Abstract
A dipicolonic acid fluorimetry assay was used instead of plate counting for the assessment of spore yields for enhanced optimization efficiency. The associated parameters, including the ratio of solid substrates, composition of liquid substrates, and cultivation conditions, were systematically optimized in a shake-flask culture. The maximum spore yield of 7.24 × 1010 CFU/g of wet substrate was achieved. The optimization process produced a 25.7-fold increase in spore yields compared with those before optimization. In addition, the maximum release of bioactive metabolites during spore accumulation was subsequently obtained with 573.0 U/g of protease, 188.8 U/g of amylase, 186.8 U/g of cellulase, and 3.45 mg/g of acid-soluble protein. The experiment provides a methodological basis for the rapidly optimized production of Bacillus spores in pure solid-state fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spore-forming Bacillus species are being intensively studied as probiotics and applied in animal feed as growth promoters and disease-resistance agents [1,2,3,4,5]. The increasing interest in Bacillus probiotics is due to their potential in livestock production as an alternative to feed antibiotics, which are being gradually banned from use as growth promoters [1, 3]. Furthermore, spore-formers share the capability of surviving the gastrointestinal barrier and high stability during processing and storage without a loss of viability [6, 7].
The strategies for the production of spore-formers from Bacillus have been studied both in submerged fermentation (SMF) and solid-state fermentation (SSF) [8,9,10,11]. Compared with SMF, SSF offers the advantage of less energy consumption, lower cost, and simpler techniques [12,13,14]. In particular, some local agro-industrial biomasses, such as wheat bran, soybean meal, and some other residues, can be fully used as the solid substrates in SSF [8, 13]. Bacillus spp. can produce abundant extracellular enzymes such as protease, amylase, and cellulase [15]. During the growth process of Bacillus in SSF, the accumulation of spores is often concomitant with the digestion of agro-industrial substrates and the release of bioactive metabolites [10]. Therefore, SSF often results in lowering antinutritional factors and improving the bioavailability of feed ingredients [16, 17]. This is why the preparation of probiotics by SSF is better than by SMF in terms of practical feeding effects for piglet and broiler production [18, 19].
With whichever fermentation technique, high spore yields are often achieved based on careful experimental designs for industrial exploitation [8, 9, 11, 20]. In our previous study, a rapid and simple method on endospore detection based on the DPA marker was presented and the method was successfully applied in the rapid optimization of spore production in SMF [20, 21]. The assay provided a high throughput method for the detection of many samples from a statistically designed group in response surface methodology (RSM). Under this method, the efficiency in screening key factors associated with spore production can be greatly improved.
In the present study, the objective was to further investigate the feasibility of using a DPA-fluorimetry response variable (measured in arbitrary units, AUs) as an alternative to plate counting (measured in colony forming units, CFUs) in the optimization procedures for SSF (Fig. 1). In addition, the production of bioactive metabolites during spore accumulation was subsequently analyzed for possible enhanced bioavailability as feed additives.
Materials and Methods
Bacterial Strains and Inocula Preparation
The strain of B. amyloliquefaciens BS-20, applied as spore-forming probiotics, was used in the present study. The strain was deposited in the China Center of Industrial Culture Collection (CICC; Beijing, China) as CICC 24265. The colonies of strain on slope culture were picked and maintained at −80 °C in 20% sterile glycerol until needed. It was activated on commercial Luria-Bertani (LB) agar (Oxoid) at 37 °C. A loopful of the activated strain was inoculated in a 250-ml Erlenmeyer flask containing 50 ml of LB broth (Oxoid). The strain was cultured on a rotating shaker at 37 °C and 200 rpm for 12 h. Ready for the inoculum, the cells were collected by centrifugation at 2500g at 4 °C for 10 min. After twice washes with 0.85% NaCl, the cells were then suspended in 0.85% NaCl solution with an OD600 nm of 1.0.
