Abstract
Next-generation microorganisms have recently gained prominence in the scientific community, mainly due to their probiotic and postbiotic potentials. However, there are few studies that investigate these potentials in food allergy models. Therefore, the present study was designed to evaluate the probiotic potential of Akkermansia muciniphila BAA-835 in an ovalbumin food allergy (OVA) model and also analyse possible postbiotic potential. To access the probiotic potential, clinical, immunological, microbiological, and histological parameters were evaluated. In addition, the postbiotic potential was also evaluated by immunological parameters. Treatment with viable A. muciniphila was able to mitigate weight loss and serum levels of IgE and IgG1 anti-OVA in allergic mice. In addition, the ability of the bacteria to reduce the injury of the proximal jejunum, the eosinophil and neutrophil influx, and the levels of eotaxin-1, CXCL1/KC, IL4, IL6, IL9, IL13, IL17, and TNF, was clear. Furthermore, A. muciniphila was able to attenuate dysbiotic signs of food allergy by mitigating Staphylococcus levels and yeast frequency in the gut microbiota. In addition, the administration of the inactivated bacteria attenuated the levels of IgE anti-OVA and eosinophils, indicating its postbiotic effect. Our data demonstrate for the first time that the oral administration of viable and inactivated A. muciniphila BAA-835 promotes a systemic immunomodulatory protective effect in an in vivo model of food allergy to ovalbumin, which suggests its probiotic and postbiotic properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food allergy is defined as an exacerbated activation of the immune system by proteins that would normally be classified as innocuous. These proteins are usually present in eggs, seafood, milk, and peanuts [1]. There are some risk factors for developing food allergy such as ethnicity, genetic factors, some dietary patterns, and a dysbiotic microbiota [2, 3]. Altered patterns in the composition of the intestinal microbiota can culminate in the development of food allergy [3], which emphasizes the importance of the modulation of the microbiota by probiotics. Probiotics can exert their effects on allergic processes by several mechanisms. Among them are the restoration of the Th1/Th2 response, activation of regulatory T cells (Treg), attenuation of specific allergen IgE, and maintenance of tissue integrity of the epithelium [4, 5]. Furthermore, short-chain fatty acids (SCFA) produced by some probiotics by fermentation of dietary fibre may attenuate Th2 responses in asthma and food allergy [6, 7]. Therefore, due to the importance of maintaining the microbial composition, several studies that aimed at its manipulation have been carried out. The most traditional probiotics investigated are Lactobacillus and Bifidobacterium species, generally isolated from fermented dairy products and faecal microbiome [8,9,10]. However, in the last decade, the concept of next-generation probiotic is emerging. These microorganisms were identified using next-generation sequencing techniques and bioinformatic methods. Therefore, they are defined as “live microorganisms identified based on comparative analyses of the microbiota that, when administrated in adequate amounts, confer a health benefit on the host” [11]. Among them, Akkermansia muciniphila has stood out for its beneficial effects [12].
A. muciniphila is a Gram-negative, strict anaerobic, oval-shaped bacterium, initially isolated from a faecal sample of a healthy individual. The intestinal microbiota generally contains 1–4% of A. muciniphila. Reduced levels of the bacteria have been associated with diabetes, obesity, hypertension, liver diseases, intestinal inflammation, ulcerative colitis, and Crohn’s disease [13].
Some studies have shown that A. muciniphila is capable of surviving the simulated conditions of the gastrointestinal tract, a basic condition for considering the microorganism as a potential probiotic [14]. Some studies have pointed out its ability to produce SCFA, stimulate the production of IL10, and increase the levels of Treg cells. In addition, it stimulates the production of antimicrobial peptides and regulates the intestinal barrier integrity [15,16,17]. Furthermore, recent studies have shown that some probiotics are capable of exerting beneficial effects even when inactivated by heat [18]. There is a growing interest in these microorganisms, called postbiotics, mainly because of their ability to increase the shelf life of probiotic products and because they are safer when administered to immunocompromised individuals [19]. There is some evidence that the administration of heat-killed A. muciniphila promoted a significant improvement in murine models of obesity and type 2 diabetes. The authors attribute these beneficial effects to the presence of an outer membrane protein called Amuc_1100. This protein is stable after pasteurization and has been shown to interact with the Toll-like receptor 2 [20]. In addition, it is important to mention that pasteurized A. muciniphila is the first next-generation microorganism that has been approved by the European Food Safety Authority (EFSA) [13, 21].
Therefore, the aim of this study was to evaluate the probiotic and postbiotic effect of A. muciniphila in a murine model of food allergy to ovalbumin by analysing clinical, histological, microbiological, and immunological parameters.
Materials and Methods
Microorganism
To perform the experiment, A. muciniphila BAA-835 (DSM 22,959) was used. The bacterium was obtained from the German Collection of Microorganisms and Cell Cultures GmbH (DSMZ), Braunschweig, Germany. It was maintained in brain heart infusion (BHI) broth (Sigma-Aldrich, St. Louis, MO, USA) and preserved in 20% glycerol at − 80 °C. The cultivation method was carried out by incubating A. muciniphila in an anaerobic atmosphere, using the commercial kit ANAEROBAC® (PROBAC, São Paulo, SP, Brazil), for 48 h, at 37 °C, in BHI broth supplemented with hemin (0.1%), menadione (0.1%), yeast extract (5 g/L), L-cysteine (0.5 g/L), and mucin (0.1%). For bacterial inactivation, the sample was centrifuged (9500 rpm, for 10 min) (Heraeus Megafuge 8R Centrifuge, Thermo Fisher Scientific, Waltham, MA, USA), resuspended in saline (0.9% NaCl) solution, and subsequently concentrated to obtain 1010 colony-forming units (CFU)/mL. The concentrated sample was subjected to a water bath at 75 °C for 10 min. Subsequently, a sample was incubated in BHI supplemented broth and incubated as described above. The turbidance was analysed to confirm the inactivation process.
Mice
Conventional, 6–8 weeks old, female, BALB/c mice, were used. They were distributed in mini-isolators (Alesco, Monte Mor, SP, Brazil), which were placed in a ventilated caging cabinet (Alesco) and kept under light (12-h light–dark cycle), humidity (60–80%), and temperature control (22 ± 1 °C). Food (AIN-93G based diet [22, 23]) and water were offered ad libitum. Animal manipulations were executed following the Brazilian National Council for the Control of Animal Experimentation (CONCEA) protocols (available at http://www.mctic.gov.br/concea). All the experiment was approved by the Ethics Committee on Animal Experimentation (CEUA) of the Federal University of Minas Gerais under the protocol # 110/2019.
