Abstract
The skin is the largest organ in the human body, and it orchestrates many functions that are fundamentally important for our survival. Although the skin might appear to present a relatively inhospitable or even hostile environment, a multitude of commensals and also some potentially pathogenic microorganisms have successfully adapted to survive and/or thrive within the diverse ecological niches created by the skin’s topographical architecture. Dysbiosis within these microbial populations can result in the emergence and pathological progression of skin diseases. Unsurprisingly, this has led to a new focus of research both for the medical dermatology and cosmetic industries that is concerned with modulation of the skin microbiome to help address common microbially mediated or modulated conditions such as acne, body odour, and atopic dermatitis. This review presents an overview of our current understanding of the complex relationship of the skin with its microbiome and then introduces the concept of probiotic intervention for the management of microbial dysbiosis within the skin ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skin comprises around 15% of the total adult human body weight and on average has a surface area of 1.5–2 m2 [1]. A primary function of the skin is to act as a physical barrier to pathogenic microbes and toxic substances, and indeed, it can be considered to be one of the body’s key first lines of defence against infection, along with the innate and adaptive immune systems [2]. Its other functions include preventing transepidermal water loss (TEWL), thermoregulation, structural support, and production of vitamins, all of which help to sustain a healthy body [2,3,4]. From a microbiological viewpoint, the surface of the skin can be considered to provide an overlapping spectrum of specialized ecosystems, each comprised of its own menagerie of bacteria, fungi, viruses, and skin mites, and as for all of the other anatomical sites in the body, the skin-adapted organisms and viruses have specifically evolved and adapted to inhabit a highly specific ecological niche. The numbers of bacteria on the skin are considerably lower in comparison with those present in the gut: some estimates ranging from an average of 1 million (106) per cm2 [5] compared to more than 1 trillion (1012) microorganisms in the large intestine [6]. The composition of the skin microbiota varies widely in different body sites (Table 1) with the differences mediated by a multitude of factors including tissue-specific topographical considerations, internal host-mediated factors, and the external environment [7]. The diversity of bacteria within a particular body site and interpretation of the potential implications of variations between different sites can be studied in terms of alpha and beta diversity. “Alpha diversity” is a measure of bacterial diversity within a sample, whereas “beta diversity” is a measure of bacterial diversity between different regions [8]. Major organs, including the gut, skin, and oral mucosa, harbour high levels of alpha diversity, compared to the low levels of alpha diversity in the vaginal mucosa. An interesting example in which both beta and alpha diversity exist within a single region is the oral cavity, which comprises a variety of hard and soft surfaces, differing atmospheres, and exposure to a heterogeneous supply of dietary nutrients [9].
Structure of the Skin
The skin comprises three main layers: epidermis, dermis, and subcutaneous layer (Fig. 1). Each layer contributes to help provide a barrier between the exterior environment and the internal body and each may contain additional “sub” layers [2]. The epidermis is composed of four distinct layers: stratum corneum, stratum granulosum, stratum spinosum, and stratum germinativum (from the outermost to innermost). Keratinocytes make up 80% of the epidermis layer and provide structural support, aid in sustaining the microbiota, and help counter pathogens through modulation of the innate immune system [10]. The structure of the stratum corneum contributes to the skin’s remarkable barrier function, helps prevent water loss, regulates the synthesis of epidermal DNA and lipids, and also aids defence against pathogen penetration [11]. Other cells present in the epidermis include Langerhans and T epidermal cells, which contribute to the detection of antigens in the epidermis for the innate and adaptive immune system; melanocytes, which synthesize the pigment melanin; and Merkle cells, which act as slow-adapting mechanoreceptors that respond to stimuli on the skin [12, 13].
Physical structure of the skin with corresponding immune cells, bacteria, and appendages that inhabit each layer (adapted from “Anatomy of the Skin”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates)
The dermis is the second major layer of the skin. It is around 2–5 mm thick and contains connective tissue, a network of nerves, and vascular structures as well as additional skin appendages including hair follicles as well as a variety of eccrine, sebaceous, and apocrine glands. Immune cells, including macrophages and dendritic cells, are also present, and these help initiate innate immune responses within the skin. Dermal collagen and elastic fibres provide pliability, tensile strength, and elasticity to the skin, whereas nerve and vasculature networks maintain thermoregulation and allow for the recruitment of immune cells and the detection of stimuli on the skin surface [10].
The third and innermost layer is the subcutaneous fat layer which provides the skin with its characteristic buoyancy and functions as an energy storehouse [10].
Skin Microbiome
The human skin microbiome is the community of microorganisms (i.e. the microbiota) which inhabits the human skin together with its “theatre of activity” (i.e. the associated microbial structural elements such as proteins, lipids, polysaccharides, and nucleic acids. The skin microbiota comprises bacteria, fungi, viruses, and skin mites [14]. The four main bacterial phyla identified on the skin are Actinobacteria (52%), Firmicutes (24%), Proteobacteria (17%), and Bacteroidetes (7%) [14]. Predominant genera include Corynebacterium, Propionibacterium, and Staphylococcus [4, 15]. Colonization within the human microbiome is initiated at birth, and the composition of the pioneer communities is strongly influenced by the route of delivery [7, 16]. The composition of the skin microbiota is then determined by a multitude of internal and external host factors generating inter- and intrapersonal variations. However, a core set of microbial species are present across all individuals. Rare and transient species of microbes account for interpersonal variations which can be attributed to host lifestyle and environmental and genetic factors [2]. Intrapersonal variation is frequently seen on the forearms and palms of the hand, and this can be attributed to the specific niche, lifestyle factors, hygiene rituals, and cosmetic products (e.g. hand creams) used in these areas, e.g. [8]. Characteristic microbiota differences have also been detected between males and females in certain regions of the body such as the axilla [2].
Types of Skin
Skin type can be broadly classified into three main categories: oily, moist, and dry with each type hosting a distinctive microbial community (Fig. 2).
Oily skin can be found commonly on areas such as the forehead and nose, forming the T-zone. These areas contain a high density of pilosebaceous units which produce sebum, a lipid-rich substance that gives this skin an oily composition. Sebum secreted by the sebaceous glands act as a moisture barrier that prevents the skin from desiccation and supplies the contiguous microbiota with nutrients. Cutibacterium acnes (formerly known as Propionibacterium acnes), a common commensal of the skin, is particularly abundant in sebaceous environments within the pilosebaceous units. C. acnes has adapted to thrive in an oily, anaerobic environment, accessing sebum as a source of nutrients through the action of the enzyme lipase which degrades sebum and releases free fatty acids, thereby contributing to the acidic nature of the surface of the skin [16, 19]. The resulting acidic environment supports the growth of characteristic commensal bacteria whilst inhibiting proliferation by opportunistic pathogens such as Staphylococcus aureus and Streptococcus pyogenes [2, 20].
Moist skin is typically found in unexposed areas of the body and includes the armpits, skin folds such as the elbows, between toes, and groyne areas. Increased moisture in these areas is due to the increased production of sweat. Moist environments favour a more diverse community of Gramme-negative and Gramme-positive commensal and pathogenic bacteria [21] with a predominance of Staphylococcus spp. and Corynebacterium spp. [22]. Some pathogens, such as the ubiquitous Pseudomonas aeruginosa, have also adapted to thrive in these conditions [21].
Dry skin sites such as the forearms, hands, legs, and parts of the feet harbour a vast community of microbes, the principal phyla being the Actinobacteria, Proteobacteria, Firmicutes, and Bacteroidetes. Several fungal species are also commonly present and these especially include Malassezia spp., Aspergillus spp., Cryptococcus spp., Rhodotorula spp., and Epicoccum spp. Malassezia spp. are particularly common, sometimes accounting for up to 80% of the entire fungal community [18, 19].
