Abstract
Bacillus sp. DU-106, a potential probiotic, has been proved to activate innate immunity, reduce hypercholesterolemia, and regulate the gut microbiota of mice. In the present study, we investigated the therapeutic effect of strain DU-106 in antibiotic-associated diarrhea (AAD) via analyzing the changes in gut microbial composition in mice. The results indicated that supplementation of strain DU-106 alleviated gastrointestinal symptoms, improved gut barrier integrity and immunoglobulin-A level of mice with AAD. A 16S rRNA sequencing showed that antibiotics decreased bacterial diversity and the abundances of Alistipes, Roseburia, Hungatella, Eubacterium-xylanophilum, Lachnospiraceae-UCG-001, Intestinimonas, and Lachnospiraceae-NK4A136, but increased the abundance of Klebsiella, Bacteroidota, and Verrucomicrobiota. However, strain DU-106 treatment reversed these alternations in mice with AAD. In conclusion, strain DU-106 could alleviate AAD in association with the regulation of intestinal microbiota and could be used as an alternative treatment for AAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diarrhea was a commonly multifactorial gastrointestinal disorder, clinically characterized by thin feces and increased fecal moisture [1]. There were nearly 1.7 billion cases of childhood diarrheal disease annually [2]. Among them, most bacterial infectious diarrhea treated with antibiotics, which also severely destroyed the diversity, uniformity, and functionality of gut microbiota when antibiotics were misused, which led to a common complication in clinical treatment [3], called “antibiotic-associated diarrhea (AAD).” Although patients could recover spontaneously in most cases, targeted intervention could promote patients’ recovery and reduce their pain [4]. Recently, drug therapy is a commonly used method for the treatment of AAD. However, the problem of safety and efficacy of drug therapy [5] is controversial. Thus, new effective and safe strategies are needed to treat AAD.
In recent years, increasing research has evidenced the close relationship between diarrhea and gut microbiota [6]. Literature shows that the occurrence and recovery of AAD are usually along with the disruption and restoration of intestinal flora respectively [7], suggesting intestinal flora may be the target of AAD treatment. Those presented a new avenue of treatment for intestinal diseases AAD [8]. The ability of test substances to regulate intestinal flora is a pivotal factor in the intervention of AAD. Probiotics are intended to confer health benefits when consumed by humans, generally by restoring the gut flora diversity or modulating gut microbiota with a decrease of the relative abundance of harmful bacteria [9], such as opportunistic pathogens Clostridium difficile [10], Klebsiella pneumonia [11], and Staphylococcus aureus [12]. Therefore, the administration of probiotics is a reasonable therapeutic strategy for the treatment of AAD by regulating or restoring the intestinal microbiota.
Clinical studies have shown that probiotics such as Bifidobacterium animalis subsp. lactis BB-12 [13], Saccharomyces boulardii CNCM I-745 [14], and Lactobacillus rhamnosus GG [15] have a positive effect on the intestinal flora regulation and the prevention and treatment of AAD. Although traditional probiotics mentioned above show outstanding probiotic activities that increasing beneficial bacteria, considering their survivability in an extremely harsh environment, its utility may not achieve satisfactory results. Interestingly, as spore-forming probiotic bacteria, some Bacillus strains own high resistance in the gut and are more likely to exert their probiotic role in the intestinal tract than traditional live probiotics. They are more suitable candidates for health care than commercial probiotics [16], and the administration of Bacillus strains may be an attractive therapeutic approach for AAD. In the current research, Bacillus coagulants can regulate host symbiotic microbiota and inhibit the growth of pathogenic bacteria [16], even act as probiotics on intestinal microflora populations of broiler chickens to improve their growth performance [17]. While the effect of Bacillus strains for AAD is still not reported.
We previously isolated Bacillus sp. DU-106, which possessed the ability to produce lactic acid, exerted a positive effect on gut dysbiosis diarrhea. It could resist bile salt and low pH of simulated gastric juice in vitro experiments, and the acute oral toxicity test of mice certificated the safety of strain DU-106 for humans [18]. These characteristics indicate that DU-106 is more stable than other probiotics [19, 20]. We previously showed a beneficial effect in hypercholesterolemia and gut dysbiosis in high-fat diet rats [21] or activating innate immunity [22] in mice. Therefore, we speculated that Bacillus sp. DU-106 had the potential to become probiotics to treat diarrhea resulting from changes in gut microbial communities. The current study initially investigated the anti-AAD ability of strain DU-106 and evaluated its effects on the gut microbiota in AAD mice compared with three known probiotic strains. The results of this work provide a safe and effective probiotic for the alleviation of AAD and gut dysbiosis, which facilitated its development as probiotics in the food industry.
Materials and Methods
Bacterial Strain
The bacterial strain used in the study was Bacillus sp. DU-106; it is a newly isolated member of Bacillus cereus group according to our previous report [18] and preserved in our laboratory with powder form. The other three comparative strains were Bifidobacterium animalis subsp. lactis BB-12, Lactobacillus rhamnosus LGG and Lactobacillus acidophilus LA05, combined into a probiotic complex, purchased from Infinitus (Guangzhou, China) Co., Ltd.