Experimental Design
Mice were distributed into the five following groups (n = 6/group): CTL (untreated and non-sensitized); Akk (A. muciniphila treated and unsensitized); OVA (untreated and OVA sensitized); OVA + Akk (treated with viable A. muciniphila cells and OVA sensitized); and OVA + Akk In (treated with inactivated A. muciniphila cells and OVA sensitized).
The sensitize process was executed administrating subcutaneously 0.2 mL of a solution containing 10 μg of adsorbed on aluminium hydroxide, in mice, at day 0. The control group received only the adjuvant. A booster (composed by ovalbumin and saline only) was administrated 14 days later. The control group received only saline. Seven days after the booster (day 21), mice were challenged with a modified AING93 diet containing 20% OVA, offered ad libitum, until the end of the experiment.
The probiotic intervention was performed 4 days after the booster (day 18) and until the end of the experiment. For this purpose, mice received by intragastric gavage 0.1 mL containing 1010 CFU/mL of viable A. muciniphila cells or 1010 cells/mL of inactivated A. muciniphila. The control group received 0.1 mL of sterile saline solution.
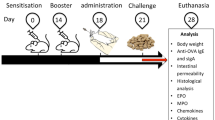
Mice were submitted to a general anaesthesia (ketamine 100 mg/kg plus xylazine 10 mg/kg), euthanized by exsanguination after 28 days since the first sensitization [24], and subsequently, samples were collected for analysis (Fig. 1).
Experimental design. On day 0, mice (n = 6) were sensitized with a subcutaneous injection of 0.2 mL of saline with 10 µg of ovalbumin adsorbed on aluminium hydroxide. Two weeks later (day 14), a booster was administrated. The control group received saline with the adjuvant on day 0 and only saline on day 14. From day 18 to the end of the experiment, 0.1 mL of viable or inactivated A. muciniphila was administered by intragastric gavage. On day 21, mice were challenged with OVA diet and received this diet until the end of the experiment (day 28), when mice were euthanized
Clinical Analysis
Body weight was measured daily, at the same time. The body weight on day 0 was considered as 100%, and the results of the last day of the experiment (day 28) were expressed as a percentage of variation in relation to body weight on day 0 [24].
Serum Anti-OVA IgE and IgG1
The analysis of serum levels of anti-OVA IgE and IgG1 was performed by the ELISA method [25]. For anti-OVA IgE analysis, polystyrene microplates (Nunc, Roskilde, Denmark) were coated with 50 μL per well of rat anti-mouse IgE antibody (Southern Biotechnology, Birmingham, AL, USA) diluted 1:250 in carbonate buffer pH 9.6, and incubated at – 4 °C overnight. Streptavidin-peroxidase conjugate (Southern Biotechnology), o-phenylenediamine (OPD), and hydrogen peroxide were used. On the other hand, IgG1 analysis was performed by coating the microplates with an OVA solution. The protocol was performed using 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (Sigma). Absorbance was determined at 405 nm with an ELISA reader (Epoch, BioTek Instruments, Inc., Winooski, VT, USA). Both results were expressed as optical density (OD).
Histological Analysis
Proximal jejunum samples were rolled up (Swiss rolls) and fixed in Bouin solution. Slides were coded, stained with haematoxylin–eosin (HE) or periodic acid Schiff (PAS), and analysed. Photodocumentation was performed using an Olympus BX-40 microscopy/spot basic microcamera, and the software Image J (v.1.47f, Wayne Rasband/National Institutes of Health, USA) was used to analyse the files. A grading system was used to determinate the histopathological scores in which values between 0 and 6 were given according to the histopathological findings (score 0: normal mucosa; score 1: mucosa showing mild oedema and little or no inflammatory infiltrate; score 2: mucosa showing slight inflammatory infiltrate, increased mucus production, 10% decrease in villus length, crypts showing slight hypertrophy of Paneth cells and submucosa without cellular and vascular alterations; score 3: mucosa showing moderate inflammatory infiltrate, increased mucus production classified as mild to moderate, 15% decrease in villus length, crypts showing hypertrophy of Paneth cells, classified as discrete to moderate, and submucosa without any vascular or cellular alterations; score 4: mucosa showing moderate inflammatory infiltrate, increased mucus production, 20% decrease in villus length, crypts with moderate hypertrophy of Paneth cells and submucosa showing discrete inflammatory infiltrate and dilated vessels; score 5: mucosa showing inflammatory infiltrate, increased mucus production classified as moderate to severe, 25% decrease in villus length, crypts showing hypertrophy of Paneth cells classified as moderate to severe, submucosa showing mild inflammatory infiltrate and dilated vessels; score 6: mucosa showing intense inflammatory infiltrate, increased mucus production, 30% decrease in villus length, crypts showing evident hypertrophy of Paneth cells, submucosa showing moderate inflammatory infiltrate and dilated vessels) [24, 26].
Eosinophil Peroxidase Activity
The presence of eosinophil can be detected indirectly by the analysis of eosinophil peroxidase (EPO) activity. Therefore, samples of proximal jejunum were collected (100 mg) and homogenized and centrifuged at 10,000 g for 10 min, and the pellet was resuspended. Subsequently, the samples were freeze and thawed with liquid nitrogen and centrifuged at 4 °C and 10,000 g for 10 min. OPD diluted in Tris–HCl and H2O2 were added to the supernatant and incubated for 30 min at 20 °C. To stop the reaction, 50 μL of 1 M H2SO4 was added. Absorbance was determined at 492 nm on a microplate spectrophotometer (Epoch). Results were expressed as OD [27].
Myeloperoxidase Activity
Myeloperoxidase activity was analysed to indirectly determine the neutrophil infiltrate. For this purpose, a sample of proximal jejunum (100 mg) was homogenized in 1.9 mL of PBS and centrifuged at 10,000 g for 10 min. Subsequently, the samples were taken to a lysis process and the supernatants collected for the enzymatic assay. To perform the assay, 25 µL of supernatant was added to 25 µL of 3,3′,5,5′-tetramethylbenzidine (TMB) (T2885, Sigma-Aldrich) diluted in DMSO and H2O2 and incubated at 37 °C for 5 min. To stop the reaction, 1 M H2SO4 was added. Absorbance was determined at 450 nm on a microplate spectrophotometer (Epoch). The results were expressed as OD [24].
