 44-46). Of these, 12 trials (57.14%) were done in the healthy (30-33, 36, 37, 39, 41, 42, 44-46), leaving that sarcopenic in 4 trials (19.05%)(21, 35, 38, 43), obese in 2 trials (9.52%)(20, 47) and frail(40), mobility-limited(19), non-frail(34) in 1 trial separately.
Body composition
We analyzed changes in body composition, including BD (Body Weight), BMI (Body Mass Index), muscle as well as fat mass. Several techniques were applied in body composition assessment, DXA (dual energy x-ray) as the gold standard methods was the most frequently used, 15 of the 21 studies used DXA (19, 20, 30, 31, 33, 34, 36-38, 40, 42-46), except two that used BIA (bioelectrical impedance analysis)(21, 35) and the other two used ADP (air-displacement plethysmography)(32, 47).
Changes in muscle mass
A total of 18 RCTs reported muscle mass changes (19-21, 30, 32-38, 40, 42-47), with a total of 1,039 patients, including 526 (50.63%) in the EG and 513 (49.37%) in the CG. Among which, studies that reported changes in FFM included 13 RCTs (61.90%) in total(19, 30, 32-38, 40, 43, 44, 47), the effect sizes obtained from our studies indicated that greater increases in EG compared with CG, with the SMD being 0.23 kg (95% CI: 0.09, 0.38; P=0.002), the analysis did not revealed significant heterogeneity between studies (I2=3.8%, P=0.409) (Figure 2). A similar result was observed for ASMM (20, 21, 30, 32, 35, 38, 40, 42, 45, 46), with a larger improvement of 0.39 kg (95% CI: 0.14, 0.64; P=0.003) in EG (Figure 2), no evidence of significant heterogeneity between studies was found (I2=45.9%, P=0.055).
Changes in BD, BMI and fat mass
The meta-analysis results in BD, BMI, fat-mass and fat percentage between EG and CG were showed in Figure S1. Compared with the CG, the body weight and BMI did not show any significant difference within the intervention groups. But the individuals assigned to the EG existed a greater reduction in fat-mass (SMD= 0.19 kg, 95% CI: 0.36, 0.01; P=0.04) and fat percentage (SMD= 0.40 kg, 95% CI: 0.69, 0.12; P=0.005).
Muscle strength
Out of seventeen RCTs that were included in exploring muscle strength changes(19-21, 30–34, 39-47), involves 911 participants, 469 (51.48%) in the EG, 442 (48.52%) in the CG. Changes in muscle strength between groups were investigated by measuring the hand grip strength in 6 RCTs (20, 21, 31, 39, 40, 47), knee extension strength in 11 RCTs (19, 21, 30, 31, 33, 40, 42-46) and leg press strength in 5 RCTs(19, 32, 34, 40, 41). The meta-analysis revealed positive effect on muscle strength in the EG, with the handgrip strength increased by 0.29 kg (95% CI: 0.08, 0.50; P=0.008), knee extension strength increased by 0.27 kg (95% CI: 0.06, 0.47; P=0.013) and leg press strength increased by 0.33 kg (95% CI: 0.01, 0.64; P=0.04). All studies did not demonstrate significant heterogeneity (Figure 3).
Muscle function
A variety of methods were used to evaluate muscle function, we pooled the gait speed test in 9 RCTs (19-21, 30, 35, 36, 38, 39, 47), time up and go test in 4 RCTs(30, 33, 38, 41), chair rise test in 7 RCTs (19, 20, 38, 40, 41, 46, 47), and SPPB in 2 RCTs(19, 40), stair climb test (19) in 1 RCT separately, since these are the most common assessment measures in muscle function. Between the two interventions, no significant differences in gait speed(P=0.83), time up and go test(P=0.10), chair rise time(P=0.80), SPPB (P=0.07) and stair climb test were found (Figure 4). The heterogeneity was prominent between the studies. To explore the sources of the heterogeneity, we performed the sensitivity analysis (Figure S2). Once the study “Kim2016” and “Maltais2016” was excluded in the gait speed test, the heterogeneity would be reversed, but the results kept no statistically significant, with the SMD being 0.11 (P= 0.25). Same method was used in the chair rise test, we found that after excluding the literature that caused heterogeneity, the overall result remained stable.