Animals and Experimental Design
In total, 50 SPF KM mice (4-week-old, body weight ∼18–22 g) were obtained from the Guangdong Medical Laboratory Animal Center (Animal production license number: SYXK (yue) 2018–0002). All mice were housed five per cage in Laboratory Animal Center of Guangzhou University of Chinese Medicine (Animal Use License number: SYXK (yue) 2018–0001), with specific pathogen-free (SPF) conditions under a 12-h dark/light cycle at an appropriate temperature (23 ± 3 °C), and relative humidity between 40 and 70%. The mice were fed with a normal chow diet (Research diets LF10B, Guangdong Medical Laboratory Animal Center, Foshan, China). We comply with the guidelines for the care and use of laboratory animals as described by the U.S. National Institutes of Health.
After a 3-day acclimation period, the mice were randomly allocated to five groups (n = 10, each group consisted of male and female mice equally): (1) blank control group (BC, administered intragastrically at 10 mL/kg bw/day for saline), (2) model control group (MC, administered intragastrically at 10 mL/kg bw/day for saline after modeling), (3) probiotics control group (PC, administered intragastrically for probiotic complex supplement (Bifido subsp. lactis BB12 6 × 109 CFU/kg, Lact. LGG 6 × 109 CFU/kg, Lact. LA05 6 × 109 CFU/kg) after modeling,), (4) high-dose group (HD, administered intragastrically at 3.5 × 109 CFU/kg bw/day for Bacillus sp. DU-106 supplement after modeling), and (5) low-dose group (LD, administered intragastrically at 1.75 × 109 CFU/kg bw/day for Bacillus sp. DU-106 supplement after modeling).
Subsequently, group MC, group PC, group HD, and group LD were given triple antibiotics (clindamycin 700 mg/kg bw/day, ampicillin 790 mg/kg bw/day, streptomycin 395 mg/kg bw/day, all purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd, Shanghai, China) by intragastric administration to establish antibiotic-associated diarrhea (AAD) mouse model according to previous research [19]. Different amounts of the powdered Bacillus sp. DU-106, triple antibiotic, and probiotic complex were dissolved in saline to prepare a solution with different concentrations. All the groups received an equivalent volume of 10-mL/kg bw/day solution. Model establishment and test substance treatment both lasted for 2 weeks. Bodyweight, fecal morphology, and the difference in dry/wet weight ratio were recorded daily for each mouse. At the end of the treatment, all the mice were allowed to fast overnight, then their eyeballs were picked for blood, killed with spinal dislocation, and dissected. 2-cm jejunum intestine of each mouse was taken.
Diarrhea Rate Statistics and Evaluation of Diarrhea Model
The fecal morphology and diarrhea rate were the key to judge the success of modeling. The feces of each mouse were collected by squeezing the root of the mouse tail, and its morphology was divided into three grades (Fig. S1A from supplementary materials): level 0 (brown, shaped, hard stool), level 1 (yellow or brown, shaped, soft stool), and level 2 (yellow or brown, pulpy, unshaped stool).
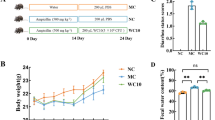
A certain amount of feces was collected for wet weighing and dry weighing (dry the feces in the oven until the mass change is less than 1%); its dry–wet weight ratio (reflect the water content of feces) was calculated. The minimum dry–wet ratio in the BC group was taken as the reference value, and those with a dry–wet ratio less than the reference value were defined as positive diarrhea. The diarrhea rates of the BC group, MC group, PC group, and treatment (HD, LD) group were recorded, and the differences among the groups were statistically compared.
ELISA for Biochemical Determination
Serum samples were separated from blood samples (overnight at 2 to 8 ℃) after centrifugation(3000 r/min) for 1 min under 4 °C and stored at − 20 ℃ in 1.5-mL centrifuge tubes. The IgA, IgG, IL-4, IL-6, and IFN-γ levels in the serum samples were analyzed according to the manufacturer’s instructions of Sandwich ELISA kit (Shanghai Enzyme-linked Biotechnology Co., Ltd, Shanghai, China).
Histopathological Evaluation
Two centimeters of jejunum intestine tube of each mouse were taken during dissection. Then rinsed with saline and fixed with 10% formalin immediately. The jejunum was stained with H.E. after dehydration and embedding. Histopathological sections were made to observe the changes of villi, crypt, and intestinal glands in the small intestine of mice under a light microscope. At least three fields under a 40 × optical microscope of the section from each mouse were randomly selected to record the length of villi and crypt on average, and the ratio of above two was calculated. The morphology of intestinal villi in pathological sections was observed under 100 × and 200 × optical microscope, and histological changes of villi, crypt, and intestinal glands in the jejunum were evaluated.