Chemokines and Cytokines Levels
The ELISA method was used to determine the levels of chemokines (eotaxin-1 and CXCL1/KC) and cytokines (IL4, IL6, IL9, IL10, IL13, IL17, and TNF) present in proximal jejunum samples. For this, they were homogenized and suspended in 1 mL of cytokine extraction mixture containing an anti-protease cocktail (0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A) and 0.05% of Tween 20. The supernatant was collected, and chemokine and cytokine levels were measured in accordance to the protocols provided by the manufacturer (R&D Systems, Minneapolis, MN, USA) [28].
Microbiota Analysis
Some studies demonstrated that the gut microbiota composition of allergic individual is different from non-allergic ones [29]. A culture-dependent method was used to investigate the intestinal microbiota composition. Fresh faeces were aseptically collected, weighted, homogenized, and diluted in sterile saline. To analyse the anaerobe group, samples were cultured onto blood agar (Brucella agar supplemented with 0.1% hemin, 0.1% menadione, 0.5% yeast extract, and 5% sheep blood), Bacteroides bile esculin agar (BBE, Difco, Sparks, USA), and de Man, Rogosa, and Sharpe agar (MRS, Merck, Darmstadt, Germany), for total anaerobes, Bacteroides sp., and lactic acid bacteria, respectively. Plates were incubated at 37 °C for 48–72 h in an anaerobic chamber (Forma Scientific Company, Marietta, USA) containing an atmosphere of 85% N2, 10% H2, and 5% CO2. For investigation of facultative anaerobes, the samples were cultured onto blood agar, brain heart infusion agar (BHI, Difco) enriched with sodium azide (0.02%), MacConkey agar (Difco), hypertonic salt mannitol agar (Difco), and YPD agar supplemented with 0.2 g/L chloramphenicol, for total aerobes, Enterococcus, Enterobacteria, Staphylococcus, and yeast, respectively. The agar plates were incubated at 37 °C for 24–48 h under aerobic conditions. Bacterial and yeast counts were expressed as log10 of CFU per gramme of faeces [24].
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism software (GraphPad Software, San Diego, CA, USA). The results were submitted to one-way ANOVA followed by Tukey test. The results were considered significant for P < 0.05 [24].
Results
A. muciniphila Reduced Body Weight Loss and Serum Levels of Anti-OVA IgE and IgG1 in Allergic Mice
As expected for a murine model of ovalbumin allergy [24, 26], our data showed that untreated mice sensitized with OVA presented a significant loss of body weight (Fig. 2A) and increased serum levels of anti-OVA IgE (Fig. 2B) and anti-OVA IgG1 (Fig. 2C) when compared to the control (CTL) group (P < 0.05). These parameters were reduced with the daily administration of 9.0 log10 CFU of viable A. muciniphila (Fig. 2).
Body weight variation (A) and anti-OVA IgE (B) and anti-OVA IgG1 (C) serum levels in mice: only treated with sterile saline (CTL); treated with sterile saline and OVA sensitized (OVA); treated with A. muciniphila and not sensitized (Akk); and treated with viable A. muciniphila cells and OVA sensitized (OVA + Akk). Vertical lines represent standard error. a and b indicate statistical difference in relation to, respectively, the control (CTL) and sensitized (OVA) groups (P < 0.05 by one-way ANOVA) (n = 6 per group)
A. muciniphila Mitigated Intestinal Injury and Mucus Production in Allergic Mice
Sensitized mice exposed to OVA diet showed a disruption of the integrity of the intestinal epithelium culminating in tissue damage. The histopathological analysis of the small intestine showed that the CTL group presented a normal aspect of the intestinal mucosa (Fig. 3A). On the other hand, the sensitized group presented an inflammatory infiltrate in the mucosa, predominantly composed of lymphocytes, neutrophils, and eosinophils (Fig. 3B and E), reduced villus length (Fig. 3B), hyperplasia and hypertrophy of the goblet cells (increasing the production of mucus), and increased Paneth cell activity (evidenced by increased cell size and colour intensity of PAS staining) (Fig. 4B and E). In addition, the sensitized group had submucosa with a slight inflammatory infiltrate and some dilated vessels. These signs of intestinal injury were attenuated in the group treated with A. muciniphila, which presented a mucosa with mild to moderate inflammatory infiltrate, with a slight increase in mucus production. The reduction in villus length was also discrete (Fig. 3D), as was the activity of Paneth cells, evidenced by the attenuation of the PAS staining colour intensity (Fig. 4D). Furthermore, there were no cellular or vascular alterations in the submucosa layer (Fig. 3D). Treatment with A. muciniphila also led to a significant reduction in the number of goblet cells/field (P < 0.05), which indicates depletion in mucus production (Fig. 4F). These results are summarized as histopathological score in Fig. 3F. In summary, the administration of viable A. muciniphila was able to significantly reduce (P < 0.05) the signs of inflammation promoted by food allergy.
Histological aspect of the proximal jejunum of mice A only treated with sterile saline (CTL); B treated with sterile saline and OVA sensitized (OVA); C treated with A. muciniphila and not sensitized (Akk); D treated with viable A. muciniphila cells and OVA sensitized (OVA + Akk); E in this figure, eosinophils and Paneth cells are indicated by the white and black arrows, respectively, in the OVA group; F Histopathological score of tissue damage in the small intestine for each group analysed. B presents a key that highlights the reduction in villus height found in the proximal jejunum of mice exposed to food allergy. Vertical lines represent standard error. a and b indicate statistical difference in relation to, respectively, the control (CTL) and sensitized (OVA) groups (P < 0.05 by one-way ANOVA). H.E. (n = 6 per group)
Histological aspect of the proximal jejunum of mice A only treated with sterile saline (CTL); B treated with sterile saline and OVA sensitized (OVA); C treated with A. muciniphila and not sensitized (Akk); D treated with viable A. muciniphila cells and OVA sensitized (OVA + Akk); E in this figure, short arrows point to goblet cells evidencing its hypertrophy, and long arrows point to Paneth cells evidencing its increased colour intensity in the OVA group; F number of goblet cells/field for each group. Vertical lines represent standard error. a and b indicate statistical difference in relation to, respectively, the control (CTL) and sensitized (OVA) groups (P < 0.05 by one-way ANOVA). Periodic acid-Schiff (n = 6 per group)
A. muciniphila Reduced Intestinal Mucosa Leukocyte Infiltration in Allergic Mice
To assess leukocyte infiltration, analysis of eosinophilic peroxidase (EPO) and myeloperoxidase (MPO) activity in proximal jejunum samples was performed. These protocols are used as an indirect analysis of the presence of eosinophils and neutrophils in the intestinal mucosa [26], respectively. Not treated mice and sensitized mice with OVA showed a significant (P < 0.05) increase in EPO and MPO activities when compared to CTL (Fig. 5A and C). The administration of 9.0 log10 CFU of A. muciniphila promoted a significant attenuation of both parameters (P < 0.05). It is well known that CCL11/eotaxin-1 and CXCL1/KC are responsible for recruiting, respectively, eosinophils and neutrophils to the site of inflammation. Corroborating the EPO and MPO data, a significant reduction (P < 0.05) was observed in the levels of CCL11/eotaxin-1 and CXCL1/KC in the proximal jejunum of the group treated with A. muciniphila when compared to sensitized and untreated animals (Fig. 5B and D).