Stability of Skin Microbiome
The stability of the microbiome is influenced by the skin environment and the phylogeny of colonization. The stability of microbial niches is inversely proportional to their microbial diversity; the higher the microbial diversity, the less stable is the community composition [3]. Oily environments harbour more stable microbial niches than dryer sites due to the high perturbation and exposure to external stimuli [3]. Factors which can further affect this stability include exposure to antimicrobial treatments such as soaps and shampoos, application of skin care products, and changes to lifestyle factors including relocations to new environments, e.g. urban to rural, diet, immunosuppressive drugs, and illness. Changes in the stability of the microbiome suggest that there is microbial-host intercommunication which maintains a stable equilibrium [18].
Immune Modulation
Intercommunication between host and the microbiome helps to maintain a stable microbiota through modulation of the innate immune system [18]. For example, keratinocytes interact with microbes through pattern expression receptors (PRR) on the cell surface, constantly sampling the skin microbiota [2]. Recognition by PRRs can result in the release of antimicrobial peptides (AMPs), cytokines, and chemokines in response to microbial growth to help control the skin microbiota, reduce skin dysbiosis, and educate the immune system [2]. PPRs become desensitized when exposed to long-term commensal microbes like Staphylococcus epidermidis, which can help the skin distinguish between commensal and pathogenic microbes [20]. Skin appendages such as the pilosebaceous unit and eccrine secretions have immune modulation abilities [18]. Pilosebaceous units release AMPs in response to Gramme-positive bacterial activity, and the presence of free fatty acids provides antimicrobial effects. Eccrine secretions contain dermcidin, a weak AMP, providing further control over the microbiota [19].
The skin surface engages in symbiosis with diverse microbial niches working together to improve the overall innate immune response. Commensal bacteria contribute greatly to this relationship via the production of AMPs, selective bacteriocins, and phenol-soluble modulins which selectively inhibit the growth of pathogenic bacteria without harming themselves [19]. AMPs are important chemical modulators continuously released from cells in a controlled manner. In the presence of a pathogen, their concentration increases signalling to the immune system that something is wrong, and thus, they are a vital component of microbial-host communication [18].
Microbiome Analysis
Microbial Sampling Methods
Invasive and non-invasive techniques are involved in the collection of microbial samples from the skin (Fig. 3). Non-invasive techniques known as “flock sampling” require a cotton swab, to be rubbed against the skin surface to remove skin and bacterial cells. To collect samples from deeper layers of the skin, keratin tape or super glue has been found to be useful [18]. A punch biopsy is an invasive technique; however, due to its nature, it is used only to identify infections and to inform their appropriate antibiotic treatment.
Potential methods for sampling the skin microbiome: skin swab, tape strip, and punch biopsy (adapted from “Anatomy of the Skin”, and “Skin Biopsy” by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates)
Analytical Tools and Techniques
Swab Culture
A traditional approach to microbiota analysis involves the enumeration of the microbes present in swab samples using selective agar-based growth media. This technique is simple and does not require sophisticated equipment so it can be replicated in many laboratories. However, it is very species-specific and time-consuming. There are also limitations to the range of microbial species that can be cultured. For example, Staphylococcus spp. are relatively easily cultivated when compared with Cutibacterium and Corynebacterium species potentially resulting in inaccurate representations of their numbers in samples [7, 16].
DNA Amplicons
DNA amplicon sequencing allows for the specific regions of genomic material to be amplified. For bacteria, the highly conserved 16S rRNA primer is used to amplify certain regions, whereas in fungi, ITS1 is preferred [18]. The 16S rRNA primer is universal and found in almost all prokaryotes, aiding in assessing the complexity and diversity of microbial communities [18]. This method is limited, however, by its short-read lengths, sequencing errors, and its ability to distinguish between related species within the same phyla due to limited resolution [23]. This method also provides no information concerning microbe-host interactions or whether the organism is dead or alive at the time of sequencing. It is also expensive and time-consuming [18, 23].
Shot Gun Sequencing
Shot gun sequencing is a technique used to target the entire genomic material of a microorganism rather than a specific section. Whole genome sequencing involves breaking the DNA into segments of various sizes and then cloning the fragments into vectors [24]. This method offers a global view of microbial communities, allowing for better assessment of phylogenetic diversity regarding species and strains within phyla and the actions they are responsible for [16]. Limitations to this technique include the requirement for large DNA samples, which is difficult with normal skin swabs as they typically harvest only a relatively small biomass of microbes [24]. The requirement for analysis of large quantities of genomic information results in incomplete genomic analysis for some microbial populations [7].
Skin Commensal Bacteria
Commensal microbes inhabit different regions of the skin and contribute to symbiosis in various ways (Table 1). Something important to note is the switch from a homeostatic state to a disease state that can be affected by microorganisms. It is not fully understood what initiates this process and if it is microbe-dependent or environmentally triggered. Potential effectors include physical damage to the mucosa, disruption of the indigenous microbiota with antimicrobials, expression of specific virulence factors, and access to the site by excessive numbers of pathogens [7, 25].
Microbial Imbalance
Dysbiosis
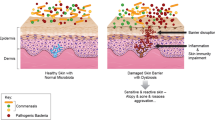
Dysbiosis is a state of microbial imbalance within areas of the body sometimes impacting on the vitality and health status of those areas. Illness is thought to be associated with dysbiosis due to a loss of microbial diversity and an increase in the relative numbers of pathogenic bacteria [27]. Dysbiosis can be induced and influenced by a wide variety of factors including the route by which a baby is born, hormonal shifts, undue stress, hygiene practices, personal care products, medicine use, nature, and food [4, 34]. Gender-linked differences may be based on physiological and anatomical differences such as hormone production, sebum production, soaps, fragrances, and personal care products that contain harsh preservatives and synthetic ingredients that can influence the microbiome by altering the pH of the skin [4, 34]. Many human skin disorders and diseases have been linked to changes within the skin microbiome, including acne, atopic dermatitis, and psoriasis [4, 27, 30, 31]. However, it is generally not established whether dysbiosis is the cause of these condition or the consequences.
Putative Inducers of Dysbiosis
Antibiotics
Antibiotics are one of the greatest medical discoveries of all time, having transformed the treatment of many previously incurable conditions. However, the long-term adverse effects of antibiotic administration have relatively seldom been given scientific consideration, including their effect on the body’s microbial composition, which can take many years to re-equilibrate [4, 35]. Antibiotics are the most common type of medication prescribed to children in the Western World [35]. In the USA alone, antibiotics account for 25% of all prescriptions for paediatric populations, with amoxicillin, azithromycin, and amoxicillin/clavulanate most commonly utilized [35]. The type and duration of antibiotic treatment differentially alter microbial communities by targeting and eradicating susceptible taxa [35, 36]. This can affect the microbiome in many ways such as contributing to the elimination of key commensal taxa and a generalized loss of biodiversity which can contribute to specific health issues [27, 36]. Communities of bacteria that have been eradicated from their specific niches leave these areas open for pathogenic species to colonize and flourish in, further disrupting microbiota homeostasis. Long-term therapy with topical antibiotics to treat acne has led to the development of resistant bacterial strains, decreasing clinical improvement of acne lesions [37]. A wide variety of health consequences including increased risk of excessive weight gain, asthma, allergies, and autoimmune diseases have been associated with early childhood antibiotic exposure [35, 38]. However, the literature is limited in directly implicating microbial dysbiosis as the link between childhood antibiotics and conditions later in life as there are a multitude of confounding factors including genetic predisposition and immune interactions [36].