DNA Extraction and PCR Amplification
After the treatment, bacterial DNA was extracted from the fecal samples (collected aseptically in a sterile EP tube and stored at − 80 °C) of each mouse (randomly selected from each group) by using cetyltrimethylammonium ammonium bromide (CTAB) method. The purity of extracted DNA was determined by 1% agarose gel electrophoresis. Then DNA was diluted to 1 ng/μL with sterile water in a centrifuge tube before subsequent processing.
The bacterial 16S rRNA V4 region was amplified from extracted DNA using barcoded specific primer 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), Phusion High-Fidelity PCR Master Mix with GC Buffer, and high-efficiency high-fidelity enzymes. The PCR products were detected by electrophoresis on 2% agarose gel and mixed with the same volume of 1 × loading buffer (contained SYB green), then mixture PCR products were purified with Qiagen Gel Extraction Kit (Qiagen, Germany).
Library Construction and Sequencing
Sequencing libraries were generated using TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, United States), and its quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Then, 250 bp paired-end reads were generated after the libraries were sequenced on an Illumina Novaseq 6000 platform at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China). Paired-end reads were merged using FLASH [23] (Version 1.2.7, Baltimore, MD, USA), and the splicing sequences were called raw tags. After quality filtration using QIIME [24] (Version 1.9.1) and chimera removal using UCHIME algorithm [25], high-quality clean tags [26] were finally obtained. Operational taxonomic units (OTUs) were obtained from sequences with ≥ 97% similarity using Uparse software [27] (Uparse v7.0.1001). Representative sequences were selected for each OTU, and the Silva Database [28] was used to annotate taxonomic information based on Mothur algorithm.
Alpha diversity metrics (Observed-species, Chao1, Shannon, Simpson, ACE, Good-coverage) and beta diversity on the weighted Unifrac distance were calculated by QIIME software based on normalized OTU data and both displayed with R software (Version 2.15.3). The Venn diagram, heatmap analysis of the major genus, and principal coordinates analysis (PCoA) results were displayed by WGCNA package, stat packages, and ggplot2 package in R software (Version 2.15.3). The Anosim and multi-response permutation procedure (MRPP) tests for significance were performed.
Statistical Analysis
All statistically significant differences were analyzed by a one-way ANOVA procedure followed by Tukey’s test with SPSS 24 software (IBM, Chicago, USA). Unless otherwise stated, data points were expressed as mean values and error bars represent standard deviations of biological replicates. The results were considered statistically significant at P < 0.05.
Results
Bacillus sp. DU-106 Reduces AAD-Related Gastrointestinal Symptoms
The dry–wet weight ratio (Fig. 1A) of the BC group was extremely significantly higher than the other groups at the beginning of the intervention period. The fecal morphology of the mice in the model group was all level 1 or above, while the BC group was all level 0 (Fig. S1A from supplementary materials). These results supported that the mice were in a diarrhea state, and the model was established successfully.
Dry–wet weight ratio of feces from mice (A). The dry–wet weight ratio of feces is negatively correlated with its moisture content. Changes of diarrhea rate in each group during the intervention period (B), the diarrhea rates were obtained from the number of mice with diarrhea/the number of mice in the group × 100%
The probiotic complex significantly increased the dry–wet ratio (Fig. 1A) and decreased the diarrhea rate (Fig. 1B) (P < 0.05) compare with the MC group at day 3, indicating that the compound probiotics worked. The dry–wet ratio and diarrhea rate of the DU-106 group was significantly different (P < 0.05) from the MC group at day 4, indicating that the Bacillus sp. DU-106 began to take effect and could accelerate the recovery of diarrhea in mice. The difference in the diarrhea rate between the HD, LD, and PC group denoted that the Bacillus sp. DU-106 had a dose–response relationship and the improvement in the diarrhea of mice was similar with the compound probiotics.
Bacillus sp. DU-106 Enhances the Immune Function of AAD Mice
The level of IgA (Fig. 2A) in treatment group and the IL-4 level in MC group (Fig. S2C from supplementary materials) were both increased (P < 0.01) compared with the BC group, but compound probiotics and high dose of DU-106 alleviated the IL-4 tendency of AAD mice. Besides, the IgG, IFN-γ, and IL-6 (Fig. S2 from supplementary materials) had no significant changes between each group. These results supported that the tested substance, and the compound probiotics had a certain effect of enhancing immunity.
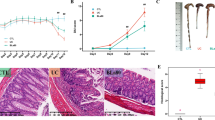
Effects of Bacillus sp. DU-106 supplement on Immunoglobulin A (A). The V/C ratio (D) was based on the length of villi (B) and the depth of crypt (C) in the small intestine. The values are presented as mean ± SD (n = 10). Differences were assessed by ANOVA. *P < 0.05, **P < 0.01 vs the BC group; ΔP < 0.05, ΔΔP < 0.01 vs the MC group
Bacillus sp. DU-106 Improves Gut Barrier Integrity in AAD Mice
Compared with BC group, villus length (Fig. 2B) (P > 0.05) and crypt depth (Fig. 2C) (P < 0.05) were both increased in MC group, but the value of V/C ratio (Fig. 2D) was decreased (P < 0.05). After 2 weeks of intervention of compound probiotics and DU-106, compared with the MC group, the crypt of AAD mice shallowed, but the villus length (P < 0.05) and the ratio of V/C (P < 0.01) were both markedly increased. The results of significance analysis showed that DU-106 restored the damage of intestinal villi caused by antibiotics, and the effect of high dose DU-106 was similar to compound probiotics.