Adverse event
In majority of the studies, serious adverse events were scarce, but several milder events occurred during interventions. Fourteen people fell down in two studies (31, 35), with one incidence of hip fracture. Four studies (8 participants) provided information on back pain (33), knee pain (32, 35), osteoarthritis (33) or physical pain (41) in different degrees. Additionally, Three participants in one study suffered minor injuries related to training (34). One study reported a heart attack and a transient ischemic attack respectively, but it is unclear whether these events were directly related to the interventions (37).
Quality evaluation and Publication bias
Of the 21 studies included, 14/21 trials showed the random allocation sequence generation methods, 15/21 trials mentioned allocation concealment, and 17/21 clarified the incomplete outcome data, 14/21 had blinded the investigators and the participants. Because of the insufficient details, we judged an unclear risk in the blinding of outcome assessment in almost all literatures.
The Egger’s linear regression test for FFM indicated the presence of publication bias in our analysis (t= 3.06, P= 0.01). Although we have adopted broad inclusion criteria and explored various search strategies to obtain more relevant literatures, it is inevitable that some documents may be omitted.
Discussion
In this meta-analysis of RCTs, we gathered over 1200 participants, which is approximately double of the former reviews (22-24). Because of the limited number of studies based on the sarcopenia, we included participants with different baselines, involving sarcopenic, frail, obese and healthy. We evaluated current evidence looking at the combination effect of protein supplementation and RT on the muscle mass, strength and function in older adults. Our analysis showed that protein supplementation combined with RT may enhance muscle mass and strength in the elderly, but for muscle function, no distinct benefit was noted from the intervention during the entire study.
The negative findings in our analysis contrast with the widely held perception that the protein intake and RT would increase muscle function in the older population. Well documented determinants explain the variation in muscle function, including age, gender, race, body-fat, weight and muscle strength (48-50). There is no denying the fact that age is inversely proportional to muscle function. And it has been suggested that comparing with the male and Europeans as well as Americans, female and Africans experience a higher risk and incidence of poor muscle function(50). Previous meta-analysis has shown the connection between higher BMI and relatively high fat percentage with age-related decline of muscle function(51). In addition, the intermuscular fat, which is positively correlated with body fatness, can significantly reduce the function of the elderly, and it is an independent risk factor for muscle function(50). This is consistent with the prior studies that body-fat is an important predictor of the muscle function(52). What’s more, studies have shown that older adults with low muscle strength have a particularly high risk of a decline in walking speed and of developing mobility limitation, and it is well established that muscle strength is a strong predictor of muscle function, it has turned out to be more correlated with functional capacities than muscle mass(53, 54). However, the relationship between muscle mass and muscle function is less known, and unlikely to be linear. Recent cross-sectional studies have found that low muscle mass is associated with poor functional performance, but due to the close relationship of muscle mass and muscle strength, it remains unclear whether this connection is the contribution of muscle mass itself or mediated by muscle strength(50).
Although our analysis has restrictions common to the previous studies (significant heterogeneity, short terms), it also has many strengths. In our analysis, the individual studies were well powered and the number of the participants is large enough to assess the endpoint, as well as the various screening tools used to identify the muscle function. Therefore, the failure of our meta-analysis to show the benefit across the muscle function is likely to be a real finding.