Hygiene Hypothesis
The hygiene hypothesis proposes that exposure to environmental microorganisms and parasites is important for healthy development and maintenance of the immune system [35]. Within the Western world, there has been a cultural shift towards more stringent application of hygiene measures. One consequence of over-cleansing and increased antimicrobial use has been the relatively reduced exposure in early childhood to potentially pathogenic microbes, thereby reducing natural immune development and predisposing to the development of atopic conditions [39]. A study conducted in the USA looked at the effect of a farming environment on microbial exposure [40]. It was shown that farm children are exposed to a higher diversity of environmental bacteria and fungi. Dust collected in Amish communities who adhere to a traditional type of farming, with minimal changes in the last 2 centuries, prevented airway hyperresponsiveness, a trademark sign of asthma, whereas dust from farming communities that had industrialized with the times did not [40]. The concept behind over-cleansing has been fostered by the notion that all bacteria are bad bacteria. Increased hygiene not only causes changes within the taxa comprising the human microbiota, but it is also hypothesized to be one of the factors responsible for a rapid increase in the prevalence of atopic dermatitis (AD) over the past two decades [41].
Birth
In utero, a child’s skin is sterile, with colonization occurring during the birthing process, whilst over time, a complex ecosystem forms made up of primitive and transient microbes [5, 26]. Caesarean (C) section birth greatly affects the variety and composition of microbes colonizing the neonate as these babies are not directly exposed to the mothers vaginal and faecal microbiota [34, 42]. Instead, they immediately come in contact with the mother’s skin microbiota, as well as microbes shed by the hospital staff or present in the operating room. Microbes acquired by infants that are associated with vaginal delivery include Lactobacillus, Prevotella, and Candida albicans as compared to infants born via C-section that are predominantly colonized by maternal commensal skin bacteria: Staphylococcus, Streptococcus, and Cutibacterium [36, 42,43,44]. C-section delivery has the potential to interrupt the “normal” colonization by skin microbes that is achieved during labour and vaginal delivery [44]. The lack of exposure to maternal vaginal and faecal bacteria results in different microbial communities on the skin of the infant [45]. A study conducted by Chu looked at the differences in microbial communities on infant skin at birth and 6 weeks of age [28]. At birth, the microbial composition of neonates was influenced by the mode of delivery; however, these differences were absent at 6 weeks of age once the infants had been exposed to similar conditions, e.g. breastfeeding and cuddling with the parents [43]. Interestingly, studies show that neonates born via C-section who were swabbed with the mother’s vaginal secretions were more microbially like infants born vaginally versus those who experienced the standard C-section birth [46].
C-section delivery impinges not only on the microbiota but also on immune development. Labour induces immune responses within the uterine cavity that are absent in the case of elective C-sections, which affects the immune environment of the neonate [47]. Levels of cytokines have been shown to be reduced in C-section delivery, cells which are vitally important in initiating and maintaining the immune response [42]. Differences in microbial colonization patterns are important as they not only impact upon the final composition of the microbiota but they also influence the development of the infant’s immune system in the first months of life. Given that the first few months of life represent a vital time window in the ontogenesis of the immune system, including establishing tolerance, C-section delivery may impact on the lifelong risk of developing immune disease [42, 47]. Immune disorders known to be impacted via C-section delivery include type I diabetes and allergen development [46, 48]. A large meta-analysis detected a 19% increase in type I diabetes in children born by C-section after controlling for confounders such as gestational age, maternal age, and birth weight [47]. Researchers speculate that initial alterations in the microbiome of infants following C-section birth may play some role in the association between chronic disease and route of birth; however, not all findings are conclusive [46]. The influence of the microbiota hence highlights the importance of the development in early life and how it can impact upon individuals later in life.
Personal Care Products
Studies show that the exposure of normal, western skin to commonly used cosmetics, soaps, steroids etc. alters the natural skin microbiota, especially in the developed world [2, 49]. Cosmetic products that contain preservatives (e.g. izothiasoline in makeup, moisturizers), alcohols (e.g. propylene glycol in deodorants and antiperspirants), and paraffins and fragrances (in moisturizers, soaps, and perfumes) can non-selectively kill or inhibit the growth of commensal skin bacteria alongside pathogenic ones, disturbing the equilibrium and creating dysbiosis [50]. Therefore, it is important to choose products that contain ingredients that benefit not only the host but also the microorganisms existing symbiotically with the host. However, there is a lack of research into what ingredients disrupt specific species of bacteria and the effects of this disruption. More research is needed to generate more conclusive findings.
Skin Surface Acidity
The pH of the skin surface, known as the acid mantle, changes to suit the needs of the specific region and protects the skin against certain pathogens (Fig. 4) [27]. The acidity of the skin’s surface is important for maintaining a healthy ecosystem, skin barrier support, optimal conditions for physiological processes, and the growth of skin microbes [18, 51]. The acidic environment is established through microbial metabolites, appendageal secretions, free fatty acid production, local generation of protons in the lower stratum corneum, and production of urocanic acid [11]. The pH of the skin ranges between 4.7 and 5.75. Low-exposure, higher occluded regions like the axilla have higher pH values ranging as high as 7.9 and are considered to be anomalies sometimes referred to as “holes in the mantle” [18].
Differing pH across different regions of the skin to suit the needs of the microorganisms and physiological functions (adapted from “Adult Male”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates)
The topographical environment of the skin is important in determining microbial colonization at each site. Hair density, occlusion level, and the presence of specific gland appendages contribute to the acidity of the skin [52]. There is a positive relationship between lower pH values and preservation of the skin microflora, with a pH of 5 recognized as an optimal level [18]. Lower pH environments favour hydrophobic and electrostatic attractions between microbes and the skin, lowering overall bacterial dispersal rates from the skin [18]. Despite the skins harsh landscape, the acidic mantle has allowed for many different types of bacteria to thrive [52]. For example, the acidity of the skin favours the growth of Staphylococcus epidermis, a skin commensal bacterium whilst inhibiting the growth of the relatively pathogenic S. aureus and S. pyogenes [18, 22].
Conditions Arising from Dysbiosis
Factors influencing the skin microbiome either alone or in combination result in several skin disorders and diseases. These skin anomalies affect people of different age groups, gender, and race and require symptomatic and therapeutic interventions. Some of the common skin ailments have an underlying microbial element leading to overpopulation of pathogenic bacteria or fungi and contributing to infection and dysbiosis.
Acne
Acne vulgaris is a chronic skin condition involving hair follicles and sebaceous glands that affects up to 80% of adolescents [26, 53]. The microbiome of acne sufferers is characterized by a diverse group of microorganisms, but C. acnes and Malassezia have been linked to the development of acne lesions [53]. C. acnes, an anaerobic Gramme-positive bacterium, influences the development of acne via sebum commensalism, comedone formation (whiteheads and blackheads), hyperproliferation of keratinocytes, and aggravating inflammation in the skin [54]. C. acnes lesions create an anaerobic microenvironment that aids the growth of C. acnes further [54]. Malassezia are lipophilic fungi associated with sebum-rich areas of skin [4].
