Abstract
Omega-3 and probiotics were shown to improve periodontal health by modulating the host immune response. Recently, the combination of omega-3 and probiotics has been shown to have a potential synergistic effect on host modulation. The aim of this study was to evaluate the prophylactic role of an omega-3 and probiotic combination on alveolar bone loss (ABL) via inflammatory response in an experimental periodontitis model. Forty-three rats were divided into 5 groups as control (C, n = 8), periodontitis (P, n = 8), omega-3 + periodontitis (O, n = 8), probiotic + periodontitis (Pro, n = 10), and omega-3 + probiotic + periodontitis (OPro, n = 9). Additionally to a standardized diet, omega-3 and/or probiotics were supplemented with oral gavage to the O, Pro, and OPro groups for 44 days. Periodontitis was induced by ligature to the P, O, Pro, and OPro groups on the 30th day for 2 weeks. ABL levels were measured histopathologically, and serum interleukin (IL) 1β, IL6, and IL10 levels were analysed by enzyme-linked immunosorbent assay. ABL increased in all periodontitis groups (P, O, Pro, and OPro), compared to C group. Compared to P group, all oral gavage groups (O, Pro, and OPro) revealed decreased ABL, which was lowest in OPro group. IL1β and IL6 decreased and IL10 increased in OPro group, compared to P group. In conclusion, prophylactic administration of omega-3 and probiotic combination reduced ABL and improved serum IL1β, IL6, and IL10 levels more than their single use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontitis is a multifactorial chronic disease that includes infection on the one hand and individual susceptibility related to immune and inflammatory response on the other. Low-grade inflammation has an important role for many systemic conditions and in the relationship between periodontal disease and systemic diseases [1].
Omega-3, high amounts of which are found in fish oil, is a very important essential fatty acid in the regulation of inflammatory response [2]. It affects the host immune system by various mechanisms, including inhibition of leucocyte chemotaxis and eicosanoids, increasing production of proresolving lipid mediators [2]. It has been shown that omega-6, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) ratios may change in response to diet, leading to an altered cell signal–mediated gene expression and lipid mediator production pattern [2].
Probiotics are microorganisms that provide beneficial effects to the host by regulating factors associated with the host or the surrounding microbial community [3]. It has been indicated that probiotics can contribute to the host defence system and have beneficial effects on the treatment of metabolic disorders through many mechanisms, including suppression of harmful pathogens, regulation of inflammatory mediators, and maintaining oxidative balance [4]. Multi strains (Lactobacillus [L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subspecies bulgaricus (L. bulgaricus)], Bifidobacterium (B. longum, B. breve and B. infantis), and Streptococcus salivarius subspecies thermophilus (S. thermophilus) containing probiotic (VSL#3, VSL Pharmaceuticals, Gaithersburg, Maryland, USA) were also reported to be more efficient than single species via possible synergistic mechanisms [5].
The effects of omega-3 [6,7,8,9,10] and probiotics [11,12,13,14,15,16,17] on periodontal disease pathogenesis have been indicated in many reports. It can be deduced that both omega-3 [18, 19] and probiotics [13] may improve periodontal health, mainly by modulating host defence and inhibiting bacterial pathogens. In experimental studies, omega-3 [6, 7] or probiotics [11, 12, 14, 15] were shown to reduce bone loss by modulating immune response and/or bacterial microbiota.
In terms of periodontal destruction, cytokines such as interleukin (IL) 1β, IL6, IL18, and their regulators IL10 and IL11 come to the fore [20]. IL1β and IL6 were shown to be increased in patients with periodontitis and decreased after treatment [21]. On the contrary, IL10 was reported to inhibit bone loss and could have a therapeutic effect on periodontitis [22].
Recently, omega-3 and probiotics have been reported to have a potential effect on reducing low-grade inflammation and combination of omega-3, and probiotics may be beneficial on immune response and inflammatory diseases [23]. Therefore, we hypothesized that a combination of omega-3 and probiotics may have positive synergistic effects on periodontal pathogenesis. The aim of this study was to evaluate the preventive role of the combination of omega-3 and probiotics on alveolar bone loss (ABL) in a ligature-induced periodontitis model and to investigate the role of serum inflammatory biomarkers (IL1 β, IL6, and IL10) in this relationship.