As shown in Fig. 3, obvious swelling, shortening, thickening, fracture, and other phenomena occurred in AAD mice, along with crypt deepened, intestinal mucosa damaged, resulted in a decrease in villi height and density. The pathological symptoms of jejunum villi in treatment group were significantly relieved, indicating that DU-106 and compound probiotics could ameliorate the damage of intestinal mucosa in AAD mice.
Sequencing Data Quality Assessment and Alpha Diversity Analysis
A total of 2,879,716 paired-end reads were obtained by sequencing of 30 fecal samples, and an average of 79,593 ± 6699 clean tags were produced from each sample after selection. The rank abundance curves (Fig. 4A) were approaching the saturation plateau. These results revealed that the sequencing depth covered the majority of species as well as reflected most of the diversity and the true state of samples. The overall differences in gut microbiota composition and structure were evaluated by α and β diversities.
Alpha diversity analysis of each group. The smoother the rank abundance curve (A), the more uniform the distribution of OTUs. The longer the span of curve A on the horizontal axis, the more abundant the species content of the sample. Shannon index (B) and Venn diagram (D) are positively correlated with species diversity, F/B ratio of different groups (C) is the ratio of Firmicutes to Bacteroidetes. * P < 0.05 vs. the BC group
It can be found (Fig. 4B–D; Fig. S3B–D from supplementary materials) that alpha diversity indexes were generally decreased in AAD mice. The Shannon index was significantly decreased in the MC group, while the high dose DU-106 treatment could reverse this reduction, and the HD group could improve alpha diversity index compared with the LD group, but the above two changes did not reach the significant level. The alpha diversity was related to the richness of bacterial species and the number of bacteria in the gut [29]. The Venn diagram (Fig. 4D) showed that DU-106 and other groups shared 283 OTU, and L, H group owned 7, 16 OTU for unique respectively. These results showed that Bacillus sp. DU-106 intervention concentrated the community structure and increased the diversity and richness of intestinal flora.
Bacillus sp. DU-106 Alters Gut Microbiota Composition in AAD Mice
The PCoA plot (Fig. 5A) presented a distinct clustering of microbiota structure for five groups based on Bray–Curtis distances. Group BC was concentrated in the upper right quadrant while other groups were gathered in the left lower quadrant, but group HD shifted the overall compositions of the gut microbiota in the MC group toward the composition of the BC group, suggesting that high-dose Bacillus sp. DU-106 had played a positive role in restoring the change caused by the antibiotic. Both Anosim and MRPP analyses (Fig. 5B) supported that statistically significant separation was found between MC and the microbiota of BC and LD group; while we did not detect an obvious difference among PC, HD, and MC. UPGMA cluster analysis (Fig. 5C) presented similar results.
Bacillus sp. DU-106 alters gut microbiota composition in antibiotic-associated diarrhea mice. Principal coordinates analysis (A) reflects the differences between samples directly. The contribution to variation of X-axis and Y-axis is 37.18% and 11.50%, respectively. Anosim/MRPP (B) analysis presents the difference at OTU level between each group. Relative abundance of the dominant bacterial and UPGMA cluster analysis at the phylum level (C)
The overall microbial composition of all groups was displayed at the phylum and genus levels (Figs. 5C and 6A–B). Bacteroidota, Firmicutes, Proteobacteria, and Verrucomicrobiota were the most dominant phylum across all groups (Fig. 5C). The MC group showed a significant decrease in the relative abundance of Firmicutes but increase in the relative abundance of Bacteroidota and Verrucomicrobiota compared with the BC group, but the PC and HD group changed this variation tendency. The relative abundance of Proteobacteria rose significantly in AAD mice, LD could weaken this change. Histogram (Fig. 6A) showed that the number of identifiable genus in PC group and Du-106 group was more than that in BC group and MC group. At the genus level, we found the Alistipes, Roseburia, Hungatella, Eubacterium-xylanophilum, Lachnospiraceae-UCG-001, Intestinimonas, and Lachnospiraceae-NK4A136 were decreased but Klebsiella increased significantly in the MC group compared with the BC group, whereas high-dose of Bacillus sp. DU-106 supplementation reversed these alterations (P < 0.05; Fig. 6B).