Initially increased sebum production is related to a change in hormones which first occurs around puberty; hence, teenagers can be the initial sufferers of acne [54]. It is also the important trigger that provides optimal living conditions for the C. acnes to thrive [27]. Whilst there are bacteria that contribute to the formation of acne, there are also some that work in favour of the host including S. epidermidis. S. epidermidis antagonizes C. acnes by fermenting glycerol which creates zones of inhibition to repel overgrown colonies of C. acnes [34, 55]. C. acnes is a commensal in healthy, unaffected skin, yet it is unknown what triggers the bacteria to be implicated in the disease state and become pathogenic [49]. Acne is classically treated with topical and oral antibiotics, commonly macrolides, clindamycin, and tetracyclines. Treatment of acne with antibiotics has caused high levels of resistance to occur which raises serious issues across multiple areas of medicine. For example, resistance towards the antibiotic clindamycin has increased from 4% in 1999 to 90.4% in 2016 [53]. Not only does it result in increased resistance rates but the amount of C. acnes on the skin also increases and it remains longer which can manifest as a re-occurrence of acne.
Atopic Dermatitis
Atopic dermatitis (AD) is a condition resulting in dry, red, and itchy skin. Eczema is thought to develop due to barrier disruptions in the upper skin layers leading to the loss of water through the skin layers known as transepidermal water loss (TEWL) causing dryness [55]. AD is a chronic condition that usually manifests in childhood but then sometimes flaring up periodically in adulthood [56]. It is commonly accompanied by asthma and food allergies, a collective of conditions sometimes referred to as the atopic march. Prevalence has increased over the past decade with 15–20% of children now affected and 2–10% of adults [30]. There is no current cure for AD, only supportive medications to help deal with symptoms, including the application of topical corticosteroids and hydrating moisturizers. Although the exact cause of eczema is unknown, S. aureus colonization is typically present in eczema lesions [56]. Ninety percent of AD patients are colonized with S aureus on both lesioned and non-lesioned skin compared with less than 5% of healthy individuals [5, 9]. Genetic and environmental factors contribute to the disease, with skin barrier dysfunction being critical for the pathogenesis of S. aureus infection [30]. Dry skin and hyperkeratinization, characteristic features of AD, act as adherence factors for S. aureus [57]. Bacteria that reside on normal skin provide some protection against S. aureus whereas the relative absence of competitive bacteria on the skin of subjects with AD and decreased bacterial diversity in these populations was strongly associated with increased colonization by S. aureus [28, 44]. The administration of specifically selected bacteria to reduce the abundance of S. aureus is inherently superior to the use of current pharmaceutically derived antibiotics because they do not disrupt cutaneous homeostasis by nonspecific killing of the indigenous microbiota [28]. For example, the common skin commensal S. hominis was found to significantly decrease the relative abundance of S. aureus when applied to the forearm of individuals with AD compared to the use of classical treatments, e.g. lotion [28, 58].
Psoriasis
Psoriasis vulgaris (PV) is a chronic inflammatory skin condition characterized by rapid proliferation of keratinocytes and infiltration of immune cells with formation of plaques of thickened skin giving the appearance of scales [59]. It is a non-communicable disease affecting around 2% of the world’s population [4] and can affect individuals of any age [55]. Not only does psoriasis impact upon an individual physically but it can also adversely influence a patient’s quality of life and emotional status. The aetiology of PV is not well-understood but it is known to be the product of a complex interplay between an individual’s genetic predisposition and environmental factors. Bacteria characteristically found on the skin of individuals with PV include Firmicutes, Actinobacteria, and Proteobacteria [4]. Additionally, Staphylococcus and Streptococcus spp. have been detected more frequently in lesioned skin [59]. In other studies, S. aureus has been found at significantly higher levels on diseased skin whereas S. epidermidis and C. acnes are more commonly detected on non-diseased skin, an observation taken to indicate that the skin microbiota may have a role in PV pathophysiology [55]. Treatment aims to remove scales and halt the rapid growth of skin cells and this is usually done via the application of topical corticosteroids, vitamin A derivatives, phototherapy, and the management of lifestyle factors which may contribute to flare ups. In the future, the dysregulated skin microbiota may become a novel therapeutic target, whilst restoration of symbiosis may increase the efficacy of already established medical treatments [60].
Body Odour
The frequently stigmatized condition of malodorous body odour is most targeted using a wide array of deodorants and antiperspirants and sometimes a combination of both. There are three types of glands present in the axilla: apocrine, eccrine, and sebaceous. Apocrine glands secrete a hydrophobic mixture that contains fats, steroids, and proteins via hair follicles. Eccrine glands conversely provide water and a hydrophilic mix including salt and lactic acid, which is released straight onto the skin. On the other hand, sebaceous glands secrete sebum, which can be used as a metabolic substrate by certain bacteria [61]. The moist, warm environment provided by the structural anatomy of the axilla together with the dense coverage of hair fosters bacterial proliferation [53, 61]. Axilla odour represents a complex interplay between specific glands, secreted amino acid conjugates, and the products of certain enzymes associated with microorganisms colonizing the skin [61]. Interestingly, fresh axilla secretions are odourless, only developing their classic pungent scent following contact with skin bacteria which transforms them into volatile odoriferous molecules, known as bacteria-triggered odour release [53]. Common microbiota present in the axilla include members of the genera Staphylococcus, Micrococcus, and Corynebacterium [62]. However, more than 200 species have been identified within this microbial community. The diversity of these populations increases with age as well as the total bacterial numbers with senior subjects (55 +) having the highest numbers [53]. Although multiple species have been tested, only members of the genus Corynebacterium are known to cause odour [44]. Odour is generated from volatile fatty acids (VFA) via the activity of the corynebacterial enzyme Na-acyl glutamine aminoacylase which acts at Na-acyl-l-glutamine, producing 3-methyl-2-hexenoic acid (3M2H), the source of the characteristic pungent apocrine smell [44, 62]. Bacterial-triggered release is not the only way odour is generated in humans. Reactions with thioalcohols involving bacteria are also known to cause odour, like that of VFA. Deodorants work in one of three ways (1) by blocking bacterial growth via the activity of antibacterials, e.g. triclosan; (2) by using odour-masking fragrances to overpower the malodours; and (3) by reducing perspiration through the activity of various inorganic salts such as aluminium chloralhydrate, which are thought to work by clogging sweat ducts [61]. Changes in microbial diversity have been linked to deodorant use, together with a relative increase of Corynebacterium, showing that axillary products can stimulate odour-producing bacteria instead of treating the problem [58]. Deodorant-focused mechanisms are not specific to malodorous bacteria and do not treat the root problem, which highlights the need for further research and product development.
Tinea Pedis
Tinea pedis is a dermatophyte infection particularly affecting the interdigital web and/or the sides of the feet. Its distribution is extensive (> 70% of humans) and occurs independently of subject age, race, and sex [63]. It is most prevalent in the post-pubescent period and can be either a chronic or reoccurring condition [63, 64]. The environment of the skin of the feet is unique and features a large number of sweat glands and a relative absence of sebaceous glands. The wearing of occlusive footwear creates a highly relative humidity and warm conditions, conditions allowing both bacteria and fungi to thrive [64]. Other factors predisposing the development of tinea pedis include sweating, trauma, an immunocompromised state, and exposure of the feet to communal surfaces [52]. Epidermal samples from patients with tinea pedis have been shown to exhibit increased fungal diversity and decreased bacterial diversity when compared to healthy controls [63, 64]. An increase in Trichophyton rubrum (27.26%) was observed in patients with tinea pedis as compared to healthy controls (0.065%) [63]. The most prevalent bacterial phyla in swabbing’s from patients with tinea pedis were Firmicutes, Actinobacteria, and Proteobacteria, whilst Staphylococcus constituted more than 30% of the bacterial genera. Tinea pedis can be treated with topical or oral antifungals or a combination of both [63]. Topical applications of antifungals are used for 1–6 weeks depending on infection severity and the active ingredient of the antifungal. A topical corticosteroid can also be incorporated into treatment regimens if the area is inflamed. Oral antifungals tend to be reserved for patients with extensive chronic tinea pedis; however, interactions with other medications are common and pose a risk to these patients [63]. It is clear that the composition of the skin microbiota in subjects having tinea pedis is different to that of controls, prompting suggestions that skin dysbiosis may influence the occurrence and development of the disease [64].