Recent studies show that the strategies toward the decreased muscle mass, strength and function, namely the therapies for sarcopenia, can be achieved through comprehensive approaches. Based on 130 sarcopenic elderly people, Rondanelli and his colleagues verified that nutritional supplementation with whey protein, essential amino acid, vitamin D concurrent with regular, controlled physical activity, could not only boosts the muscle mass and strength, but also enhance muscle function in the sarcopenic elderly(55). Bo et al also demonstrated the combination effect of protein, vitamin D and vitamin E on the sarcopenia(56). In addition, A recent meta-analysis of the results of 32 studies indicate that nutritional treatment, such as a protein and vitamin D rich diet, and perform physical exercise regularly helps to prevent frailty(57). Silva and coworkers have most clearly shown the protective of a Mediterranean diet toward frailty, functional disability and sarcopenia(58). All of these demonstrate that the combination treatment is critical for geriatric syndrome, especially the musculoskeletal disorders.
Several limitations exist in the present meta-analysis. First, due to the diversity of the supplementary products, there was a lack of unified protein supplementary dosage. In our analysis, the EG were supplemented with whey protein (8/21), amino acids (5/21), milk (3/21) and other animal protein or compounds (5/21), and were supplemented in different quantities and frequencies. The ESPEN (European Society of Parenteral Enteral Nutrition) recommends that elderly patients with either acute or chronic conditions should be given 1.0 to 1.2 g/kg protein, half of which must contain high quality protein, and distributed amongst the three meals in a day to achieve maximum MPS rate(59). Second, the duration of the included studies was short, the intervention period ranged from 10 to 72 weeks, and 14 of 21 studies followed-up for 18 weeks or less. Literatures have found that longer training periods (≥24 weeks) are more effective in building muscle strength compared with shorter ones (≤18 weeks)(60, 61). A 7 year follow-up prospective study showed that doing RT for one year, and observation after 7 years, the participants exhibited advantages in muscle strength than the non-trained, but the RT cannot attenuate the age-related muscle weakness, therefore, the studies suggest that the RT should be maintained lifelong(62). Third, we used SMD as the statistic metric, it might be less sensitive in detecting changes in SDs in the studies. Therefore, studies of adequate amount of protein or amino acid intake and longer duration RT is required in the future.
Conclusion
In the present meta-analysis, we demonstrated that when compared with individuals assigned to a resistance training alone, the elderly assigned to a protein supplementation combined with resistance training achieve greater increase in muscle mass and strength, as well as a small but significant decrease in the fat-mass and fat percentage, and the findings do not support the benefit of combination treatment for muscle function. Thus, comprehensive approaches including multiple nutrition supplement and resistance training may be alternative tools against sarcopenia, as well as geriatric syndromes.
Disclosure Statement: Our study was supported by the National Natural Science Foundation of China (No.81870184), Major basic research projects in natural science foundation of shaanxi province (2016ZDJC-13), and National Key R&D Program of China (SQ2018YFC200004-05). All the authors have no conflict of interest.
Author Contributions: The contributions of the authors are as following: Study concept and design: XiaoMing Wang, Acquisition of data: Jie Yang and Xin Jia, Analysis and interpretation of data: Xing Li and Cong Huo, Draft the manuscript: LiMing Hou and YunZhen Lei, Critical revision of the manuscript for important intellectual content: Rong Xu and Study supervision: XiaoMing Wang.
Conflict of interest: None.
Ethical standrad: There is no ethical standard for this study design.
References
World Brain Day 2016: celebrating brain health in an ageing population. DOI:10.1016/ S1474-4422(16)30171-5.
Strandberg TE, Pitkala Kh Fau - Tilvis RS, Tilvis Rs Fau - O’Neill D, et al. Geriatric syndromes—vascular disorders? Ann Med. 2013;45(3):265–273.
DiGirolamo DJ, Clemens Tl Fau - Kousteni S, Kousteni S. The skeleton as an endocrine organ.Nature reviews. Rheumatology. 2018;8(11):674–683.
von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle. 2012;3(4):213–217. DOI:10.1007/s13539-012-0089-z.
Rosenberg IH. Sarcopenia:origins and clinical relevance. J Nutr. 1997;127(5):990S-991S.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. DOI:10.1093/ageing/afq034.
Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29 (1):i44–i48. DOI:10.1093/ fampra/cmr063.