Material and Methods
Animals and Experimental Design
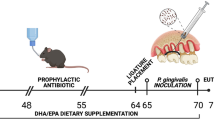
Ethical approval was obtained from the Süleyman Demirel University Animal Experiments Local Ethics Committee with the decision number 05.05.2018, 13/06. Fifty adult male Wistar albino rats (6–8 weeks old, 246.56 ± 17.4 g) were used in the study. The experimental period is demonstrated in Fig. 1. Following the 7-day acclimatization period, rats were fed a standardized ad libitum diet and water in Euro type IV cages, maintained at 21–23 ˚C heat and 55–60% humidity, with 12 h of light and 12 h of darkness for 44 days. Five groups were created randomly as: control (C, n = 10), periodontitis (P, n = 10), omega-3 + periodontitis (O, n = 10), probiotic + periodontitis (Pro, n = 10), and omega-3 + probiotic + periodontitis (OPro, n = 10). A combination of 80 mg/kg ketamine hydrochloride and 10 mg/kg xylazine was used for the induction of experimental periodontitis and scarification [14].
Induction of Periodontitis
In order to evaluate the preventive role of omega 3 and probiotics, experimental periodontitis was induced in the P, O, Pro, and OPro groups on the 30th day for 2 weeks by placing sterile 3.0 silk sutures around the maxillary 2nd molar teeth [14]. The ligatures were checked weekly, and the displaced ones were relocated.
Omega-3 and Probiotic Administration
In addition to the standardized ad libitum diet, O, Pro, and OPro groups were fed once a day, with omega-3, probiotics, or a combination in 1 ml saline for 44 days by oral gavage; 60% EPA and 40% DHA containing omega-3 fish oil (Voonka fish oil omega-3, Eczacıbaşı, İstanbul, Turkey) at a dose of 40 mg/kg [8], and L. casei, L. plantarum, L. acidophilus L. bulgaricus, B. longum, B. breve, B. infantis, and S. thermophilus strains containing probiotic (VSL#3, VSL Pharmaceuticals, Gaithersburg, Maryland, USA), at a dose of 13 × 109/kg that includes total amount of bacteria, were used [24].
Blood Serum Analyses
At the end of the study, all rats were anesthetized, approximately 10 ml of blood was taken from the vena cava inferior into separator gel-containing tubes, and the animals were sacrificed. Blood samples were centrifuged for 10 min at 3000 rpm, and serum samples were obtained and frozen at − 80 ºC until the analysis. Serum IL1β [Rat Interleukin 1β ELISA Kit (E0119Ra), Bioassay Technology Laboratory, Shanghai, China], IL6 [Rat Interleukin 6 ELISA Kit (E0135Ra), Bioassay Technology Laboratory, Shanghai, China], and IL10 [Rat Interleukin 10 ELISA Kit (E0108Ra), Bioassay Technology Laboratory, Shanghai, China] levels were measured by enzyme-linked immunosorbent assay using commercial kits. The sensitivity of the kits was 10.27 pg/ml, 0.052 ng/l, and 1.51 pg/ml, respectively.
Measurement of ABL
Maxilla was dissected, and soft tissues were removed manually, separated into halves, and kept in hydrogen peroxide (3%, 4ºC) for 24 h. Defleshed halves were cleaned with distilled water and stained with 1% methylene blue dye for 1 min to demarcate the cementoenamel junction [25]. For measurement, the occlusal plane of the 2nd molar teeth was placed perpendicular to the ground, and photographs from the buccal and lingual aspects were taken, using a stereomicroscope (Olympus CX41, Olympus Co., Tokyo, Japan) under × 20 magnification. ABL was measured as the distance from alveolar bone crest to cementoenamel junction from three points at the mesial, middle, and distal sites of both buccal and lingual aspects by a software program (Database Manual Cell Sens Life Science Imaging Software System, Olympus Co., Tokyo, Japan). Eventually, six measurements were recorded, and ABL was identified by calculating arithmetic mean values [26].