Impetigo
Impetigo is a bacterial infection of the epidermis commonly occurring in school children [57, 65]. It is a highly contagious infection, usually transmitted via direct contact. The bacteria responsible for impetigo infections are predominantly either S. pyogenes or S. aureus, or a combination of both [65]. Depending on the layer of skin that is infected, bacteria that are implicated in impetigo can also cause ecthyma or cellulitis [57]. Both of the primary bacterial agents of impetigo can produce toxins that bypass the immune system and elicit the development of characteristic lesions. The typical presentation of impetigo starts with a singular erythematous blemish which subsequently becomes vesicular or pustular [65]. The vesicles are subject to eruption leaving a “honey coloured” yellow crust over the superficial lesion. Host factors including immunosuppression and tissue damage are important in the pathological process of impetigo [57]. Often, there is a correlation between where the patient’s fingernails have scratched the skin and the development of lesions, as generally damage to the skin is required for bacterial infection [48, 65]. Mainstay treatment includes wound care and antiseptic washes such as chlorohexidine and antibiotic therapy. Topical antibiotics including fusidic acid are used in the absence of systemic symptoms. It is however more common for systemic symptoms to present, such as fever and lethargy, thereby prompting the use of systemic antibiotics [57]. There are concerns around the use of antibiotics for S. aureus infections largely prompted by the development and spread of MRSA [57, 65]. S. aureus readily acquires antimicrobial resistance, a factor limiting treatment options and contributing to the global proliferation of antimicrobial resistance.
Approaches to Establishing and Maintaining a Beneficial Skin Microbiota
Probiotics and the Current Products on the Market
The development of several key technologies has allowed researchers to evaluate the human microbiome in greater depth and to achieve a better understanding of the connections between specific microbes and a variety of disease presentations, leading to the development of many novel and emerging therapies to treat commonly occurring ailments [49]. Whilst pharmaceutical companies continually assess candidate site-specific bioactive molecules for their capability to improve skin function or to effect amelioration of disease processes, the increased information relating to the skin microbiome has also stimulated probiotic development. The World Health Organization (WHO) 2001 has defined probiotics as “Live micro-organisms which when administered in adequate amounts, confer a health benefit on the host”. The importance of maintaining the balance of good (probiotic) and bad (disease-causing) bacteria has been highlighted for many years in relationship to the gastrointestinal tract and more recently also in the oral cavity and the vaginal tract. The time for the advent of effective skin probiotics is now at hand.
Gut probiotics have now been in use for hundreds of years with early studies utilizing fermented milk dating back to Roman times. Scientist Elie Metchnikoff has been credited with the first successful promotion of the value of probiotics in relationship to his assessing of their role in promoting longevity in impoverished rural Bulgarian communities [66]. His work on phagocytosis, for which he was awarded a Nobel Prize in 1907, lead him to theorize that body changes associated with old age were at least in part due to the work of phagocytes transformed by colonic bacteria from being defenders of the gut into tissue destroyers [66]. From this, a large interest in the role bacteria can play in providing beneficial effects on gut health ensued with a focus on their ability to improve digestion, relieve post-antibiotic and traveller’s diarrhoea, and generally boost immunity. Microbial homeostasis in the gut is influenced by many factors including diet, physical condition, susceptibility to diseases, hormonal status, and antibiotic exposure. Genera commonly sourced for gut probiotics such as Bifidobacterium and Lactobacillus function as probiotics through multiple actions including the sequestering of essential nutrients required by undesirable bacteria, outcompeting others for attachment and production of anti-competitor molecules such as bacteriocins, all of which effectively limits the proliferation of undesirable bacteria and leads to the development of a microbial community rich in beneficial bacteria in the gut.
As our understanding on the role microbes play on human health progresses, the concept of applying probiotics to other sites of the body evolved with a focus on the vaginal tract and the oral cavity becoming key scientific and commercial targets. Progression of probiotics or probiotic technology (i.e. supernatants, lysates or killed probiotics, or immuno- or zombie biotics) for topical skin applications has only evolved within the past decade. All have a focus on altering the skin microbiome to promote a healthier skin. Prior to this, some research had focused on evaluating the ability of gut-targeted probiotics to also provide beneficial outcomes on the skin [67]. Recent work has evaluated direct topical application of some of these probiotics. There are indications that there is a link between certain skin pathologies and poor gut health and that the taking of high-quality probiotics and the consumption of probiotic-rich foods improve the skin hydration conditions [68]. Although the concept of improving skin health from inside the body is highly commendable, a more logical approach could be of combating skin conditions on the target site, i.e. surface of the skin. Therefore, more recent research is focussing upon developing topical probiotics suitable for maintaining skin health [32, 37, 69].
These topical probiotic approaches are being considered non-antibacterial antibiotic alternatives with potential to relieve and prevent common skin disorders and over time may become established as integral components of an individual’s natural skin microbiota. Such organisms may also contribute to the boosting of local skin immunity. To date, however, most of the so-called probiotic products for the skin do not appear to actually contain live bacteria. Instead, their formulations comprise bacterial lysates, extracts, or ferments with only very preliminary data documenting their precise mechanism of action. Considering that these products do not contain any live bacteria and with an array of terminology arising define this, the International Scientific Association for Probiotics and Prebiotics (ISAPP) defined the term “postbiotic” in 2021 [70]. This ensures a clear distinction with live probiotics which can signal in consumer products. Small-scale, poorly designed clinical studies further obscure any clinical efficacy outcomes of the products, and rather, the emphasis is on cosmetic findings that may potentially reflect the action of excipients present in the formulations rather than of the putative probiotic component [71].
Interestingly, for a number of products which do actually contain live bacteria, these probiotics have been derived from sources other than the skin. These so-called probiotic bacteria include a lactobacillus used as a yogurt starter culture [72]—the ammonia-oxidizing bacterium Nitrosomonas eutropa [73] and a combination of Lactobacillus and Bifidobacterium strains [73]. More examples are shown in Table 2. Typically, these bacteria are not considered to be typical members of the natural skin microbiota and their role in benefiting skin disorders has not been established.
Prebiotics
The ISAPP have defined a prebiotic as: “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [76]. Prebiotics can change the microbial composition by stimulating the growth of certain species which can improve host health [34, 76]. Prebiotics are mainly derived from complex carbohydrates known as oligosaccharides, and the objective of their application as nutrients is to selectively support the growth of beneficial bacteria over that of pathogenic bacteria [34]. The human body does not digest prebiotic carbohydrates due to the orientation of their osidic bonds which are able to resist hydrolysis by salivary and intestinal digestive enzymes. Oligosaccharides primarily enhance the growth of and activity of specific Bifidobacterium spp. [77]. Their bifidogenic effect varies depending on the type of prebiotic [55]. Breast milk is key prebiotic as it is the first food for infants and provides the initial intestinal microbiota with optimal nutrients for growth [55]. Examples of prebiotics commonly found in the human diet include bananas, grains, honey, and onion. A common prebiotic for skin bacteria is sucrose, which is found in fresh fruit and honey. Sucrose actively feeds S. epidermidis but not C. acnes, thereby relatively impeding the growth of the pathogenic bacteria in acne-affected skin [34]. A synbiotic is a “synergistic mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and colonization of live beneficial microorganisms in the gastrointestinal tract” [77].