Distribution and comparison of bacterial genus among groups. Histogram of relative abundance at the genus level (A). The heatmap analysis of each group at the genus level (B). Compared with the MC group, the bacteria that increased significantly and decreased significantly were marked red and blue, respectively
Discussion
Excessive antibiotic intake could impair resistance to pathogens and intestinal mucosal injury as well as gut microbiota dysbiosis and lead to diarrhea [19]. Several lines of evidence supported the beneficial effects of probiotics on AAD [9, 19, 30]. Bacillus strains are emerging probiotics in recent years, but the effectiveness of Bacillus on AAD improvement remains poorly understood. In the present study, AAD mice were intervened by Bacillus sp. DU-106, using enteropathology, immunomics, and gut microbiome, to determine whether and if yes, how potential probiotics DU-106 was able to ameliorate the gastrointestinal symptoms in AAD mice. Our result showed that Bacillus sp. DU-106 administration sped up the restoration of AAD mice. It might be connected with the regulation of intestinal flora, improvement of gut barrier integrity, and enhancement of the immune function.
The dry–wet ratio of feces and diarrhea rate directly reflected the diarrhea condition of mice [21]. On the 6th day of the intervention period, there is no significant difference (P < 0.05) in the dry–wet ratio or diarrhea rate among all groups, pointing that the natural recovery of the mice took 6 days, and the gastrointestinal symptoms persisted for a span after antibiotics stopped. We can notice from Fig. 1 that the strain DU-106 and compound probiotics began to work on the 3rd day and significantly shortened the diarrhea process in AAD mice, which strongly supported the strain DU-106 possessed the ability to treat AAD. During the experiment, we found that a steady increase and no significant difference in body weight among all the groups presented in Fig. S1B (supplementary materials), which differed from other research [20, 30], whose mice experienced a significant weight loss during the modeling period. The discrepancy might be attributed to many reasons including but not limited to the dosage of antibiotics. A lower dose of ceftriaxone did not cause body weight loss in mice [31].
Cytokines were the mediators between immune cells and other types of cells. Interleukin series function in intestinal immunity, inflammation, and other processes. The inflammatory response of body is generally caused by disequilibrium between cytokines [32]. The results showed that IL-4 increased significantly in the MC group, as the level of IL-6 and IFN-γ did not change, lead dynamic balance disrupted, indicating that inflammation occurred in the AAD mice, which meant the body’s immunity weakened. The tendency to destroy the balance was alleviated by probiotics or high-dose DU-106, which were similar to the results of Qi’s research [33]. IgA and IgG were the two highest content immune proteins produced by plasma cells. They were the key to the immune response, and their content positively correlated with the body’s immune function. The IgA content in the treatment groups increased significantly, which suggested that the immunity of AAD mice was improved by strain DU-106, which was in line with our previous study on DU-106 to enhance immunity [20]. These results suggest that AAD was associated with fluctuations in cytokines. And the strain DU-106 might possess the anti-inflammatory ability. The acceleration of DU-106 to AAD restoration may be related to its enhancement of the immune function.
The intestinal villi and crypt were essential physical barriers preventing luminal pathogens from entering the bloodstream and provided a living environment for gut microbiota [34]. The invasion of foreign substances can destroy the intestinal villi and increase the depth of the crypt. The ratio of villi length to crypt depth indicates the functional status of the intestinal mucosa, and a significant decrease in this ratio represents that intestinal mucosa is damaged, which leads to the disturbance of intestinal flora, closely linked to diarrhea [35]. Unlike the previous results [36], the length of intestinal villi in our AAD mice was increased, which might be related to the individual diversity of mice or the difference in section making. As the villi and crypt were difficult to ensure that they were completely vertical cut during section making, which caused a discrepancy in villi length and crypt depth. However, the antibiotics significantly deepened the crypt and decreased the V/C ratio in mice. We also observed that the AAD mice exhibited a rise of frequency on edema, rupture, and fragmentation of intestinal villus interstitium. Villi were damaged while its fragments appeared in the intestinal lumen simultaneously, which confirmed that antibiotics could destroy the intestinal physical barrier. Similar to compound probiotics, we found that strain DU-106 could promote the growth of intestinal villi and recovery of crypt damage or intestinal mucosa injury in AAD mice. The intestinal epithelial barrier was an essential part of gut immunity [37]. Correspondingly, strain DU-106 could improve the intestinal immunity of AAD mice. This result indicated that the underlying mechanism of DU-106 speed up AAD recovery involves the improvement of intestinal barrier or intestinal immunity.
The development of diarrhea was generally accompanied by the perturbation of intestinal flora [38]. In the present study, the OTUs and Shannon index were used to measure the density and diversity of bacterial populations, respectively. The decline in the diversity of gut microbiota was regarded as one of the bases of the development of AAD [39]. We found that the AAD mice had lower unique OTUs and alpha diversity index compared with the BC group and treated group. These results showed that the intestinal bacterial density and diversity were decreased upon AAD but restored by high-dose DU-106 or compound probiotic treatment, and both have similar recovery effects. Our findings suggest that the strain DU-106 may inhibit AAD associated with the improved bacterial growth and proliferation.