Beaudart C, Dawson A, Shaw SC, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporosis Int. 2017;28(6):1817–1833. DOI:10.1007/s00198-017-3980-9.
Yoshiharu Shimomura YY, Gustavo Bajotto. Branched-Chain Amino Acids in Exercise. Am Soci Nutr. 2006;136:529S–532s.
Borack MS, Volpi E. Efficacy and Safety of Leucine Supplementation in the Elderly. J Nutr. 2016;146(12):2625S–2629S. DOI:10.3945/jn.116.230771.
Ispoglou T, White H, Preston T, et al. Double-blind, placebo-controlled pilot trial of L-Leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65–75 years. Eur J Clin Nutr. 2015;70(2):182–188. DOI:10.1038/ejcn.2015.91.
Solerte SB, Gazzaruso C, Bonacasa R, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101(11A):69E–77E. DOI:10.1016/j. amjcard.2008.03.004.
Cramer JT, Cruz-Jentoft AJ, Landi F, et al. Impacts of High-Protein Oral Nutritional Supplements Among Malnourished Men and Women with Sarcopenia: A Multicenter, Randomized, Double-Blinded, Controlled Trial. J Am Med Dir Assoc. 2016;17(11):1044–1055. DOI:10.1016/j.jamda.2016.08.009.
Greiwe JS, Cheng B, Rubin DC, et al. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2001;15(2):475–482. DOI:10.1096/fj.00-0274com.
Frontera WR, Hughes VA, Krivickas LS, et al. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve. 2003;28(5):601–608. DOI:10.1002/mus.10480.
Kemmler W, Wittke A, Bebenek M, et al. High Intensity Resistance Training Methods with and without Protein Supplementation to Fight Cardiometabolic Risk in Middle-Aged Males: A Randomized Controlled Trial. BioMed research international. 2016;2016:9705287. DOI:10.1155/2016/9705287.
SINGHa MAF. Combined Exercise and Dietary Intervention to Optimize Body Composition in Aging. Ann N Y Acad Sci. 1998;854:378-393.
Aleman-Mateo H, Carreon VR, Macias L, et al. Nutrient-rich dairy proteins improve appendicular skeletal muscle mass and physical performance, and attenuate the loss of muscle strength in older men and women subjects: a single-blind randomized clinical trial. Clin Interv Aging. 2014;9:1517–1525. DOI:10.2147/cia.s67449.
Chale A, Cloutier GJ, Hau C, et al. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2013;68(6):682–690. DOI:10.1093/gerona/gls221.
Verreijen AM, Verlaan S, Engberink MF, et al. A high whey protein–, leucine-, and vitamin D–enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101(2):279–286. DOI:10.3945/ajcn.114.090290.
Kim H, Kim M, Kojima N, et al. Exercise and Nutritional Supplementation on Community-Dwelling Elderly Japanese Women With Sarcopenic Obesity: A Randomized Controlled Trial. J Am Med Dir Assoc. 2016;17(11):1011–1019. DOI:10.1016/j.jamda.2016.06.016.
Finger. DG, F.R., Umpierre, D. Effects of Protein Supplementation in Older Adults Undergoing Resistance Training: A Systematic Review and Meta-Analysis. Sports Med. 2015;45(2):pp 245–255.
Cermak NM, Res PT, de Groot LC, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a metaanalysis. Am J Clin Nutr. 2012;96(6):1454–1464. DOI:10.3945/ajcn.112.037556.
Liao C-D, Tsauo J-Y, Wu Y-T, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106(4):1078–1091. DOI:10.3945/ajcn.116.143594.
Robert W Morton KTM, Sean R McKellar, Brad J Schoenfeld, Menno Henselmans, Eric Helms, Alan A Aragon, Michaela C Devries, Laura Banfield JWK, Stuart M Phillips. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384.