Postbiotics
The development of commercial research containing bacterial metabolites has led to the new term of postbiotics which has been defined by ISAPP as “preparation of inanimate microorganisms and/or their component that confers a health benefit on their host” [70]. This unified term has been proposed to give a clear definition and usurp an array of other commonly utilized terms for the same thing, such as “parabiotics”, “ghost biotics”, “bacterial lysates”, and “tyndallized probiotics” as examples.
Postbiotic efficacy may be attributable to a multitude of factors including “microbial metabolites, proteins, lipids, carbohydrates, vitamins, organic acids, cell wall components, or other complex molecules that are generated in the matrix that is fermented” [77]. The underlying mechanisms of postbiotics are not fully understood, yet they seem to be mediated via interactions between the host and microbial products which stimulate an immune response [77]. Effects within cells that support this notion include increased adhesion to intestinal cells and secretion of various metabolites [78]. A benefit of postbiotics is their relative tolerance to a range of storage conditions and transportation stringencies as they are not subject to degradation to the same extent that live bacteria are. Safety advantages are also evident, as postbiotics reduce the risk of microbial translocation, infection, and reduce the occurrence and extent of inflammatory responses [78]. The introduction of postbiotics on to the market constitutes a new development in the application of microorganisms in food products and supplements. They provide an important alternative for products where probiotics are damaged, or shelf life viability is minimal as they do not require refrigeration [78].
What Are the Challenges to Topical Probiotic Applications?
Early research has demonstrated that topical applications of probiotics have a potential benefit for skin-related conditions, yet there are still some challenges to their use [79]. The generation of a probiotic requires the isolation and then comprehensive characterization of a bacterium using an exhaustive combination of genotypic and phenotypic tests [80]. There are few probiotic strains on the market that have been selected to be effective in the management of specific maladies. Unfortunately, the embryonic field of commercially developed skin probiotics is at present inadequately controlled due to the large number of poorly researched products carrying unproven claims that have gained entry to the market. Major issues include failure to establish a complete probiotic profile for the putative probiotic constituents of the product in accord with the specifications of regulatory groups [48]. Since the regulations concerning labelling are often inadequate, false claims concerning putative benefits have also been made, e.g. claims may be made for benefits relating to skin health for a generic gut probiotic [80].
Effective topical delivery of bioactives has proven to be a challenge due to the strict natural and protective barrier function of the skin maintained by the stratum corneum which serves to control the absorption of molecules into the deeper layers of the skin, thereby also limiting therapeutic options [81]. The formulation requirements for topical products containing viable microorganisms differ markedly from those solely containing relatively small molecules due to the requirement to maintain the stability of the microorganisms. Critical factors requiring control for microorganisms include pH and osmolality levels as well as the temperature and humidity levels of storage conditions [82].
A common issue in probiotic therapy is the utility (if any) of non-viable, or inactivated bacteria present in the probiotic formulations. Unfortunately, it appears that relatively few commercial probiotic formulations for skin applications actually contain viable cells (Table 2). This is because live probiotics are sensitive to heat and moisture, making it difficult to keep them alive in a formulation for skin application (such as aqueous or emulsion-based topical creams, lotions, or sprays). The additional costs required for the inclusion and maintenance of viable probiotic cells in a product in order to increase its efficacy and shelf life impose considerable commercial constraints. Therefore, most companies typically resort to the marketing of products containing heat-killed bacteria or bacterial lysates and extracts. There have been few studies conducted to determine the relative effectiveness of non-viable bacteria as alternatives to probiotics [83].
Wide variations characterize the distribution of individual microbial species within the skin microbiota, reflecting the influence of interpersonal environmental factors, ethnicity, age, and body site-specific parameters. An important question relates to the likelihood of functional efficacy in an epidermal environment of probiotics that are of non-skin origin such as the intestinally derived Lactobacillus and Bifidobacterium probiotics or non-human origin probiotics. It appears that at present, very few probiotics marketed for epidermal application have actually been sourced from the skin microbiome or are well-documented skin commensals. Exceptions include Micrococcus luteus Q24 and S. hominis [28]. It is inferred that microbes not naturally found on the skin surface are less likely to survive or colonize efficiently on the skin surface in comparison with common skin commensals. This means that any beneficial activity associated with the topical application of gut probiotics is probably akin to the relatively shorter term benefits mediated by postbiotics or prebiotics and thus is unlikely to be maintained in the manner associated with use of a colonizing probiotic.
Conclusions
The complex interplay between commensal and pathogenic bacteria, as modulated by the various downstream mediators of disease progression, plays a major role in the genesis of many of the common pathologies of the skin. There is as yet only very limited clinical research that is focused upon the topical application and efficacy of probiotics and postbiotic, but the available information highlights the imminent considerable benefits to be obtained from their application as convenient and effective preventative and treatment options for a broad spectrum of skin pathologies. Acne, atopic dermatitis, psoriasis, body odour, tinea pedis, and impetigo all exhibit putative or proven links between dermal-based dysbiosis and the progression of disease. The mechanistic details of the relationship between dysbiosis and pathogenesis have sometimes not yet been fully established. Further study is in some cases required to answer the following questions: what is the specific role of the microbiome in the expression of each disease progression? Is dysbiosis an etiological factor or a consequence of pathological processes in the skin? What factors trigger previously commensal bacteria to become pathogenic? Hopefully, this timely review now stimulates further carefully controlled research into the potential disease preventative and therapeutic benefits to be derived from the controlled topical application of appropriately certified probiotic or postbiotic agents.