EPO (A) and MPO (C) activities and CCL11/eotaxin-1 (B) and CXCL1/KC (D) levels in the proximal jejunum of mice: only treated with sterile saline (CTL); treated with sterile saline and OVA sensitized (OVA); treated with A. muciniphila and not sensitized (Akk); and treated with viable A. muciniphila cells and OVA sensitized (OVA + Akk). Vertical lines represent standard error. a and b indicate statistical difference in relation to, respectively, the control (CTL) and sensitized (OVA) groups, respectively (P < 0.05 by one-way ANOVA) (n = 6 per group)
A. muciniphila Attenuated Pro-Inflammatory Cytokines Levels in Allergic Mice
Ovalbumin allergy is characterized by a significant increase in pro-inflammatory cytokines, which can be observed in the untreated sensitized group (Fig. 6A–F) in relation to the CTL group. Treatment with 9.0 log10 CFU of viable A. muciniphila promoted a significant reduction in all pro-inflammatory cytokines investigated (P < 0.05), inferring its immunomodulatory property. However, there was no significant difference in the levels of anti-inflammatory cytokine IL10 comparing the untreated and treated allergic groups (Fig. 6G). These results indicate that the possible mechanism involving the immunomodulatory effect of A. muciniphila is to mitigate the pro-inflammatory response.
Levels of IL4 (A), IL9 (B), IL13 (C), IL6 (D), IL17 (E), TNF (F), and IL10 (G) in proximal jejunum of mice: only treated with sterile saline (CTL); treated with sterile saline and OVA sensitized (OVA); treated with A. muciniphila and not sensitized (Akk); and treated with viable A. muciniphila cells and OVA sensitized (OVA + Akk). Vertical lines represent standard error. a and b indicate statistical difference in relation to, respectively, the control (CTL) and sensitized (OVA) groups, respectively (P < 0.05 by one-way ANOVA) (n = 6 per group)
A. muciniphila Attenuated Staphylococcus Levels and Yeast Frequency Contributing in the Attenuation of Intestinal Dysbiosis
Food allergy is characterized by a change in the composition of the intestinal microbiota that contributes to the disruption of oral tolerance [29]. In the present study, the allergic group showed a significant increase (P < 0.05) in the levels of total anaerobes (Fig. 7A), Bacteroides (Fig. 7C), Staphylococcus (Fig. 7E), Enterobacteria (Fig. 7G), and in the frequency of yeasts (Fig. 7H), when compared to the control group. It is important to mention in particular the increase in yeast frequency from 16% in the control group to 100% in the allergic group. These changes confirm the characteristic dysbiosis of the intestinal microbial composition due to food allergy. The treatment with A. muciniphila was able to restore eubiosis, significantly decreasing the levels and frequency of Staphylococcus and reducing the frequency of yeasts by 50% (Fig. 7H).
Intestinal microbiota composition by culture dependent method. Effect of A. muciniphila in the levels of A total anaerobes; B total aerobes; C Bacteroides; D lactic acid bacteria; E Staphylococcus; F Enterococcus; G enterobacteria; and H yeasts. Vertical lines represent standard error. a and b indicate statistical difference in relation to, respectively, the control (CTL) and sensitized (OVA) groups, respectively (P < 0.05 by one-way ANOVA) (n = 6 per group)
A. muciniphila Presents Postbiotic Potential in Allergic Mice
To investigate the postbiotic potential of A. muciniphila in the murine model of ovalbumin allergy, anti-OVA IgE levels and EPO activity were determined. Figure 8 shows that even heat-killed bacteria are able to significantly attenuate anti-OVA IgE levels and eosinophil recruitment in allergic mice. This indicates that A. muciniphila also has interesting characteristics to be used as a postbiotic.
Serum anti-OVA IgE levels (A) and EPO activity (B) in proximal jejunum of mice: only treated with sterile saline (CTL); treated with sterile saline and OVA sensitized (OVA); treated with A. muciniphila and not sensitized (Akk); treated with viable A. muciniphila cells and OVA sensitized (OVA + Akk); and treated with inactivated A. muciniphila cells and OVA sensitized (OVA + Akk In). Vertical lines represent standard error. a, b indicate statistical difference in relation to, respectively, the control (CTL) and sensitized (OVA) groups, respectively (P < 0.05 by one-way ANOVA) (n = 6 per group)
Discussion
In murine models of food allergy, one of the most evident features of the inflammation process is the weight loss of sensitized animals after ingestion of the allergen. Our results revealed that OVA-sensitized animals treated or not presented weight loss compared to non-sensitized groups (Fig. 2A). This phenomenon may be related to the increase in IL6. This cytokine is associated with increased lipolysis, decreased adipose tissue weight and adipocyte size, and increased energy required to maintain the inflammatory process, culminating in the recruitment of reserve lipids [23]. In addition, some studies have shown that mice when sensitized and exposed to the allergen develop an aversion behaviour. This process is characterized by a decrease in food consumption due to the perception of a noxious stimulus [30]. Similar results were found in a murine model of peanut allergy [31], indicating that allergy induction occurred as expected. Furthermore, oral administration of A. muciniphila was able to attenuate weight loss (Fig. 2A), indicating its probiotic potential.