Changes in the intestinal microbiota were related to gut diseases development such as diarrhea [39] and inflammatory bowel disease (IBD) [40]. In our study, Bacteroidota, Firmicutes, Proteobacteria, and Verrucomicrobiota were the predominant gut microbiota of all groups at the phylum level. Figure 4C shows that antibiotics decreased the relative abundance of Firmicutes but increased the Bacteroidetes, caused a decrease in the F/B ratio, and notably influenced the dominating components of intestinal microbiota, consistent with the previous study [41]. By contrast, high-dose DU-106 and probiotics reduce the relative abundances of Bacteroidota, Proteobacteria, and Verrucomicrobiota but increase the Firmicutes, which brought a huge alternation to microbial community structure and made the bacterial composition of AAD mice closer to the BC mice at the phylum level.
The alteration of some genus is the pivotal mechanism to ameliorate AAD [21]. Prior studies noted that the AAD increased the relative abundance of Salmonella [19], Cl. difficile [39], and other pathogenic bacteria that colonized the intestinal tract, which induced disruption of the gut microbiome and lasted for a long time. At the genus level, antibiotics decreased the relative abundance of Alistipes, Roseburia, Hungatella, Eu. xylanophilum, Lach.UCG-001, Intestinimonas, and Lach. NK4A136, but increased the Klebsiella. Opportunistic pathogenic bacteria did not cause disease under normal circumstances, but it would disorder the gut microbiota of mice after the interference of antibiotics, leading to diarrhea and other symptoms [41]. Klebsiella was one of the opportunistic pathogens, which associated with AAD [42]. Other reduced bacteria genera in AAD mice were also associated with health. Alistipes is a relatively new genus of bacteria, isolated from clinical samples. A review suggested it might have a protective effect against colitis and cardiovascular fibrotic disorders [43]. Roseburia, Intestinimonas [44], and Eu. xylanophilum [45] were common SCFA producing bacteria, which contributed to the recovery of tight junction barrier by affecting the expression of claudin-2, occludin, cingulin, and zonula occludens proteins-1, 2 [46]. The relative abundance of Lach. UCG-001 [47] and Lach. NK4A136 [48] decreased significantly in dysbacteriosis mice, and the rebound occurred in the two genera after the intervention of inulin or the conversion to a normal diet. Hungatella hathewayi normalize the blood taurine levels and significantly reduced the risk of intracranial aneurysm formation and rupture in mice [49]. However, the aforesaid variable bacteria in AAD mice were reversed and normalized by high-dose DU-106 and compound probiotics. Thus, it is possible to state that the strain DU-106 could increase the beneficial community relevant to the gut barrier integrity and decrease the adverse flora, which can be an effective and probiotic candidate with a therapeutical effect on AAD.
Conclusion
Taken collectively, dietary supplementation of Bacillus sp. DU-106 exerted health beneficial effects against AAD by alleviating diarrhea symptoms, improving gut barrier integrity and immunoglobulin-A content as well as restoring the diversity and composition of gut microbiota. Thus Bacillus sp. DU-106 could serve as probiotics to treat AAD and more detailed molecular mechanisms of Bacillus sp. DU-106 on alleviating AAD needed to be explored.
Data Availability
All 16 s rRNA Illumina amplicon sequencing data presented in this study are openly available in The National Centre for Biotechnology Information (NCBI) Sequence Read Archive (SRA) with accession number PRJNA730997 (SUB9677559).
References
Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R (2016) Bowel disorders Gastroenterol 150(6):1393–1407. https://doi.org/10.1053/j.gastro.2016.02.031
Alemayehu B, Ayele BT, Valsangiacomo C, Ambelu A (2020) Spatiotemporal and hotspot detection of U5-children diarrhea in resource-limited areas of Ethiopia. Sci Rep 10:10997. https://doi.org/10.1038/s41598-020-67623-0
Lu JJ, Mao DC, Li X, Li X, Ma YQ, Luan YQ, Cao Y, Luan YP (2020) Changes of intestinal microflora diversity in diarrhea model of KM mice and effects of Psidium guajava L. as the treatment agent for diarrhea. J Infect Public Heal 13(1):6–26. https://doi.org/10.1016/j.jiph.2019.04.015
Ebrahim NB, Atteraya MS (2021) Oral rehydration salts therapy use among children under five years of age with diarrhea in Ethiopia. J Public Heal Res 10(1). https://doi.org/10.4081/jphr.2021.1732
Bastard QL, Ward T, Sidiropoulos T, Hillmann BM, Chun CL, Sadowsky MJ, Knights D, Montassier E (2018) Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci Rep 8(1):6219. https://doi.org/10.1038/s41598-018-24342-x