References
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14(8):e1002533. https://doi.org/10.1371/journal.pbio.1002533
Grice EA, Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9:244–253. https://doi.org/10.1038/nrmicro2537
Oh J, Byrd AL, Park M, Kong HH, Segre JA (2016) Temporal stability of the human skin microbiome. Cell 165(4):854–866. https://doi.org/10.1016/j.cell.2016.04.008
Schommer NN, Gallo RL (2013) Structure and function of the human skin microbiome. Trends in Microbiology 21:660–668. https://doi.org/10.1016/j.tim.2013.10.001
Cooper AJ, Weyrich LS, Dixit S, Farrer AG (2015) The skin microbiome: associations between altered microbial communities and disease. Aust J Dermatol 56:268–274. https://doi.org/10.1111/ajd.12253
Iizumi T, Battaglia T, Ruiz V, Perez Perez GI (2017) Gut microbiome and antibiotics. Arch Med Res 48:727–734. https://doi.org/10.1016/j.arcmed.2017.11.004
Kong HH (2011) Skin microbiome: genomics-based insights into the diversity and role of skin microbes [Internet]. Trends Mol Med 17:320–328. https://doi.org/10.1016/j.molmed.2011.01.013
Ursell LK, Clemente JC, Rideout JR, Gevers D, Caporaso JG, Knight R (2012) The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J Allergy Clin Immunol [Internet] 129(5):1204–1208. https://doi.org/10.1016/j.jaci.2012.03.010
Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT et al (2012) Structure, function and diversity of the healthy human microbiome. Nature 486(7402):207–214. https://doi.org/10.1038/nature11234
Kolarsick P, Kolarsick M, Goodwin C (2011) Anatomy and physiology of the skin. J Dermatol Nurses Assoc 3:203–213. https://doi.org/10.1097/JDN.0b013e3182274a98
Prausnitz MR, Elias PM, Franz TJ, Schmuth M, Tsai J-C, Menon GK, Holleran WM, Feingold KR (2012) Skin barrier and transdermal drug delivery. Dermatology 3:2065–2073
Losquadro WD (2017) Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plastic Surgery Clinics of North America. W.B. Saunders 25:283–289. https://doi.org/10.1016/j.fsc.2017.03.001
Tobin DJ (2011) The anatomy and physiology of the skin. Springer Publishing Company, New York
Niemeyer - van der Kolk T, van der Wall HEC, Balmforth C, Van Doorn MBA, Rissmann R (2018) A systematic literature review of the human skin microbiome as biomarker for dermatological drug development. Br J Clin Pharmacol 84:2178–2193. https://doi.org/10.1111/bcp.13662
Tavaria FK (2017) Topical use of probiotics: the natural balance. Porto Biomed J [Internet] 2(3):69–70. https://doi.org/10.1007/s13555-020-00476-7
Byrd AL, Belkaid Y, Segre JA (2018) The human skin microbiome. Nat Rev Microbiol 16:143–155. https://doi.org/10.1038/nrmicro.2017.157
Belkaid Y, Segre JA (2014) Dialogue between skin microbiota and immunity. Science 346:954–959. https://doi.org/10.1126/science.1260144
Dréno B, Araviiskaia E, Berardesca E, Gontijo G, Sanchez Viera M, Xiang LF et al (2016) Microbiome in healthy skin, update for dermatologists. J Eur Acad Dermatol Venereol 30:2038–2047. https://doi.org/10.1111/jdv.13965
Friedrich AD, Paz ML, Leoni J, Maglio DHG (2017) Message in a bottle: dialog between intestine and skin modulated by probiotics. Int J Mol Sci MDPI AG 18. https://doi.org/10.3390/ijms18061067
Sanford JA, Gallo RL (2013) Functions of the skin microbiota in health and disease. Seminars in Immunology. Semin Immunol 25:370–377. https://doi.org/10.1016/j.smim.2013.09.005
Chiller K, Selkin BA, Murakawa GJ (2001) Skin microflora and bacterial infections of the skin. J Investig Dermatology Symp Proc 6(3):170–174. https://doi.org/10.1046/j.0022-202x.2001.00043.x
Nakamizo S, Egawa G, Honda T, Nakajima S, Belkaid Y, Kabashima K (2015) Commensal bacteria and cutaneous immunity. Semin Immunopathol 37:73–80. https://doi.org/10.1007/s00281-014-0452-6
Poretsky R, Rodriguez-R LM, Luo C, Tsementzi D, Konstantinidis KT (2014) Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS One 9(4). https://doi.org/10.1371/journal.pone.0093827
Venter JC, Adams MD, Sutton GG, Kerlavage AR, Smith HO, Hunkapiller M (1998) Shotgun sequencing of the human genome. Science 280:1540–1542. https://doi.org/10.1126/science.280.5369.1540
Burton JP, Wescombe PA, Macklaim JM, Chai MHC, MacDonald K, Hale JDF et al (2013) Persistence of the oral probiotic Streptococcus salivarius M18 is dose dependent and megaplasmid transfer can augment their bacteriocin production and adhesion characteristics. PLoS One 8(6). https://doi.org/10.1371/journal.pone.0065991
Christensen GJM, Brüggemann H (2014) Bacterial skin commensals and their role as host guardians. Benef Microbes 5:201–215. https://doi.org/10.3920/BM2012.0062
Egert M, Simmering R, Riedel CU (2017) The association of the skin microbiota with health, immunity, and disease. Clin Pharmacol Ther 102(1):62–69. https://doi.org/10.1002/cpt.698
Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T et al (2017) Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9(378). https://doi.org/10.1126/scitranslmed.aah4680
Kloos WE, Musselwhite MS (1975) Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol 30(3):381–385. https://doi.org/10.1128/am.30.3.381-395.1975
Clausen ML, Edslev SM, Andersen PS, Clemmensen K, Krogfelt KA, Agner T (2017) Staphylococcus aureus colonization in atopic eczema and its association with filaggrin gene mutations. Br J Dermatol 177(5):1394–400. https://doi.org/10.1111/bjd.15470
Rather IA, Bajpai VK, Kumar S, Lim J, Paek WK, Park YH (2016) Probiotics and atopic dermatitis: an overview. Front Microbiol 7:507. https://doi.org/10.3389/fmicb.2016.00507
Knackstedt R, Knackstedt T, Gatherwright J (2020) The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol 29:15–21. https://doi.org/10.1111/exd.14032
Noble SM, Gianetti BA, Witchley JN (2017) Candida albicans cell-type switching and functional plasticity in the mammalian host. Nat Rev Microbiol 15:96–108. https://doi.org/10.1038/nrmicro.2016.157
Maguire M, Maguire G (2017) The role of microbiota, and probiotics and prebiotics in skin health. Arch Dermatol Res 309(6):411–421. https://doi.org/10.1007/s00403-017-1750-3
Neuman H, Forsythe P, Uzan A, Avni O, Koren O (2018) Antibiotics in early life: dysbiosis and the damage done. FEMS Microbiol Rev 42:489–499. https://doi.org/10.1093/femsre/fuy018
Vangay P, Ward T, Gerber JS, Knights D (2015) Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe 17:553–564. https://doi.org/10.1016/j.chom.2015.04.006
Kang BS, Seo JG, Lee GS, Kim JH, Kim SY, Han YW et al (2009) Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol 47(1):101–109. https://doi.org/10.1007/s12275-008-0179-y
Flohr C, Pascoe D, Williams HC (2005) Atopic dermatitis and the “hygiene hypothesis”: too clean to be true? Br J Dermatol 152:202–216. https://doi.org/10.1111/j.1365-2133.2004.06436.x
Lee GR, Maarouf M, Hendricks AJ, Lee DE, Shi VY (2019) Topical probiotics: the unknowns behind their rising popularity. Dermatol 25(5). https://doi.org/10.5070/D3255044062
Ege MJ (2017) The hygiene hypothesis in the age of the microbiome. Ann Am Thorac Soc 14:S348–S353. https://doi.org/10.1513/AnnalsATS.201702-139AW
Gibbs S, Surridge H, Adamson R, Cohen B, Bentham G, Reading R (2004) Atopic dermatitis and the hygiene hypothesis: a case-control study. Int J Epidemiol 33(1):199–207. https://doi.org/10.1093/ije/dyg267
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J et al (2017) The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev 81(4). https://doi.org/10.1128/mmbr.00036-17
Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM (2017) Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 23(3):314–326. https://doi.org/10.1038/nm.4272
Schneider AM, Nelson AM (2019) Skin microbiota: friend or foe in pediatric skin health and skin disease. Pediatr Dermatol 36:815–22. https://doi.org/10.1111/pde.13955
Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH et al (2019) Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol 29(9):1335–1340. https://doi.org/10.4014/jmb.1906.06064
Dunn AB, Jordan S, Baker BJ, Carlson NS (2017) The maternal infant microbiome: considerations for labor and birth. MCN Am J Matern Nurs 42(6):318–325. https://doi.org/10.1097/NMC.0000000000000373
Francino MP (2018) Birth mode-related differences in gut microbiota colonization and immune system development. Ann Nutr Metab 73:12–16. https://doi.org/10.1159/000490842
Reid G, Younes JA, Van Der Mei HC, Gloor GB, Knight R, Busscher HJ (2011) Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 9:27–38. https://doi.org/10.1038/nrmicro2473
Wallen-Russell C, Wallen-Russell S (2017) Meta analysis of skin microbiome: new link between skin microbiota diversity and skin health with proposal to use this as a future mechanism to determine whether cosmetic products damage the skin. Cosmetics 4. https://doi.org/10.3390/cosmetics4020014
Salverda JGW, Bragt PJC, De Wit-Bos L, Rustemeyer T, Coenraads PJ, Tupker RA et al (2013) Results of a cosmetovigilance survey in the Netherlands. Contact Dermatitis 68(3):139–148. https://doi.org/10.1111/cod.12005
Lambers H, Piessens S, Bloem A, Pronk H, Finkel P (2006) Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci 28(5):359–370. https://doi.org/10.1111/j.1467-2494.2006.00344.x
Scharschmidt TC, Fischbach MA (2013) What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov Today Dis Mech 10. https://doi.org/10.1016/j.ddmec.2012.12.003
Xu H, Li H (2019) Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol 20:335–44. https://doi.org/10.1007/s40257-018-00417-3
Mottin VHM, Suyenaga ES (2018) An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis. Int J Dermatol 57:1425–32. https://doi.org/10.1111/ijd.13972
Lolou V, Panayiotidis MI (2019) Functional role of probiotics and prebiotics on skin health and disease. Fermentation 5. https://doi.org/10.3390/fermentation5020041
Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH et al (2015) Dysbiosis and Staphyloccus aureus colonization drives inflammation in atopic dermatitis. Immunity 42(4):756–766. https://doi.org/10.1016/j.immuni.2015.03.014
Pereira LB (2014) Impetigo - Review. Anais Brasileiros de Dermatologia. Sociedade Brasileira de Dermatologia 89:293–299. https://doi.org/10.1590/abd1806-4841.20142283
Callewaert C, Hutapea P, Van de Wiele T, Boon N (2014) Deodorants and antiperspirants affect the axillary bacterial community. Arch Dermatol Res 306(8):701–710. https://doi.org/10.1007/s00403-014-1487-1
Visser MJE, Kell DB, Pretorius E (2019) Bacterial dysbiosis and translocation in psoriasis vulgaris. Front Cell Infect Microbiol 9:7. https://doi.org/10.3389/fcimb.2019.00007
Benhadou F, Mintoff D, Schnebert B, Thio HB (2018) Psoriasis and microbiota: a systematic review. Dis (Basel, Switzerland) 6(2):47. https://doi.org/10.3390/diseases6020047
Natsch A (2015) What makes us smell: the biochemistry of body odour and the design of new deodorant ingredients. Chimia (Aarau) 69(7):414–420. https://doi.org/10.2533/chimia.2015.414
James AG, Austin CJ, Cox DS, Taylor D, Calvert R (2013) Microbiological and biochemical origins of human axillary odour. FEMS Microbiol Ecol 83:527–540. https://doi.org/10.1111/1574-6941.12054
Ilkit M, Durdu M (2015) Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol 41:374–88. https://doi.org/10.3109/1040841X.2013.856853
Liu X, Tan J, Yang H, Gao Z, Cai Q, Meng L et al (2019) Characterization of skin microbiome in tinea pedis. Indian J Microbiol 59(4):422–427. https://doi.org/10.1007/s12088-019-00816-y
Brown J, Shriner DL, Schwartz RA, Janniger CK (2003) Impetigo: an update. Int J Dermatol 251–255. https://doi.org/10.1046/j.1365-4362.2003.01647.x
Mackowiak PA (2013) Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Heal. https://doi.org/10.3389/fpubh.2013.00052
Wescombe PA, Burton JP, Cadieux PA, Klesse NA, Hyink O, Heng NC et al (2006) Megaplasmids encode differing combinations of lantibiotics in Streptococcus salivarius. Antonie Van Leeuwenhoek. https://doi.org/10.1007/s10482-006-9081-y
Lee DE, Huh CS, Ra J, Choi ID, Jeong JW, Kim SH et al (2015) Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: a randomized, double blind, placebo-controlled study. J Microbiol Biotechnol 25(12):2160–2168. https://doi.org/10.4014/jmb.1509.09021
Scariya L, Nagarathna DV, Varghese M (2015) Probiotics in periodontal therapy. Int J Pharma Bio Sci 6(1):P242–P250. https://doi.org/10.5455/musbed.20141106034910
Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM et al (2021) The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-021-00440-6
Blanchet-Réthoré S, Bourdès V, Mercenier A, Haddar CH, Verhoeven PO, Andres P (2017) Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol 10:249–257. https://doi.org/10.2147/CCID.S135529
Lebeer S, Oerlemans E, Claes I, Wuyts S, Henkens T, Spacova I et al (2018) Topical cream with live lactobacilli modulates the skin microbiome and reduce acne symptoms. bioRxiv 463307. https://doi.org/10.1101/463307
Lopes EG, Moreira DA, Gullón P, Gullón B, Cardelle-Cobas A, Tavaria FK (2017) Topical application of probiotics in skin: adhesion, antimicrobial and antibiofilm in vitro assays. J Appl Microbiol 122(2):450–461. https://doi.org/10.1111/jam.13349
Karska-Wysocki B, Bazo M, Smoragiewicz W (2010) Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol Res 165(8):674–686. https://doi.org/10.1016/j.micres.2009.11.008
Benic GZ, Farella M, Morgan XC, Viswam J, Heng NC, Cannon RD et al (2019) Oral probiotics reduce halitosis in patients wearing orthodontic braces: a randomized, triple-blind, placebo-controlled trial. J Breath Res 13(3):36010. https://doi.org/10.1016/j.micres.2009.11.008
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ et al (2017) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14(8):491–502. https://doi.org/10.1038/nrgastro.2017.75
Wegh CAM, Geerlings SY, Knol J, Roeselers G, Belzer C (2019) Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci 20. https://doi.org/10.3390/ijms20194673
Akter S, Park JH, Jung HK (2020) Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells. J Microbiol Biotechnol 30:477–481. https://doi.org/10.4014/JMB.1911.11019
Osborne DW, Tan PI, Varma T, Carbol J (2018) Formulating topical products containing live microorganisms as the active ingredient. Pharm Technol 42(3):32–36
Dolan KE, Pizano JM, Gossard CM, Williamson CB, Burns CM, Gasta MG et al (2017) Probiotics and disease: a comprehensive summary-part 6, skin health. Integr Med 16(4):32–41
Marto J, Ascenso A, Simoes S, Almeida AJ, Ribeiro HM (2016) Pickering emulsions: challenges and opportunities in topical delivery. Expert Opin Drug Deliv 13:1093–107. https://doi.org/10.1080/17425247.2016.1182489
Sreeja V, Prajapati JB (2013) Probiotic formulations: application and status as pharmaceuticals-a review. Probiotics Antimicrob Proteins 5(2):81–91. https://doi.org/10.1080/17425247.2016.1182489
Makinen K, Berger B, Bel-Rhlid R, Ananta E (2012) Science and technology for the mastership of probiotic applications in food products. J Biotechnol 162(4):356–365. https://doi.org/10.1016/j.jbiotec.2012.07.006
Acknowledgements
The authors acknowledge that the figures were created with Biorender.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
I.J.M. and E.M.W. were interns at Blis Technologies, the manufacturer of a skin probiotic.
R.J., J.R.T. and J.D.F.H. are employees of Blis Technologies.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McLoughlin, I.J., Wright, E.M., Tagg, J.R. et al. Skin Microbiome—The Next Frontier for Probiotic Intervention. Probiotics & Antimicro. Prot. 14, 630–647 (2022). https://doi.org/10.1007/s12602-021-09824-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09824-1