Abrams KR, Gillies CL, Lambert PC. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med. 2005;24(24):3823–3844. DOI:10.1002/ sim.2423.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. DOI:10.1186/1471-2288-5-13.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. DOI:10.1186/1471-2288-14-135.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10):e1–34. DOI:10.1016/j.jclinepi.2009.06.006.
Arnarson A, Gudny GO, Ramel A, et al. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: double blind, randomised controlled trial. Eur J Clin Nutr. 2013;67(8):821–826. DOI:10.1038/ejcn.2013.40.
Bunout. Effects of nutritional supplementation and resistance training on muscle strength in free living elders. Results of one year follow. Journal of nutrition, health & aging. 2004;8(2):68-75.
Candow DG, Chilibeck PD, Facci M, et al. Protein supplementation before and after resistance training in older men. Eur J Appl Physiol. 2006;97(5):548–556. DOI:10.1007/ s00421-006-0223-8.
Daly RM, O’Connell SL, Mundell NL, et al. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am J Clin Nutr. 2014;99(4):899–910. DOI:10.3945/ ajcn.113.064154.
Karelis AD, Messier V, Suppere C, et al. Effect of cysteine-rich whey protein (ImmunocalA (R)) supplementation in combination with resistance training on muscle strength and lean body mass in non-frail elderly subjects: A randomized, double-blind controlled study. J Nutr Health Aging. 2015;19(5):531–536. DOI:10.1007/s12603-015-0442-y.
Kim HK, Suzuki T, Saito K, et al. Effects of Exercise and Amino Acid Supplementation on Body Composition and Physical Function in Community-Dwelling Elderly Japanese Sarcopenic Women: A Randomized Controlled Trial. J Am Geriatr Soc. 2012;60(1):16–23. DOI:10.1111/j.1532-5415.2011.03776.x.
Kukuljan S, Nowson CA, Sanders K, et al. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol. 2009;107(6):1864–1873. DOI:10.1152/japplphysiol.00392.2009.
Leenders M, Verdijk LB, Van der Hoeven L, et al. Protein Supplementation during Resistance-Type Exercise Training in the Elderly. Med Sci Sport Exer. 2013;45(3):542–552. DOI:10.1249/MSS.0b013e318272fcdb.
Maltais ML, Ladouceur JP, Dionne IJ. The Effect of Resistance Training and Different Sources of Postexercise Protein Supplementation on Muscle Mass and Physical Capacity in Sarcopenic Elderly Men. J Strength Cond Res. 2016;30(6):1680–1687. DOI:10.1519/jsc.0000000000001255.
Oesen S, Halper B, Hofmann M, et al. Effects of elastic band resistance training and nutritional supplementation on physical performance of institutionalised elderly — A randomized controlled trial. Exp Gerontol. 2015;72:99–108. DOI:10.1016/j. exger.2015.08.013.
Tieland M, Dirks ML, van der Zwaluw N, et al. Protein Supplementation Increases Muscle Mass Gain During Prolonged Resistance-Type Exercise Training in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J Am Med Dir Assoc. 2012;13(8):713–719. DOI:10.1016/j.jamda.2012.05.020.
Trabal J, Forga M, Leyes P, et al. Effects of free leucine supplementation and resistance training on muscle strength and functional status in older adults: a randomized controlled trial. Clin Interv Aging. 2015;10:713–723. DOI:10.2147/cia. s75271.
Weisgarber. Whey protein and high-volume resistance training in postmenopausal women. J Nutr Health Aging. 2015;19(5):511-517.
Zdzieblik D, Oesser S, Baumstark MW, et al. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Brit J Nutr. 2015;114(8):1237–1245. DOI:10.1017/s0007114515002810.
LT.Rossato PN FM. S. de Branco. Higher Protein Intake Does Not Improve Lean Mass Gain When Compared with RDA Recommendation in Postmenopausal Women Following Resistance Exercise Protocol: A Randomized Clinical Trial. Nutrients. 2017;9(9):1007-1022. DOI:10.3390/nu9091007.