Statistical Analysis
The estimated sample size was determined based on previous reports [8, 16]. A power of > 95% (effect size = 1.21, α = 0.05) was achieved for ABL using a digital program (G*power, v.3.1.9.2 for Windows, University of Kiel, Kiel, Germany). Variables were analysed by a statistics packet program (SPSS 15.0, SPSS Inc., Chicago, IL, USA). Results are given as mean ± standard deviation. A nonparametric Kruskal–Wallis test was performed for comparisons, and intergroup differences were detected by Mann–Whitney U test. P < 0.05 was considered to indicate statistical difference.
Results
Two rats from C, P, and O groups and a rat from OPro group died from anaesthesia-related complications during the induction of periodontitis, and the study was completed with 43 rats. Body weights at baseline and at the end of the study were similar among groups (P > 0.05). The ligature-induced periodontitis model was successfully accomplished, and ABL was increased in all periodontitis groups (P, O, Pro, and OPro), compared to C group (P < 0.05) (Fig. 2). Compared to P group, O (P = 0.009), Pro (P = 0.001), and OPro (P = 0.001) groups indicated lower levels of ABL. Additionally, OPro group had decreased levels of ABL compared to O (P = 0.027) and Pro groups (P = 0.000), although there was no significant difference between O and Pro groups (P = 0.424) (Table 1).
Histomorphometric appearance of alveolar bone loss (ABL). Increased ABL levels are shown in (b), (c), (d), and (e), compared to (a), and decreased levels are revealed in (c), (d), and (e), compared to (b). Arrows indicate cementoenamel junction. Bars = 1 mm. (a): control group; (b): periodontitis group; (c): omega-3 + periodontitis group; (d): probiotic + periodontitis group; (e) omega-3 + probiotic + periodontitis group
The levels of serum inflammatory parameters are shown in Table 1. IL1β increased in P (P = 0.016) and O (P = 0.021) groups, compared to C group and decreased in O (P = 0.793), Pro (P = 0.008), and OPro (P = 0.016) groups, compared to P group. Similarly, IL6 increased in P (P = 0.009) and O (P = 0.012) groups compared to C group and decreased in Pro (P = 0.374) and OPro (P = 0.003) groups, compared to P group. The lowest levels of IL1β and IL6 were shown in OPro group. By contrast, the level of IL10 was lower in P (P = 0.005) and O groups (P = 0.003), compared to C group, and higher in Pro (P = 0.374) and OPro groups (P = 0.027), compared to P group.
Discussion
To date, there has been no report investigating the combined effects of omega-3 and probiotics on periodontal pathogenesis. In our study, we demonstrated, for the first time, that prophylactic administration of a combination of omega-3 and probiotics decreased ABL more than single usage of them, and serum inflammatory biomarkers may play a role in this relationship.
Omega-3 and probiotics were thought to have similar positive effects on the host immune system, so the combination of omega-3 and probiotics may have synergistic effects [27]. Actually, probiotics were reported to change the fatty acid composition of tissue [28, 29] and increase DHA levels [28]. On the other hand, omega-3 may help the probiotic bacteria attach to the intestinal wall [30]. Recently, it was indicated that combination of omega-3 and probiotics may be beneficial in regulating immune response and gut-brain-axis communication, and combining of omega-3 and probiotics could have a potential effect on preventing low-grade inflammation [23] which has also a crucial role in the pathogenesis of periodontal diseases [1].
It is stated that the main factor for the onset and spread of periodontitis is specific bacterial plaque, but the resulting tissue destruction occurs predominantly through the host inflammatory response [31]. To evaluate the effects of omega-3 and probiotics on ABL via inflammatory response, an experimental periodontitis model was used. The ligature model has been reported to induce bone loss and inflammation similar to periodontitis in humans and is more effective than oral gavage methods [32]. Although the ligature induction period varies between studies, a 14-day period was shown to be sufficient for ABL [25]. Similarly, we indicated that ABL increased in all ligature-induced periodontitis groups (P, O, Pro, and OPro), compared to C group. Also, elevated IL1β and IL6 together with decreased IL10 levels in P group compared to C group are in accordance with the literature [21, 33].