During an allergic process, B cells in response to an antigen stimulus produce specific IgE antigen, and when a second exposure to the allergen occurs, basophils and mast cells degranulate, culminating in the release of inflammatory mediators. IgG is also another relevant immunoglobulin involved in allergic processes. Some studies have shown that IgG1 interacts with allergens forming an immunocomplex. This immune complex interacts with neutrophils and basophils promoting the release of platelet-activating factor (PAF), which is involved in anaphylactic processes [32,33,34,35,36,37]. Our results showed a remarkable ability of A. muciniphila to suppress these immunoglobulins (Fig. 2B and C), indicating a systemic probiotic effect. Fu et al. [38] obtained similar results using a Lacticaseibacillus casei strain in a murine model of tropomyosin allergy (protein responsible for triggering food allergy to crustaceans). Furthermore, studies carried out by Fu et al. [39] also demonstrated the attenuation of these immunoglobulins when shrimp tropomyosin-allergic mice were treated with Bacillus coagulans or Lactiplantibacillus plantarum. In addition, Shin et al. [40] demonstrated that a combination of Lactococcus lactis, Pediococcus pentosaceus, Lactiplantibacillus pentosus, Lacticaseibacillus paracasei, and Bacillus amyloliquefaciens also had similar effects in a murine model of food allergy to ovalbumin, evidencing the auspicious role of probiotics in the treatment of food allergy.
In an allergic process, eosinophils are recruited by some chemokines, such as CCL11 (eotaxin-1), to inflamed tissues, and once in the tissue, they release their granules, which contain some proteins such as the EPO enzyme. This enzyme can catalyse the oxidation of some substances generating reactive oxygen species (ROS). Such a mechanism can promote tissue damage, contributing to the installation of pro-inflammatory reactions. In addition, the EPO enzyme is a protein present only in the cytoplasmic granules of eosinophils, making it a mechanism for indirect assessment of the presence of eosinophils in the tissue [41,42,43,44,45,46,47,48]. This process has been demonstrated in our results in which OVA-sensitized group showed increased levels of eosinophils and tissue damage on histopathological analysis (Fig. 3E), eotaxin-1 (Fig. 5B), and increased eosinophil peroxidase activity (Fig. 5A). Furthermore, treatment with A. muciniphila was able to mitigate all these parameters. In summary, our data demonstrated that A. muciniphila has an immunomodulatory property that decreases eotaxin-1 levels, leading to an attenuation of eosinophil recruitment in the inflammatory infiltrate, mitigating the release of EPO, contributing to a less tissue damage.
Neutrophils are also recruited to the inflammation site during the allergic process. Molecules such as CXCL1 (KC) carry out this chemoattractant activity. Once in the tissue, these cells can degranulate releasing some molecules such as myeloperoxidase (MPO). This enzyme is associated with the production of ROS, which can intensify tissue damage [49,50,51,52]. These aspects were demonstrated in the present study, which revealed a significant increase of neutrophils and tissue damage in the histopathological analysis (Fig. 3B), as well as of CXCL1/KC levels (Fig. 5D) and MPO activity when compared to the CTL group (Fig. 5C). In addition, there is some evidence that neutrophils are involved in the development of anaphylaxis [53], highlighting the importance of investigating their role in the allergic process. Regarding the effects of A. muciniphila, our data suggest that the bacterium was able to significantly reduce KC levels, consequently attenuating the neutrophil infiltrate, in addition to reducing MPO release and contributing to decrease tissue damage. These findings indicate that the treatment can contribute as an adjunct to the management of the anaphylaxis process and can also help to reduce tissue damage caused by allergic reactions.
Tissue damage caused by allergic reactions culminates in gut barrier integrity disruption. Some studies pointed that the outer membrane protein, Amuc_1100, present in A. muciniphila can promote amelioration on gut barrier integrity [15]. Our data demonstrated that the allergic group presented reduced villus length (Fig. 3B), hyperplasia and hypertrophy of the goblet cells (increasing the production of mucus), and increased Paneth cell activity (Fig. 4B and E). These features demonstrated the gut barrier integrity disruption present in food allergy reactions. On the other hand, the group treated with A. muciniphila presented amelioration in all of these features, indicating the role of A. muciniphila in maintaining gut barrier integrity.
The most notable feature of IgE-mediated food allergy is the disruption of oral tolerance, culminating in the activation and differentiation of naïve T cells into Th2 effector cells. These cells are responsible for releasing Th2-type cytokines (IL4, IL6, IL9, IL13, IL17, and TNF) leading to the activation of B cells, promoting anti-OVA IgE production, intensifying weight loss, eosinophilia, activation of goblet cells and consequently mucus production, eosinophils and neutrophils recruitment (forming the inflammatory infiltrate), and tissue damage [26, 54]. All these characteristics were demonstrated in our data. Therefore, it is important to mention that the group treated with A. muciniphila presented reduced levels of IL4, IL6, IL9, IL13, IL17, and TNF, indicating that the bacterium has an immunomodulatory property that helps to restore oral tolerance. In regard to IL10, it is well known that the Foxp3 + Treg cells attenuate the exacerbated activation of Th2 cells diminishing the allergic response. Some studies demonstrated that A. muciniphila contributes to IL10 production culminating in the preservation of the gut immunological homeostasis [55]. However, there was no significant alteration in IL10 levels comparing the treated group to the allergic group, indicating that the mechanism by which the microorganism exerts its immunological effect is by suppressing the Th2 cytokines and not increasing IL10 levels.