Desjeux JF, Heyman M (1997) The acute infectious diarrhoeas as diseases of the intestinal mucosa. J Diarrhoeal Dis Res 15(4):224–231. https://doi.org/10.1097/00004836-199712000-00049
Coccorullo P, Strisciuglio C, Martinelli M, Miele E, Greco L, Staiano A (2010) Lactobacillus reuteri (DSM 17938) in infants with functional chronic constipation: a double-blind, randomized, placebo-controlled study. J Pediatr 157:598–602. https://doi.org/10.1016/j.jpeds.2010.04.066
Mantegazza C, Molinari P, D’Auria E, Sonnino M, Morelli L, Zuccotti GV (2018) Probiotics and antibiotic-associated diarrhea in children: a review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol Res 128:63–72. https://doi.org/10.1016/j.phrs.2017.08.001
Mekonnen SA, Merenstein D, Fraser CM, Marco ML (2020) Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr Opin Biotechnol 61:226–234. https://doi.org/10.1016/j.copbio.2020.01.005
Badger VO, Ledeboer NA, Graham MB, Edmiston CE (2012) Clostridium difficile. J Parenter Enter Nutr 36:645–662. https://doi.org/10.1177/0148607112446703
Xiao JZ, Zhang Y, Yang ZN (2014) Lactic acid bacteria in health and disease. In: Zhang H (eds) Lactic acid bacteria. Springer, Dordrecht, pp 303–374. https://doi.org/10.1007/978-94-017-8841-0_5
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Tang C, Xie BJ, Zong Q, Sun ZD (2019) Proanthocyanidins and probiotics combination supplementation ameliorated intestinal injury in Enterotoxigenic Escherichia coli infected diarrhea mice. J Funct Foods 62:103521. https://doi.org/10.1016/j.jff.2019.103521
Czerucka D, Rampal P (2019) Diversity of Saccharomyces boulardii CNCM I-745 mechanisms of action against intestinal infections. World J Gastroenterol 25(18):2188–2203. https://doi.org/10.3748/wjg.v25.i18.2188
Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D (2007) Meta-analysis: Lactobacillus GG for treating acute diarrhoea in children. Aliment Pharmacol Ther 25(8):871–881. https://doi.org/10.1111/j.1365-2036.2007.03282.x
Cao J, Yu ZM, Liu WY, Zhao JX, Zhang H, Zhai QX, Chen W (2020) Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J Funct Foods 64:103643. https://doi.org/10.1016/j.jff.2019.103643
Lin SY, Hung ATY, Lu JJ (2011) Effects of supplement with different level of Bacillus coagulans as probiotics on growth performance and intestinal microflora populations of broiler chickens. J Anim Vet Adv 10(1):111–114. https://doi.org/10.3923/javaa.2011.111.114
Li P, Tian WN, Jiang Z, Liang ZH, Wu XY, Du B (2018) Genomic characterization and probiotic potency of Bacillus sp. DU-106, a highly effective producer of l-lactic acid isolated from fermented yogurt. Front Microbiol 9:2216. https://doi.org/10.3389/fmicb.2018.02216
Zhang W, Zhu B, Xu J, Liu YY, Qiu EQ, Li ZJ, Li ZC, He Y, Zhou HW, Bai Y, Zhi FC et al (2018) Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front Immunol 9:1040. https://doi.org/10.3389/fimmu.2018.01040
Lai YJ, Chen SM, Luo PH, Li P, Du B (2020) Dietary supplementation of Bacillus sp. DU106 activates innate immunity and regulates intestinal microbiota in mice. J Funct Foods 75:104247. https://doi.org/10.1016/j.jff.2020.104247
Zhang N, Liang TS, Jin Q, Shen C, Zhang YF, Jing P (2019) Chinese yam (Dioscorea opposita Thunb.) alleviates antibiotic-associated diarrhea, modifies intestinal microbiota, and increases the level of short-chain fatty acids in mice. Food Res Int 122:191–198. https://doi.org/10.1016/j.foodres.2019.04.016
Huang JZ, Xiao N, Sun YY, Wu SS, Tian WN, Lai YJ, Li P, Du B (2021) Supplementation of Bacillus sp. DU-106 reduces hypercholesterolemia and ameliorates gut dysbiosis in high-fat diet rats. Appl Microbiol Biotechnol 105:287–299. https://doi.org/10.1007/s00253-020-10977-2
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinform 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinform 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, Mills DA, Caporaso JG (2013) Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat Methods 10:57–59. https://doi.org/10.1038/nmeth.2276
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596. https://doi.org/10.1093/nar/gks1219
Wang Y, Chen ZD, Tang MJ, Zhou HX, Yuan XL, Ashraf MA, Mao ST, Wang J (2017) Expression of Ldh-c (sperm-specific lactate dehydrogenase gene) in skeletal muscle of plateau pika, Ochotona curzoniae, and its effect on anaerobic glycolysis. Pak J Zool 49:905–913. https://doi.org/10.17582/journal.pjz/2017.49.3.905.913
Kambale RM, Nancy FI, Ngaboyeka GA, Kasengi JB, Bindels LB, Linden DVD (2020) Effects of probiotics and synbiotics on diarrhea in undernourished children: systematic review with meta-analysis. Clin Nutr 40(5):3158–3169. https://doi.org/10.1016/j.clnu.2020.12.026
König J, Jerry W, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ (2016) Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 7(10):e196. https://doi.org/10.1038/ctg.2016.54