Many reports have been presented evaluating the effects of omega-3 or probiotics on periodontal disease. Dietary supplementation with 10% fish oil was reported to reduce ABL and have potential benefits as a host modulatory agent in periodontitis [6]. Umrania et al. [9] demonstrated that dietary omega-3 supplementation, as an adjunct to scaling and root planing (SRP), reduced salivary IL1β levels in patients with chronic periodontitis and has been suggested for use as an adjunctive management of chronic periodontitis. Omega-3 plus low-dose aspirin were shown to decrease gingival crevicular fluid (GCF) IL1β and IL10 levels and improve clinical periodontal parameters [10]. Azuma et al. [34] indicated decreased periapical bone IL1β and IL6 and increased IL10 levels following omega-3 administration. Kesavalu et al. [7] presented that rats treated with fish oil had significantly less ABL and decreased IL1β gene expression, although there was no apparent effect on IL6 and IL10 gene expressions.
By contrast, Vardar-Sengul et al. [8, 35], who also supplemented a dose of 40 mg/kg omega-3 by oral gavage, indicated that omega-3 administration was ineffective at reducing ABL, while increasing serum IL1β levels. It has been demonstrated that omega-3 fatty acids reach maximum membrane concentration on the 14th day and show an anti-inflammatory effect [36], and to achieve an anti-inflammatory effect, the amount of omega-3 fatty acids must be at least 30 mg/kg [2]. Similarly to Vardar-Sengul et al. [8, 35], omega-3 at a dose of 40 mg/kg was applied in our study. Unlike Vardar-Sengul et al. [8, 35], who performed omega-3 supplementation for a period ranging from 14 to 28 days, a 44-day period was applied in our study.
Garcia et al. [11] reported that SRP with Saccharomyces cerevisiae reduced ABL and IL1β levels and increased IL10 levels in a ligature-induced periodontitis model. They concluded that probiotics were effective at controlling periodontitis. Oliveira et al. [15] presented that Bifidobacterium reduced ABL and IL1β, IL1β/IL10 but did not affect IL10 levels. Maekawa and Hajishengallis [12] indicated that topical administration of L. brevis reduced ABL and decreased levels of IL1β and IL6 in an experimental study. In an apical periodontitis model, lactobacillus species containing probiotic groups were shown to have a smaller periapical lesion area, lower IL1β and IL6, and higher IL10 levels, as compared with the control group [37]. Contrary to Cosme-Silva et al. [37], L. reuteri containing chewing gum did not show any significant effect on GCF IL1β, IL6, and IL10 levels, although bleeding on probing was improved, and GCF volume was decreased in patients with moderate gingival inflammation [17].
It has been demonstrated that probiotic supplementations in drinking water in ligature-induced periodontitis models significantly reduced ABL [14]. In addition to decreased ABL levels, Messora et al. [14] also indicated reduced IL1β and increased IL10 levels. Similarly to Messora et al. [14], whose study includes 44 days of bacillus species administration in drinking water, which started 30 days before the induction of ligature-induced periodontitis, and we followed the same protocol, but used multi probiotics in oral gavage.
A high concentration of multi species (L. casei, L. plantarum, L. acidophilus, L. bulgaricus, B. longum, B. breve, B. infantis, and S. thermophilus) containing probiotic was used in this study. The purpose of using large numbers of different strains together was to obtain high efficiency on probiotic action including competing with pathogenic bacteria, inhibition of bacterial translocation, reinforcement of mucosal defence, and modulating of mucosal cytokine production via possible synergistic mechanisms among them. As a matter of fact, multi species bacteria containing VSL#3 were reported to have an immunomodulatory function such as decreased neutrophil activity and proinflammatory cytokine production, together with an increased anti-inflammatory response [5]. Also, Salmonella-induced disintegrated intestinal barrier was shown to be prevented by VSL#3 [38]. Hence, it can be concluded that immunomodulatory function, maintaining of epithelial integrity, and metabolic effects have been thought to be the key factors in the action mechanisms of probiotic bacteria [5].