Optimization for Spore Production in SSF
Effect of the Ratio of Solid Substrates
The fermentations were carried out in 250 ml Erlenmeyer flasks containing 30 g (dry weight) of solid substrates with a proper proportion of nutritious solution to match the initial moisture contents. Each flask after mixing solid and liquid substrates was autoclaved at 121 °C for 30 min ensure sterility. The optimization strategy was developed by consecutively determining the ratio of solid substrates, the composition of liquid substrates, and the cultivation condition parameters. The initial optimized conditions were employed in subsequent experiments. In the process of solid-substrates and liquid-substrates optimization, the fermentations were carried out at 37 °C for 48 h with 50% moisture content and an inocula volume of 4%. The final results for statistical analysis were based on three replication experiments.
The solid dry substrates for the SSF were composed of feed-grade soybean meal, corn flour, and wheat bran, which were bought from the local market of feedstuffs in Jiangxia District, Wuhan City. The solid substrates were divided into groups that consisted of soybean meal, corn flour, and wheat bran in the proportions of 0:0:1, 1:1:0, 1:1:1, 1:1:2, 1:1:3, 1:1:4, and 1:1:5, respectively. The proportion of 0:0:1 was used as a control.
Effect of Different Liquid Substrates and the Combination of Optimized Substrates
First, a single-factor experiment was used for screening liquid substrates containing carbon sources, nitrogen sources, and mineral salts at optimal concentrations for maximum spore yields (Table 1 and Table 2). The materials were of analytical reagent grade and were purchased from Shanghai Guoyao Chemical, Ltd. The liquids were separately mixed with solid material to reach a 50%-moisture level, and the production of spores was tested. The selection of single liquid substrates was based on statistical analysis of three replication experiments. The optimized liquid substrates’ combined effect on spore production was studied through response surface methodology (RSM) with two replications based on the statistical software JMP 11 (SAS Institute Inc., USA).
Effect of Cultivation Conditions
Five process parameters affecting spore production during SSF were optimized, including the initial moisture levels, initial pH, inocula volume, dry flask content, and cultivation time. Each parameter was optimized in sequential order. The results of three replication experiments were compared by statistical analyses.
Extraction of Spores and Bioactive Metabolites
At the end of SSF, all of the fermented substrates in one flask were immersed in sterile phosphate-buffered saline (PBS; 0.144% Na2HPO4, 0.024% KH2PO4, and pH 7.0) for a 10-fold dilution (1:10, w/v). The mixed material was shaken thoroughly in a shaker at 250 rpm for 20 min. Then the mixture was filtered through an autoclaved two-layer gauze. The spore-containing filtered solution was collected for spore determination. The bioactive metabolite–containing supernatant was prepared by centrifugation at 2500g at 4 °C for 10 min.
Spore Determination
The spore concentration was quantified based on the optimized DPA fluorimetry method [20, 21]. Briefly, the spore suspensions were treated by centrifugation (2500×g for 10 min at 4 °C) and washed twice, and finally suspended in isometric, sterile tris-HCl (50 mM, pH 8.0). The spore suspensions were then autoclaved at 121 °C for 10 min for the full release of DPA into the buffer. The DPA-containing supernatants were collected by centrifugation (2500×g for 10 min at 4 °C). With a certain dilution, the supernatants were mixed with 2 mM EuCl3 and 2 mM CyDTA (Alfa Aesar, 99.9% purity) in the proportion of 1:4.5:4.5 by a vortex oscillator. The complexes were assayed for the detection of fluorescence intensity, which was quantified by a Hitachi F-7000 spectrofluorophotometer (Hitachi Ltd., Tokyo, Japan) at a 272-nm excitation and 619-nm emission level with the pre-set parameters of a 5-nm/10-nm slit and a photo-multiplier tube voltage of 700 V. The final fluorescence intensity, chosen in arbitrary units on a scale from 0 to 1000, was used to obtain the proper dilution of DPA supernatants. If necessary, traditional plate-counting assay was also performed to detect the spore concentration based on previous reports [20].
Bioactive Metabolites Determination
Protease Activity Assay
The protease activity after SSF was determined by using 1% casein (Sigma-Aldrich) as the substrate following the methods in previous reports with some modifications [15]. Briefly, 500 μl of the supernatant (diluted by PBS buffer at pH 7.0 if necessary) was mixed with 250 μl of the substrate (1% casein) and 1.25 ml of PBS. The 2 ml mixture was incubated at 37 °C for 30 min and was then terminated by adding 5 ml of 10% trichloroacetic acid (TCA; Shanghai Guoyao, China). After standing for 20 min, the mixture was centrifuged at 2500g for 10 min at 4 °C, and the supernatant was analyzed for protein content by Lowry’s method [22]. The protease activity (U/g) is expressed as the amount in micromoles of tyrosine equivalents released from casein per minute at 37 °C and pH 7.0 per gram of wet weight of fermented material.