Some studies have demonstrated that the microbiota may contribute to maintain the homeostasis of IgE and the control of allergic responses triggered by IgE [56]. Nakayama et al. [57] demonstrated consistent differences between intestinal microbial compositions in allergic individuals comparing to non-allergic ones. Therefore, strategies that aim its manipulation could be considered as a target in the treatment of food allergy. In this way, the microbial composition is evaluated by culture-dependent method, and the results are demonstrated in Fig. 7. In the present study, the allergic group presented an increase in faecal levels of total anaerobes (Fig. 7A), Bacteroides (Fig. 7C), Staphylococcus (Fig. 7E), and Enterobacteria (Fig. 7G) and in yeast frequency (Fig. 7H) compared to the control group. These alterations indicated a misbalance of the intestinal microbial composition and, therefore, an establishment of the dysbiosis process. The dysbiosis is defined as alterations in function and composition of the intestinal microbiota [58]. Tsilochristou et al. [59] demonstrated that S. aureus is associated with the sensitization process and disruption of oral tolerance. In the present study, we demonstrated that A. muciniphila was able to reverse this parameter, diminishing Staphylococcus levels and frequency, matching the levels of the control group. Another alteration in microbial composition was the increase of Enterobacteria (Fig. 7G). Azad et al. [60] demonstrated an increase in faecal samples of allergic child corroborating our data. This alteration was associated with the development of atopic dermatitis, which can also culminate in the disruption of oral tolerance and increase the allergen absorption. In regard to the treated group, there was no significant alteration on the levels of Enterobacteria (Fig. 7G). In addition, Bacteroides deficiency in child is associated with the development of food allergy [29, 60, 61]. However, on the present study, we demonstrated an increase of Bacteroides in the allergic group (Fig. 7C). This fact could be related to the strain- and specie-dependent effect of these microorganisms in the intestinal microbiota. Some authors revealed that B. thetaiotaomicron, for example, could trigger colitis in some murine models [62]. Furthermore, in Fig. 7A, there is an increase in total anaerobes in the allergic group comparing to the control group, which can be due to the significant increase on Bacteroides levels. In addition, it is important to mention that the frequency of yeast in the allergic group was of 100% when compared to 16% present in the control group. In regard to the treated group, it is noticeable that A. muciniphila was able to reduce in 50% the yeast frequency, suggesting that A. muciniphila can contribute to the modulation of intestinal microbial composition (Fig. 7H). All of these results suggest that A. muciniphila could exert its beneficial effects by the modification of intestinal microbiota composition.
In summary, viable A. muciniphila presents health benefits to the host in the murine model investigated. This characteristic is essential to consider it a probiotic, because by definition, probiotics are living microorganisms that confer health benefits to the host [63]. However, the concept of postbiotic is currently emerging, showing that some microorganisms are able to promote health benefits even when inactivated [18]. The most recent definition of postbiotic is “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [64]. The postbiotics have some advantages over probiotics, such as, when incorporated into industrialized products, they have a longer shelf life and are safer to be administered to immunocompromised individuals. This fact is mainly due to the translocation capacity of viable cells leading to infection in this population [19, 65]. The structural components of the bacterial cell are most responsible for these beneficial effects. It is well known that A. muciniphila has a 32 kDa extracellular protein called Amuc_1100, which is thermostable, remaining active after pasteurization processes. This protein is related to the improvement of the intestinal epithelial barrier and the parameters of obesity and type 2 diabetes [15, 20, 66, 67]. In our study, we demonstrated that heat-inactivated A. muciniphila was able to reduce anti-OVA IgE and EPO levels (Fig. 8A and B), indicating that the bacterium may have postbiotic potential to be used in the treatment of food allergy.
In conclusion, our results indicate that the oral administration of viable and inactivated A. muciniphila has a systemic immunomodulatory effect that could be used in the treatment of food allergy.
Availability of Data and Material
The datasets generated and analysed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Turnbull JL, Adams HN, Gorard DA (2015) The diagnosis and management of food allergy and food intolerances. Aliment Pharmacol Ther 41:3–25. https://doi.org/10.1111/apt.12984
Matsui T, Tanaka K, Yamashita H, Saneyasu KI, Tanaka H, Takasato Y, Sugiura S, Inagaki N, Ito K (2019) Food allergy is linked to season of birth, sun exposure, and vitamin D deficiency. Allergol Int 68:172–177. https://doi.org/10.1016/j.alit.2018.12.003
Aitoro R, Paparo L, Amoroso A, Di Costanzo M, Cosenza L, Granata V, Di Scala C, Nocerino R, Trinchese G, Montella M, Ercolini D, Berni Canani R (2017) Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients 9:672. https://doi.org/10.3390/nu9070672
Sharma G, Im SH (2018) Probiotics as a potential immunomodulating pharmabiotics in allergic diseases: current status and future prospects. Allergy, Asthma Immunol Res 10:575–590. https://doi.org/10.4168/aair.2018.10.6.575
Chernikova DA, Zhao MY, Jacobs JP (2022) Microbiome therapeutics for food allergy. Nutrients 14:5155. https://doi.org/10.3390/nu14235155
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20:159–166. https://doi.org/10.1038/nm.3444
Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR (2016) Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 15:2809–2824. https://doi.org/10.1016/j.celrep.2016.05.047
Castellazzi AM, Valsecchi C, Caimmi S, Licari A, Marseglia A, Leoni MC, Caimmi D, Del Giudice MM, Leonardi S, Rosa ML, Marseglia GL (2013) Probiotics and food allergy. Ital J Pediatr 39:1–10. https://doi.org/10.1186/1824-7288-39-47
Kim H, Kwack K, Kim DY, Ji GE (2005) Oral probiotic bacterial administration suppressed allergic responses in an ovalbumin-induced allergy mouse model. FEMS Immunol Med Microbiol 45:259–267. https://doi.org/10.1016/j.femsim.2005.05.005
Lee J, Bang J, Woo HJ (2013) Effect of orally administered Lactobacillus brevis HY7401 in a food allergy mouse model. J Microbiol Biotechnol 23:1636–1640. https://doi.org/10.4014/jmb.1306.06047