In literature, generally, a single-species probiotic was studied, and there has been no study evaluating a combination of lactobacillus, Bifidobacterium, and streptococcus species containing probiotic on periodontal pathogenesis. Multi probiotic supplementation, which was used in our study, was reported to inhibit IL1β levels [39] and to improve plasma IL6 [40] and IL10 levels [40]. Esposito et al. [24] demonstrated that multi probiotics were able to modulate the nuclear factor kappa B pathway and reduce inflammatory response in patients with non-alcoholic fatty liver disease. Similarly to Esposito et al. [24], multi probiotics, at a dose of 13 × 109/kg/day, by oral gavage were applied in our study.
The present study revealed that ABL was significantly reduced in O, Pro, and OPro groups, compared to C group. In accordance with our study results, most of the studies indicated decreased bone ABL levels after omega-3 or probiotics supplementation. Moreover, OPro group had the lowest levels of ABL, thus seeming to confirm a synergistic effect of omega-3 and probiotic on ABL. However, there were conflicting results of omega-3 and probiotics on inflammatory cytokines and bone biomarkers. We indicated that none of the serum parameters but IL1β, which was lower in Pro group, was significantly different in O or Pro groups, compared to P group. However, relative to P group, statistically insignificant decreases were observed in IL1β and IL6 levels in O and Pro groups, together with an increase in IL10 levels in Pro group. Additionally, all evaluated serum markers were only significantly different between OPro group and P group. There was a limited study evaluating the combined effects of omega-3 and probiotics on inflammatory mediators. Kobyliak et al. [41] indicated that an 8-week coadministration of omega-3 and multispecies probiotics was able to reduce serum IL1β, TNFα, IL8, IL6, and interferon gamma levels in patients with non-alcoholic fatty liver disease.
Although we did not intend to compare omega-3 and probiotics, it can be thought that probiotics contributed more than omega-3 to the significant differences observed in OPro group. Methodological variations between studies are considered to play a role on insignificant results, and to date, no periodontal study has been reported using lactobacillus, Bifidobacterium, and streptococcus species containing probiotics. Thus, we suggest that an omega-3 and probiotic combination may synergistically reduce the inflammatory response by modulating IL1β, IL6, and IL10 levels.
There has been no consensus on studies regarding the content, dose, application method, and periods of omega-3 or probiotic administrations in experimental periodontitis models. The fact that the studied markers have different effects on the immune-inflammatory system through different pathways makes it difficult to interpret our results, in terms of revealing their effects on periodontal pathogenesis. Additionally, omega-3 and/or probiotics may also modulate the bacterial environment that may affect ABL and inflammatory biomarkers, which also needs to be investigated.