α-Amylase Activity Assay
One percent of soluble starch was used as the substrate for the determination of α-amylase activity following the method described in previous reports with minor modifications [15]. Briefly, 500 μl of the supernatant (diluted by PBS buffer at pH 7.0 if necessary) was mixed with 500 μl of the substrate and incubated at 37 °C for 30 min for the release of reducing sugar. The reaction was terminated by adding 3 ml of dinitrosalicylic acid (DNS; Shanghai Guoyao, China) solution. The mixture was heated in boiling water for 5 min. After cooling to room temperature, 15 ml of water was added and mixed by an oscillator. The mixture was sampled to detect the absorbance peak at 540 nm through a spectrophotometer (7230G; Shanghai Analysis Co., China). The protease activity (U/g) is expressed as the amount in micromoles of maltose equivalents released from starch per minute at 37 °C and pH 7.0 per gram wet weight of fermented material.
Cellulase Activity Assay
The method for detecting cellulase activity was similar to that of α-amylase activity assay except that the substrate was replaced by a 1% carboxymethylcellulose sodium (CMC-Na; Shanghai Guoyao, China). The cellulase activity (U/g) is defined as the amount of maltose in micromoles liberated from the CMC-Na per minute at 37 °C and pH 7.0 per gram of wet weight of fermented material.
Estimation of Trichloroacetic Acid-soluble Protein
TCA-soluble protein was analyzed according to Rai et al.’s method [15] by precipitating 1 ml of supernatants by an equal volume of 10% TCA (w/v). The supernatants were separated from the precipitation after incubation at 37 °C for 2 h and then were centrifuged at 8000g for 10 min at 4 °C. The protein content was measured by Lowry’s method [22]. TCA-soluble protein was expressed as milligrams of tyrosine per gram of wet weight of fermented material.
Statistical Analysis
The results were expressed as means and standard deviation (SD). The counts of viable cells based on plate counting were transformed to log10 values. Differences among treatments were compared using a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer (HSD) test [23] through the software JMP 11.0 (SAS Institute Inc., USA). A P value less than 0.05 was considered to represent a significant difference.
Results
Optimization of the Ratio of Different Solid Substrates
The ratio of the three raw solid substrates was optimized by varying the wheat bran content, and the result is shown in Fig. 2. The highest fluorescence intensity was observed in the treatment with a 1:1:3 ratio of corn meal to soybean meal to wheat bran (w/w). The ratio without wheat bran (1:1:0) produced the lowest fluorescence intensity in all treatments.
Optimization of Liquid Substrates
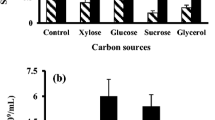
From the results in Table 1, the addition of carbon sources and nitrogen sources in liquid substrates all shared a positive effect on the spore production (P < 0.05). Out of all carbon and nitrogen sources, 8 g/l of glucose and 2 g/l of peptone produced the highest responses in fluorescence intensity, respectively. All mineral salts except ZnCl2 significantly enhanced the production of spores compared with the control without salt supplementation (P < 0.05). MnCl2 at 1 mM had the highest spore production (Table 2).
Glucose, peptone, and MnSO4 were selected as the optimal constituents in liquid substrates and were included in the central composite design (CCD) for the determination of their optimum combination. As observed from Table 3, the response variable was analyzed through RSM and a standard ANOVA (Table 4). The dataset can be fitted with a regression quadratic equation as described in Eq. (1):
The model shows that the optimization was successful in improving spore production since the coefficient of determination, R2, and adjusted determination coefficient, adj. R2, were 0.9213 and 0.82, respectively. The value of “P > F” was less than 0.05, indicating that the model was significant. The terms \( {x}_1,\kern0.5em {x}_1^2, \)\( {x}_3^2,{x}_2^2,\kern0.5em {x}_3, \) and x1x3 (arranged by ascending P values) were found to be significant (P < 0.05).