Martín R, Langella P (2019) Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol 10:1047. https://doi.org/10.3389/fmicb.2019.01047
Souza RO, Miranda VC, Quintanilha MF, Gallotti B, Oliveira SR, Silva JL, Alvarez-leite JI, Jesus LCL, Azevedo V, Vital KD, Fernandes SOA, Cardoso VN, Ferreira E, Nicoli JR, Martins FS (2023) Evaluation of the treatment with Akkermansia muciniphila BAA-835 of chemotherapy-induced mucositis in mice. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-023-10040-2
Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Hupp T, Duchnowska R, Marek-Trzonkowska N, Połom K (2022) Next-generation probiotics - do they open new therapeutic strategies for cancer patients? Gut Microbes 14:2035659. https://doi.org/10.1080/19490976.2022.2035659
Marcial-Coba MS, Cieplak T, Cahú TB, Blennow A, Knøchel S, Nielsen DS (2018) Viability of microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct 9:5868–5879. https://doi.org/10.1039/C8FO01331D
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50:e450–e450. https://doi.org/10.1038/emm.2017.282
Derrien M, Belzer C, de Vos WM (2017) Akkermansia muciniphila and its role in regulating host functions. Microb Pathog 106:171–181. https://doi.org/10.1016/j.micpath.2016.02.005
Naito Y, Uchiyama K, Takagi T (2018) A next-generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr 63:33–35. https://doi.org/10.3164/jcbn.18-57
Cuevas-González PF, Liceaga AM, Aguilar-Toalá JE (2020) Postbiotics and paraprobiotics: from concepts to applications. Food Res Int 136:109502. https://doi.org/10.1016/j.foodres.2020.109502
Akter S, Park JH, Jung HK (2020) Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J Microbiol Biotechnol 30:477–481. https://doi.org/10.4014/jmb.1911.11019
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, Van der Ark KCH, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23:107–113. https://doi.org/10.1038/nm.4236
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch Ernst KI, Knutsen HK (2021) Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J 19:e06780. https://doi.org/10.2903/j.efsa.2021.6780
Reeves P, Nielsen F, Fahey G (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Dourado LPA, Noviello MDLM, Alvarenga DM, Menezes Z, Perez DA, Batista NV, Menezes GB, Ferreira AVM, Souza DG, Cara DC (2011) Experimental food allergy leads to adipose tissue inflammation, systemic metabolic alterations and weight loss in mice. Cell Immunol 270:198–206. https://doi.org/10.1016/j.cellimm.2011.05.008
Miranda VC, Santos SS, Assis HC, Faria AMC, Quintanilha MF, Morão RP, Nicoli JR, Cara DC, Martins FS (2020) Effect of Saccharomyces cerevisiae UFMG A-905 in a murine model of food allergy. Benefic Microbes 11:255–268. https://doi.org/10.3920/BM2019.0113
Saldanha JCS, Gargiulo DL, Silva SS, Carmo-Pinto FH, Andrade MC, Alvarez-Leite JI, Teixeira MM, Cara DC (2004) A model of chronic IgE-mediated food allergy in ovalbumin-sensitized mice. Braz J Med Biol Res 37:809–816. https://doi.org/10.1590/S0100-879X2004000600005
Santos SS, Miranda VC, Trindade LM, Cardoso VN, Reis DC, Cassali GD, Nicoli JR, Cara DC, Martins FS (2021) Bifidobacterium longum subsp. longum 51A attenuates signs of inflammation in a murine model of food allergy. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-021-09846-9
Strath M, Warren DJ, Sanderson CJ (1985) Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods 83:209–215. https://doi.org/10.1016/0022-1759(85)90242-X
Martins FS, Elian SD, Vieira AT, Tiago FC, Martins AK, Silva FC, Souza EL, Sousa LP, Araújo HR, Pimenta PF, Bonjardim CA, Arantes RM, Teixeira MM, Nicoli JR (2011) Oral treatment with Saccharomyces cerevisiae strain UFMG 905 modulates immune responses and interferes with signal pathways involved in the activation of inflammation in a murine model of typhoid fever. Int J Med Microbiol 301:359–364. https://doi.org/10.1016/j.ijmm.2010.11.002
Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, Yuan L, Wang Y, Sun J, Li L, Xiang C (2014) Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol 80:2546–2554. https://doi.org/10.1128/AEM.00003-14
Cara DC, Conde AA, Vaz NM (1997) Immunological induction of flavour aversion in mice. II. Passive/adoptive transfer and pharmacological inhibition. Scand J Immunol 45:16–20. https://doi.org/10.1046/j.1365-3083.1997.d01-363.x
Cardoso CR, Provinciatto PR, Godoi DF, Fonseca MT, Ferreira BR, Teixeira G, Cunha FQ, Pinzan CF, Da Silva JS (2019) The signal transducer and activator of transcription 6 (STAT-6) mediates Th2 inflammation and tissue damage in a murine model of peanut-induced food allergy. Allergol Immunopathol 47:535–543. https://doi.org/10.1016/j.aller.2019.02.006
Kapingidza AB, Kowal K, Chruszcz M (2020) Antigen–Antibody Complexes. In: Hoeger U, Harris J (eds) Vertebrate and invertebrate respiratory proteins, lipoproteins and other body fluid proteins. Subcellular Biochemistry, vol 94. Springer, Cham. https://doi.org/10.1007/978-3-030-41769-7_19
Kelly BT, Grayson MH (2016) Immunoglobulin E, what is it good for? Ann Allergy Asthma Immunol 116:183–187. https://doi.org/10.1016/j.anai.2015.10.026
Meulenbroek LA, de Jong RJ, den Hartog Jager CF, Monsuur HN, Wouters D, Nauta AJ, Knippels LMJ, Joost van Neerven RJ, Ruiter B, Leusen JHW, Hack CE, Bruijnzeel-Koomen CAFM, Knulst AC, Garssen J, van Hoffen E (2013) IgG antibodies in food allergy influence allergen–antibody complex formation and binding to B cells: a role for complement receptors. J Immunol 191:3526–3533. https://doi.org/10.4049/jimmunol.1202398
Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ (1997) Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE-or IgG1-dependent passive anaphylaxis. J Clin Investig 99:901–914. https://doi.org/10.1172/JCI119255
Nunes MPO, van Tilburg MF, Tramontina Florean EOP, Guedes MIF (2019) Detection of serum and salivary IgE and IgG1 immunoglobulins specific for diagnosis of food allergy. PLoS ONE 14:e0214745. https://doi.org/10.1371/journal.pone.0214745
Vidarsson G, Dekkers G, Rispens T (2014) IgG subclasses and allotypes: from structure to effector functions. Front Immunol 5:520. https://doi.org/10.3389/fimmu.2014.00520
Fu L, Xie M, Wang C, Qian Y, Huang J, Sun Z, Zhang H, Wang Y (2020) Lactobacillus casei Zhang alleviates shrimp tropomyosin induced food allergy by switching antibody isotypes through the NF-κB-dependent immune tolerance. Mol Nutr Food Res 64:1900496. https://doi.org/10.1002/mnfr.201900496
Fu L, Peng J, Zhao S, Zhang Y, Su X, Wang Y (2017) Lactic acid bacteria-specific induction of CD4+Foxp3+T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling. Sci Rep 7:1987. https://doi.org/10.1038/s41598-017-02260-8