In conclusion, the results of the study indicate that a prophylactic administration of the combination of omega-3 and probiotics may reduce ABL and inflammatory response, leading to improvements of serum IL1β, IL6, and IL10 levels, in comparison to their single use. It can be stated that the combination of omega-3 and probiotics is promising in the development and prevention of periodontal disease, and our study results are likely to be supported by future studies.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- ABL:
-
Alveolar bone loss
- C:
-
Control
- P:
-
Periodontitis
- O:
-
Omega-3 + periodontitis
- Pro:
-
Probiotic + periodontitis
- OPro:
-
Omega-3 + probiotic + periodontitis
- IL:
-
Interleukin
- SRP:
-
Scaling and root planing
- GCF:
-
Gingival crevicular fluid
References
Cecoro G, Annunziata M, Iuorio MT, Nastri L, Guida L (2020) Periodontitis, low-grade inflammation and systemic health: a scoping review. Medicina (Kaunas) 56:272. https://doi.org/10.3390/medicina56060272
Calder PC (2013) Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 75:645–662. https://doi.org/10.1111/j.1365-2125.2012.04374.x
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Yoo JY, Kim SS (2016) Probiotics and prebiotics: present status and future perspectives on metabolic disorders. Nutrients 8:173. https://doi.org/10.3390/nu8030173
Gionchetti P, Lammers KM, Rizzello F, Campieri M (2005) VSL#3: an analysis of basic and clinical contributions in probiotic therapeutics. Gastroenterol Clin North Am 34:499–513. https://doi.org/10.1016/j.gtc.2005.05.003
Bendyk A, Marino V, Zilm PS, Howe P, Bartold PM (2009) Effect of dietary omega-3 polyunsaturated fatty acids on experimental periodontitis in the mouse. J Periodontal Res 44:211–216. https://doi.org/10.1111/j.1600-0765.2008.01108.x
Kesavalu L, Bakthavatchalu V, Rahman MM, Su J, Raghu B, Dawson D, Fernandes G, Ebersole JL (2007) Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol Immunol 22:232–239. https://doi.org/10.1111/j.1399-302X.2007.00346.x
Vardar-Sengul S, Buduneli N, Buduneli E, Kardesler L, Baylas H, Atilla G, Lappin D, Kinane DF (2006) Dietary supplementation of omega-3 fatty acid and circulating levels of interleukin-1beta, osteocalcin, and C-reactive protein in rats. J Periodontol 77:814–820. https://doi.org/10.1902/jop.2006.050214
Umrania VV, Deepika PCR, Kulkarni M (2017) Evaluation of dietary supplementation of omega-3 polyunsaturated fatty acids as an adjunct to scaling and root planing on salivary interleukin-1beta levels in patients with chronic periodontitis: a clinico-immunological study. J Indian Soc Periodontol 21:386–390. https://doi.org/10.4103/jisp.jisp_16_16
Elkhouli AM (2011) The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study). J Periodontal Res 46:261–268. https://doi.org/10.1111/j.1600-0765.2010.01336.x
Garcia VG, Knoll LR, Longo M, Novaes VC, Assem NZ, Ervolino E, de Toledo BE, Theodoro LH (2016) Effect of the probiotic Saccharomyces cerevisiae on ligature-induced periodontitis in rats. J Periodontal Res 51:26–37. https://doi.org/10.1111/jre.12274
Maekawa T, Hajishengallis G (2014) Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J Periodontal Res 49:785–791. https://doi.org/10.1111/jre.12164
Matsubara VH, Bandara HM, Ishikawa KH, Mayer MP, Samaranayake LP (2016) The role of probiotic bacteria in managing periodontal disease: a systematic review. Expert Rev Anti Infect Ther 14:643–655. https://doi.org/10.1080/14787210.2016.1194198
Messora MR, Pereira LJ, Foureaux R, Oliveira LF, Sordi CG, Alves AJ, Napimoga MH, Nagata MJ, Ervolino E, Furlaneto FA (2016) Favourable effects of Bacillus subtilis and Bacillus licheniformis on experimental periodontitis in rats. Arch Oral Biol 66:108–119. https://doi.org/10.1016/j.archoralbio.2016.02.014