PS Junior AR, HCG. Nabuco Effects of whey protein supplementation associated with resistance training on muscular strength, hypertrophy and muscle quality in preconditioned older women. Int J Sport Nutr Exerc Metab. 2017;1(1-8). DOI:10.1123/ ijsnem.2017-0253.
Hellen C. G. Nabuco CMT, IPaulo Sugihara Junior. Effects of Whey Protein Supplementation Pre- or Post-Resistance Training on Muscle Mass, Muscular Strength, and Functional Capacity in Pre-Conditioned Older Women: A Randomized Clinical Trial. nutrients. 2018;10 (5):pii: E563. DOI:10.3390/nu10050563.
Verreijen AM, Engberink MF, Memelink RG, et al. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutr J. 2017;16(1):10–18. DOI:10.1186/s12937-017-0229-6.
Martin HJ, Syddall HE, Dennison EM, et al. Physical performance and physical activity in older people: are developmental influences important? Gerontology. 2009;55(2):186–193. DOI:10.1159/000174823.
Woods JL, Iuliano-Burns S, King SJ, et al. Poor physical function in elderly women in low-level aged care is related to muscle strength rather than to measures of sarcopenia. Clin Interv Aging. 2011;6:67–76. DOI:10.2147/CIA.S16979.
Marjolein Visser BHG, Stephen B. Kritchevsky, Anne B. Newman, Michael Nevitt, Susan M. Rubin, Eleanor M. Simonsick, Tamara B. Harris,. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. Journal of Gerontology: MEDICAL SCIENCES. 2005;60(3):324–333.
Brady AO, Straight CR, Evans EM. Body Composition, Muscle Capacity, and Physical Function in Older Adults: An Integrated Conceptual Model. Journal of Aging and Physical Activity. 2014;22(3):441–452. DOI:10.1123/japa.2013-0009.
Marjolein Visser JL, Jack M Guralnik, Jane A Cauley, Richard A Kronmal, John Robbins. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study1,2. Am J Clin Nutr. 1998;68:584–590.
Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiologic reviews. 2013;35:51–65. DOI:10.1093/epirev/mxs006.
Koster A, Ding J, Stenholm S, et al. Does the Amount of Fat Mass Predict Age-Related Loss of Lean Mass, Muscle Strength, and Muscle Quality in Older Adults? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A(8):888–895. DOI:10.1093/gerona/glr070.
Rondanelli M, Klersy C, Terracol G, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016;103(3):830–840. DOI:10.3945/ajcn.115.113357.
Bo Y, Liu C, Ji Z, et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin Nutr. 2018. DOI:10.1016/j.clnu.2017.12.020.
Artaza-Artabe I, Saez-Lopez P, Sanchez-Hernandez N, et al. The relationship between nutrition and frailty: Effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas. 2016;93:89–99. DOI:10.1016/j.maturitas.2016.04.009.
Silva R, Pizato N, da Mata F, et al. Mediterranean Diet and Musculoskeletal-Functional Outcomes in Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J Nutr Health Aging. 2018;22(6):655–663. DOI:10.1007/s12603-017-0993-1.
Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. DOI:10.1016/j.clnu.2014.04.007.
Borde R, Hortobagyi T, Granacher U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015;45(12):1693–1720. DOI:10.1007/s40279-015-0385-9.
Silva NL, Oliveira RB, Fleck SJ, et al. Influence of strength training variables on strength gains in adults over 55 years-old: a meta-analysis of dose-response relationships. J Sci Med Sport. 2014;17(3):337–344.
Kennis E, Verschueren SM, Bogaerts A, et al. Long-term impact of strength training on muscle strength characteristics in older adults. Arch Phys Med Rehab. 2013;94(11):2054–2060.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hou, L., Lei, Y., Li, X. et al. Effect of Protein Supplementation Combined With Resistance Training on Muscle Mass, Strength and Function in the Elderly: A Systematic Review and Meta-Analysis. J Nutr Health Aging 23, 451–458 (2019). https://doi.org/10.1007/s12603-019-1181-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-019-1181-2