Shin HS, Eom JE, Shin DU, Yeon SH, Lim SI, Lee SY (2018) Preventive effects of a probiotic mixture in an ovalbumin-induced food allergy model. J Microbiol Biotechnol 28:65–76. https://doi.org/10.4014/jmb.1708.08051
Acharya KR, Ackerman SJ (2014) Eosinophil granule proteins: form and function. J Biol Chem 289:17406–17415. https://doi.org/10.1074/jbc.R113.546218
Collins PD, Marleau S, Griffiths-Johnson DA, Jose PJ, Williams TJ (1995) Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med 182:1169–1174. https://doi.org/10.1084/jem.182.4.1169
Lacy P, Latif DA, Steward M, Musat-Marcu S, Man SP, Moqbel R (2003) Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol 170:2670–2679. https://doi.org/10.4049/jimmunol.170.5.2670
Neves JS, Weller PF (2009) Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr Opin Immunol 21:694–699. https://doi.org/10.1016/j.coi.2009.07.011
Ravin KA, Loy M (2016) The eosinophil in infection. Clin Rev Allergy Immunol 50:214–227. https://doi.org/10.1007/s12016-015-8525-4
Rosenberg HF (2016) Eosinophils. Encyclopedia of. Immunobiology 1:334–344. https://doi.org/10.1016/B978-0-12-374279-7.03007-1
Rosenberg HF, Dyer KD, Foster PS (2013) Eosinophils: changing perspectives in health and disease. Nat Rev Immunol 13:9–22. https://doi.org/10.1038/nri3341
Spencer LA, Weller PF (2010) Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol 88:250–256. https://doi.org/10.1038/icb.2009.115
Aratani Y (2018) Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys 640:47–52. https://doi.org/10.1016/j.abb.2018.01.004
Petri B, Sanz MJ (2018) Neutrophil chemotaxis. Cell Tissue Res 371:425–436. https://doi.org/10.1007/s00441-017-2776-8
Sawant KV, Sepuru KM, Lowry E, Penaranda B, Frevert CW, Garofalo RP, Rajarathnam K (2021) Neutrophil recruitment by chemokines Cxcl1/KC and Cxcl2/MIP2: role of Cxcr2 activation and glycosaminoglycan interactions. J Leukoc Biol 109:777–791. https://doi.org/10.1002/JLB.3A0820-207R
Strzepa A, Pritchard KA, Dittel BN (2017) Myeloperoxidase: a new player in autoimmunity. Cell Immunol 317:1–8. https://doi.org/10.1016/j.cellimm.2017.05.002
Muñoz-Cano R, Picado C, Valero A, Bartra J (2016) Mechanisms of anaphylaxis beyond IgE. J Investig Allergol Clin Immunol 26:73–82. https://doi.org/10.18176/jiaci.0046
Yu W, Freeland DMH, Nadeau KC (2016) Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 16:751–765. https://doi.org/10.1038/nri.2016.111
Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, Cokugras H, Kocazeybek BS (2019) Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol 47:365–371. https://doi.org/10.1016/j.aller.2018.12.009
Méndez CS, Bueno SM, Kalergis AM (2021) Contribution of gut microbiota to immune tolerance in infants. J Immunol Res 2021:7823316. https://doi.org/10.1155/2021/7823316
Nakayama J, Kobayashi T, Tanaka S, Korenori Y, Tateyama A, Sakamoto N, Kiyohara S, Shirakawa T, Sonomoto K (2011) Aberrant structures of fecal bacterial community in allergic infants profiled by 16S rRNA gene pyrosequencing. FEMS Immunol Med Microbiol 63:397–406. https://doi.org/10.1111/j.1574-695X.2011.00872.x
Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E (2017) Dysbiosis and the immune system. Nat Rev Immunol 17:219–232. https://doi.org/10.1038/nri.2017.7
Tsilochristou O, du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, Bahnson HT, Radulovic S, Basting M, Plaut M, Lack G (2019) Association of Staphylococcus aureus colonization with food allergy occurs independently of eczema severity. J Allergy Clin Immunol 144:494–503. https://doi.org/10.1016/j.jaci.2019.04.025
Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, Mandhane SE, Turvey SE, Subbarao P, Becker AB, Scott JA, Kozyrskyj AL (2015) Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy 45:632–643. https://doi.org/10.1111/cea.12487
Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA (2010) Consumption of human milk oligosaccharides by gut related microbes. J Agric Food Chem 58:5334–5340. https://doi.org/10.1021/jf9044205
Atarashi K, Honda K (2011) Microbiota in autoimmunity and tolerance. Curr Opin Immunol 23:761–768. https://doi.org/10.1016/j.coi.2011.11.002
Food and Agriculture Organization / World Health Organization (FAO/WHO) Working Group (2002) Guidelines for the evaluation of probiotics in food. FAO/WHO, London, ON, Canada
Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EM, Sanders ME, Shamir R, Swann JR, Szajewska H, Vinderola G (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 18:649–667. https://doi.org/10.1038/s41575-021-00440-6
Liong MT (2008) Safety of probiotics: translocation and infection. Nutr Rev 66:192–202. https://doi.org/10.1111/j.1753-4887.2008.00024.x
Ottman N, Geerlings SY, Aalvink S, de Vos WM, Belzer C (2017) Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract Res Clin Gastroenterol 31:637–642. https://doi.org/10.1016/j.bpg.2017.10.001
Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skkurnik M, Boeren S, Satokari R, Mercenier A, Palva A, Smidt H, deVos W, Belzer C (2017) Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 12:e0173004. https://doi.org/10.1371/journal.pone.0173004
Funding
This work was supported by grants from the Brazilian National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the Research Support Foundation of the State of Minas Gerais (FAPEMIG), Brazil. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. VCM received a PhD fellowship from CNPq. AMCF, JRN, and FSM are CNPq fellowship holders.
Author information
Authors and Affiliations
Contributions
VCM and FSM analysed data and wrote the paper. FSM and DCC designed and supervised the research. VCM, ROS, MFQ, BG, and HCA, performed experiments and analysed data. AMCF, JRN, DCC, and FSM provided expertise in laboratory resources, improved the issue, and critically revised the article. All authors approved the final version of the article.
Corresponding author
Ethics declarations
Ethics Approval
All animal procedures were carried out according to the standards of the Brazilian Society of Laboratory Animal Science/Brazilian College for Animal Experimentation (available at http://www.mctic.gov.br/concea). This work was approved by the Ethics Committee in Animal Experimentation of the Federal University of Minas Gerais (CEUA/UFMG, protocol # 110/2019).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miranda, V.C., Souza, R.O., Quintanilha, M.F. et al. A Next-Generation Bacteria (Akkermansia muciniphila BAA-835) Presents Probiotic Potential Against Ovalbumin-Induced Food Allergy in Mice. Probiotics & Antimicro. Prot. 16, 737–751 (2024). https://doi.org/10.1007/s12602-023-10076-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10076-4