Huang J, Wang YL, Jiang DB, Zhou J, Huang XK (2010) The sympathetic-vagal balance against endotoxemia. J Neural Transm 117:729–735. https://doi.org/10.1007/s00702-010-0407-6
Qi YL, Chen LX, Gao K, Shao ZJ, Huo XH, Hua M, Liu SX, Sun YS, Li SS (2019) Effects of Schisandra chinensis polysaccharides on rats with antibiotic-associated diarrhea. Int J Biol Macromol 124:627–634. https://doi.org/10.1016/j.ijbiomac.2018.11.250
Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, Post SVD, Svensson F, Rodríguez-Piñeiro AM, Nyström EEL et al (2014) The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 260:8–20. https://doi.org/10.1111/imr.12182
Lange K, Buerger M, Stallmach A, Bruns T (2016) Effects of antibiotics on gut microbiota. Dig Dis 34:260–268. https://doi.org/10.1159/000443360
Li SS, Qi YL, Chen LX, Qu D, Li ZM, Gao K, Chen JB, Sun YS (2019) Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int J Biol Macromol 124:931–937. https://doi.org/10.1016/j.ijbiomac.2018.11.271
Guo Q, Goldenberg JZ, Humphrey C, Dib R, Johnston BC (2019) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 4(4):CD004827. https://doi.org/10.1002/14651858.CD004827.pub5
Young VB, Schmidt TM (2004) Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol 42:1203–1206. https://doi.org/10.1128/jcm.42.3.1203-1206.2004
Nasiri MJ, Goudarzi M, Hajikhani B, Ghazi M, Goudarzi H, Pouriran R (2018) Clostridioides (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: a systematic review and meta-analysis. Anaerobe 50:32–37. https://doi.org/10.1016/j.anaerobe.2018.01.011
Willing BP, Russell SL, Finlay BB (2011) Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 9:233–243. https://doi.org/10.1038/nrmicro2536
Bergogne-Bérézin E (2000) Treatment and prevention of antibiotic associated diarrhea. Int J Antimicrob Agents 16:521–526. https://doi.org/10.1016/S0924-8579(00)00293-4
Larcombe S, Hutton ML, Lyras D (2016) Involvement of bacteria other than Clostridium difficile in antibiotic-associated diarrhoea. Trends Microbiol 24:463–476. https://doi.org/10.1016/j.tim.2016.02.001
Parker BJ, Wearsch P, Veloo ACM, Rodriguez-Palacios A (2020) The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol 11:906. https://doi.org/10.3389/fimmu.2020.00906
Bui TPN, Troise AD, Nijsse B, Roviello GN, Fogliano V, Vos WM (2020) Intestinimonas-like bacteria are important butyrate producers that utilize Nε-fructosyllysine and lysine in formula-fed infants and adults. J Funct Foods 70:103974. https://doi.org/10.1016/j.jff.2020.103974
Ma XM, Zhou ZH, Zhang XJ, Fan MJ, Hong YY, Feng Y, Dong QH, Diao HY, Wang GY (2020) Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice. Cell Biol Toxicol 36:509–515. https://doi.org/10.1007/s10565-020-09518-4
Plöger S, Stumpff F, Penner GB, Schulzke JD, Gäbel G, Martens H, Shen ZM, Günzel D, Aschenbach JR (2012) Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann NY Acad Sci 1258:52–59. https://doi.org/10.1111/j.1749-6632.2012.06553.x
Song XF, Zhong L, Lyu N, Liu F, Li BX, Hao YN, Xue Y, Li J, Feng YQ, Ma Y et al (2019) Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Genom Proteom Bioinform 17:64–75. https://doi.org/10.1016/j.gpb.2019.03.001
Zhang ZM, Yang L, Wan Y, Liu C, Jiang S, Shang EX, Duan JA (2021) Integrated gut microbiota and fecal metabolomics reveal the renoprotective effect of rehmanniae radix preparata and corni fructus on adenine-induced CKD rats. J Chromatogr B 1174:122728. https://doi.org/10.1016/j.jchromb.2021.122728
Li H, Xu HC, Li YX, Jiang YH, Hu YM, Liu TT, Tian XQ, Zhao XH, Zhu YD, Wang SX et al (2020) Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat Commun 11:3218. https://doi.org/10.1038/s41467-020-16990-3
Acknowledgements
We would like to thank Novogene (Beijing, China) for the use of Illumina platform.
Funding
This work was financially supported by China Agriculture Research System of MOF and MARA (CARS-21) and the Natural Science Foundation of Guangdong Province (2020A1515011268).
Author information
Authors and Affiliations
Contributions
Experimental concept and design: PL and BD. Experimental operation: DH, HC, and XD. Acquisition of data: JH and SL. Data curation and analysis: DH and YC. Writing of the original draft: DH, YC, and PL. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All the experiments involving animals were in compliance with the Regulations of China on the Administration of Laboratory Animals.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, D., Chen, Y., Chen, H. et al. Supplementation of Bacillus sp. DU-106 Alleviates Antibiotic-Associated Diarrhea in Association with the Regulation of Intestinal Microbiota in Mice. Probiotics & Antimicro. Prot. 14, 372–383 (2022). https://doi.org/10.1007/s12602-022-09906-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09906-8