Oliveira LF, Salvador SL, Silva PH, Furlaneto FA, Figueiredo L, Casarin R, Ervolino E, Palioto DB, Souza SL, Taba M Jr, Novaes AB Jr, Messora MR (2017) Benefits of Bifidobacterium animalis subsp. lactis probiotic in experimental periodontitis. J Periodontol 88:197–208. https://doi.org/10.1902/jop.2016.160217
Foureaux RDC, Messora MR, de Oliveira LF, Napimoga MH, Pereira AN, Ferreira MS, Pereira LJ (2014) Effects of probiotic therapy on metabolic and inflammatory parameters of rats with ligature-induced periodontitis associated with restraint stress. J Periodontol 85:975–983. https://doi.org/10.1902/jop.2013.130356
Twetman S, Derawi B, Keller M, Ekstrand K, Yucel-Lindberg T, Stecksen-Blicks C (2009) Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand 67:19–24. https://doi.org/10.1080/00016350802516170
Chee B, Park B, Fitzsimmons T, Coates AM, Bartold PM (2016) Omega-3 fatty acids as an adjunct for periodontal therapy-a review. Clin Oral Investig 20:879–894. https://doi.org/10.1007/s00784-016-1750-2
Huang CB, Ebersole JL (2010) A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol Oral Microbiol 25:75–80. https://doi.org/10.1111/j.2041-1014.2009.00553.x
Seymour GJ, Gemmell E (2001) Cytokines in periodontal disease: where to from here? Acta Odontol Scand 59:167–173. https://doi.org/10.1080/000163501750266765
Reis C, Da Costa AV, Guimaraes JT, Tuna D, Braga AC, Pacheco JJ, Arosa FA, Salazar F, Cardoso EM (2014) Clinical improvement following therapy for periodontitis: association with a decrease in IL-1 and IL-6. Exp Ther Med 8:323–327. https://doi.org/10.3892/etm.2014.1724
Zhang Q, Chen B, Yan F, Guo J, Zhu X, Ma S, Yang W (2014) Interleukin-10 inhibits bone resorption: a potential therapeutic strategy in periodontitis and other bone loss diseases. Biomed Res Int 2014:284836. https://doi.org/10.1155/2014/284836
Hutchinson AN, Tingo L, Brummer RJ (2020) The potential effects of probiotics and omega-3 fatty acids on chronic low-grade inflammation. Nutrients 12:2402. https://doi.org/10.3390/nu12082402
Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, Canani RB, Calignano A, Raso GM, Meli R (2009) Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr 139:905–911. https://doi.org/10.3945/jn.108.101808
Kirzioglu FY, Ozmen O, Dogan B, Bulut MT, Fentoglu O, Ozdem M (2018) Effects of rosuvastatin on inducible nitric oxide synthase in rats with hyperlipidaemia and periodontitis. J Periodontal Res 53:258–266. https://doi.org/10.1111/jre.12513
Taskan MM, Balci Yuce H, Karatas O, Gevrek F, Toker H (2019) Evaluation of the effect of oleuropein on alveolar bone loss, inflammation, and apoptosis in experimental periodontitis. J Periodontal Res 54:624–632. https://doi.org/10.1111/jre.12662
Eratte D, McKnight S, Gengenbach TR, Dowling K, Barrow CJ, Adhikari BP (2015) Co-encapsulation and characterisation of omega-3 fatty acids and probiotic bacteria in whey protein isolate–gum Arabic complex coacervates. Journal of Functional Foods 19:882–892. https://doi.org/10.1016/j.jff.2015.01.037
Joffre C, Dinel AL, Aubert A, Fressange-Mazda C, Le Ruyet P, Laye S (2016) Impact of Lactobacillus fermentum and dairy lipids in the maternal diet on the fatty acid composition of pups’ brain and peripheral tissues. Prostaglandins Leukot Essent Fatty Acids 115:24–34. https://doi.org/10.1016/j.plefa.2016.10.002
Kaplas N, Isolauri E, Lampi AM, Ojala T, Laitinen K (2007) Dietary counseling and probiotic supplementation during pregnancy modify placental phospholipid fatty acids. Lipids 42:865–870. https://doi.org/10.1007/s11745-007-3094-9
Das U (2002) Essential fatty acids as possible enhancers of the beneficial actions of probiotics. Nutrition 18:786–789. https://doi.org/10.1016/s0899-9007(02)00840-7