Response surface plots were drawn to study the interactive effects of glucose, peptone, and Mn2+ on sporulation and to determine their optimum concentrations for maximum possible spore yields (Fig. 3a, b, c). The response surface and contour plots indicated that the interactions between the independent variables glucose (x1), peptone (x2), and Mn2+ (x3) were significant (P < 0.05). All three response surface plots had a convex surface with a downward opening, shown in Fig. 3. Therefore, the response surface maximal point (721.1 AU) was obtained when the optimal significant variables were at the following levels: glucose (x1) = 8.0 g/l, peptone (x2) = 2 g/l, and Mn2+ (x3) = 1 mM.
Optimization of Cultivation Conditions
Each parameter associated with cultivation conditions was studied in sequential order, and the selected five parameters significantly alter the spore yields (Table 5). The optimized process was as follows: water content, 50%; initial pH, 7.0; dry flask content, 30 g/250 ml; inocula volume, 6%; and fermentation time, at least 48 h.
Verification of Optimization Procedure for Spore Production
The predicted spore results based on the CCD analyses were further verified in practice to check the accuracy of the models over three replicates (Table 6). The results indicated that the experimental values (723.4 ± 24.0 AU) were very close to the predicted values (721.1 AU), which suggested that the optimization models were validated. From the verification experiments, the optimization processes enhanced spore yield by 25.7-fold, and the spore concentration reached 7.24 × 1010 CFU/g of wet substrate.
The Change of Production of Spores and Bioactive Metabolites under Optimized Conditions in SSF
From the results in Table 7, many bioactive metabolites, including active hydrolases and TCA-soluble protein (protease, 573.0 U/g; amylase, 188.8 U/g; cellulase, 186.8 U/g; acid-soluble protein, 3.45 mg/g), significantly increased at 48 h after the spore optimization processes. The spore-accumulation and bioactive metabolites–production process by B. amyloliquefaciens BS-20 fermentation is shown in Fig. 4. The results indicated that the spores were formed at 12 h, and there was a considerable decline in reducing sugar at the initial stage of SSF from 0 h to 24 h. Meanwhile, the active metabolites started to produce at 24 h, and the highest levels of spore and hydrolase concentrations were obtained at 48 h.
Discussion
The production of endospores depends on many factors, including the composition of solid substrates, the amount of extra nutrients from liquid substrates, and whether the cultivation conditions in SSF are optimal [8, 12]. Many early reports have focused on techniques for high yields of spore production and resulted in spore concentrations varying from 3.6 × 1010 CFU/g to 1.7 × 1011 CFU/g of dry matter [8, 11]. The optimization process for higher spore concentrations is not easy to conduct, and many factors are often included when screening designs with many experimental treatments [20, 24]. The quantification of spore concentration based on routine colony-forming counting is relatively tedious and time-consuming [25]. The optimization technique based on the DPA fluorescence intensity of spore concentrations has the advantage of enhancing the efficiency of high-throughput studies on optimized SMF designs [20]. Similar findings were also observed in the optimization procedures for SSF in the present study. Using single-factor experiments, about 200 samples from different liquid substrates at different concentrations based on three replicates were assayed for spore quantification. Moreover, in the CCD experiments, 17 runs with two replicates produced 34 samples. Therefore, a large number of samples caused significant trouble in quantifying spore concentration through colony-forming unit counting. The current study demonstrated that as a response variable, a DPA fluorimetry assay can successfully determine the number of spores in order to optimize spore production in SSF, and the spore concentration was greatly enhanced after optimization. This easy and rapid method can greatly simplify the optimization process, which provides a feasible way to enhance spore yields by screening possible factors involved in the SSF of Bacillus.
In the optimization procedure of solid substrates, raw solid substrates of corn meal, soybean meal, and wheat bran were included in SSF since the solid substrates not only served as nutrient material, but also as supporters for bacteria proliferation [12]. Corn meal is rich in starch, and soybean meal is rich in protein, respectively, and both of them can be utilized for the growth of Bacillus strains through the release of amylase and protease. Wheat bran is mainly used as a supporter for aerobic Bacillus growth with enough oxygen transfer and heat dispersion [12, 26]. Therefore, wheat bran content in the solid substrates is very important for maintaining a certain degree of sponginess in SSF. The result in Fig. 2 confirmed the role of wheat bran in SSF, which shows that solid substrates with comparatively high proportions of wheat bran (1:1:3) produced significantly higher DPA fluorescence intensity. Similar results were also reported in other, earlier studies [12, 27, 28]. The decreased spore production that resulted when the proportion of wheat bran was further increased from 1:1:3 to 1:1:5 might be associated with the lower amount of nutrients coming from corn meal and soybean meal.