Chapple IL, Matthews JB (2007) The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 43:160–232. https://doi.org/10.1111/j.1600-0757.2006.00178.x
de Molon RS, Mascarenhas VI, de Avila ED, Finoti LS, Toffoli GB, Spolidorio DM, Scarel-Caminaga RM, Tetradis S, Cirelli JA (2016) Long-term evaluation of oral gavage with periodontopathogens or ligature induction of experimental periodontal disease in mice. Clin Oral Investig 20:1203–1216. https://doi.org/10.1007/s00784-015-1607-0
Leira Y, Iglesias-Rey R, Gomez-Lado N, Aguiar P, Sobrino T, D’Aiuto F, Castillo J, Blanco J, Campos F (2020) Periodontitis and vascular inflammatory biomarkers: an experimental in vivo study in rats. Odontology 108:202–212. https://doi.org/10.1007/s10266-019-00461-3
Azuma MM, Gomes-Filho JE, Ervolino E, Cardoso CBM, Pipa CB, Kawai T, Conti LC, Cintra LTA (2018) Omega-3 fatty acids reduce inflammation in rat apical periodontitis. J Endod 44:604–608. https://doi.org/10.1016/j.joen.2017.12.008
Vardar-Sengul S, Buduneli N, Buduneli E, Baylas H, Atilla G, Lappin D, Kinane DF (2006) Effects of selective cyclooxygenase-2 inhibitor and omega-3 fatty acid on serum interleukin-1beta, osteocalcin, and C-reactive protein levels in rats. J Periodontol 77:657–663. https://doi.org/10.1902/jop.2006.050164
Croft KD, Beilin LJ, Legge FM, Vandongen R (1987) Effects of diets enriched in eicosapentaenoic or docosahexaenoic acids on prostanoid metabolism in the rat. Lipids 22:647–650. https://doi.org/10.1007/BF02533943
Cosme-Silva L, Dal-Fabbro R, Cintra LTA, Dos Santos VR, Duque C, Ervolino E, Mogami Bomfim S, Gomes-Filho JE (2019) Systemic administration of probiotics reduces the severity of apical periodontitis. Int Endod J 52:1738–1749. https://doi.org/10.1111/iej.13192
Otte JM, Podolsky DK (2004) Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol 286:G613-626. https://doi.org/10.1152/ajpgi.00341.2003
Lammers KM, Brigidi P, Vitali B, Gionchetti P, Rizzello F, Caramelli E, Matteuzzi D, Campieri M (2003) Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol 38:165–172. https://doi.org/10.1016/S0928-8244(03)00144-5
Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, Del Vecchio BC (2005) Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 39:540–543. https://doi.org/10.1097/01.mcg.0000165671.25272.0f
Kobyliak N, Abenavoli L, Falalyeyeva T, Mykhalchyshyn G, Boccuto L, Kononenko L, Kyriienko D, Komisarenko I, Dynnyk O (2018) Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: a randomized clinical study. Minerva Med 109:418–428. https://doi.org/10.23736/S0026-4806.18.05845-7
Funding
This study was financially supported by the Hatay Mustafa Kemal University Scientific Research Projects Commission (Project No. 18.M.090).
Author information
Authors and Affiliations
Contributions
Conceptualization: Burak Doğan, Esra Sinem Kemer Doğan, Özlem Özmen, Özlem Fentoğlu, Fatma Yeşim Kırzıoğlu, Mustafa Calapoğlu. Methodology: Burak Doğan, Esra Sinem Kemer Doğan, Özlem Özmen, Mustafa Calapoğlu. Formal analysis: Burak Doğan. Writing—original draft preparation: Burak Doğan, Esra Sinem Kemer Doğan. Writing—review and editing: Burak Doğan, Esra Sinem Kemer Doğan, Özlem Özmen, Özlem Fentoğlu, Fatma Yeşim Kırzıoğlu, Mustafa Calapoğlu.
Corresponding author
Ethics declarations
Ethics Approval
Süleyman Demirel University Animal Experiments Local Ethics Committee approved the study with the decision number 05.05.2018, 13/06.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doğan, B., Kemer Doğan, E.S., Özmen, Ö. et al. Synergistic Effect of Omega-3 and Probiotic Supplementation on Preventing Ligature-Induced Periodontitis. Probiotics & Antimicro. Prot. 14, 114–120 (2022). https://doi.org/10.1007/s12602-021-09803-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09803-6