In addition to the nutrients from solid substrates, extra-soluble nutrients, including carbon sources, nitrogen sources, and certain metal minerals, in optimal concentrations favored the accumulation of vegetative cells of Bacilli and the formation of endospores in SSF [8]. The selection of glucose and peptone as essential nutrients in certain concentrations benefited Bacilli proliferation and sporulation. However, extra nutrients in high concentrations could lead to the suppression of sporulation, which was observed in the present study and was also in line with some earlier reports [24]. As for metal ions, manganese plays an indispensable role in the sporulation of Bacillus [20, 29], and the findings in the present study also showed a similar effect. The optimization of liquid substrates produced a 23.4-fold increase in spore yield, which also demonstrated the advantage of optimizing SSF in contrast with the findings of earlier reports on spore production [8, 11].
In the production of probiotic Bacillus preparations, the spores are often manufactured through SMF, which often results in high costs and effluent waste [26]. In contrast, SSF techniques have the advantages of low investment; no waste released into the environment; and the application of cheap, local materials [11, 26]. Moreover, the increased production of many bioactive metabolites during spore accumulation greatly improved the bioavailability of feed probiotics through SSF. Bioactive metabolites also play important roles in promoting intestinal health and feed digestion in animals, which enhanced the utility of probiotic spore-formers, in particular those produced by SSF [18, 19]. The fermentation kinetics of B. amyloliquefaciens BS-20 in SSF indicated that the rapid increase of spores may have been caused by the exhaustion of reducing sugar since spores are mainly accumulated at the later stage of fermentation [6, 7]. The release of TCA-soluble proteins during fermentation was caused by the soybean hydrolysates, and the concentration of TCA-soluble proteins also increased over the fermentation period. Therefore, the rapid optimization process described in the study improved both the production of spores and the corresponding bioactive metabolites.
For the first time, the parameters associated with the spore production of B. amyloliquefaciens BS-20 in SSF were statistically optimized based on spore detection by a DPA fluorimetry assay. The optimization procedures described in the present study present a rapid method for spore production and following high concentrations of bioactive metabolites. This is a model study and the design of technological process will include appropriate optimization of all processes, including those preceding the actual SSF. The present study will serve as a general example for spore production under SSF conditions in industrial Bacillus probiotic fermentation.
References
Guo XH, Li DF, Lu WQ, Piao XS, Chen XL (2006) Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie Van Leeuwenhoek 90:139–146
Gupta A, Gupta P, Dhawan A (2014) Dietary supplementation of probiotics affects growth, immune response and disease resistance of Cyprinus carpio fry. Fish Shellfish Immun 41:113–119
Mingmongkolchai S, Panbangred W (2018) Bacillus probiotics: an alternative to antibiotics for livestock production. J Appl Microbiol 124:1334–1346
Ramesh D, Souissi S, Ahamed TS (2017) Effects of the potential probiotics Bacillus aerophilus KADR3 in inducing immunity and disease resistance in Labeo rohita. Fish Shellfish Immun 70:408–415
Zhang W, Zhu YH, Zhou D, Wu Q, Song D, Dicksved J, Wang JF (2017) Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype muc4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor negative. Appl Environ Microbiol 83:e02747–e02716
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28:214–220
Elshaghabee FMF, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490
Berikashvili V, Sokhadze K, Kachlishvili E, Elisashvili V, Chikindas ML (2017) Bacillus amyloliquefaciens spore production under solid-state fermentation of lignocellulosic residues. Probiotics Antimicro. https://doi.org/10.1007/s12602-017-9371-x
Khardziani T, Kachlishvili E, Sokhadze K, Elisashvili V, Weeks R, Chikindas ML, Chistyakov V (2017) Elucidation of Bacillus subtilis KATMIRA 1933 potential for spore production in submerged fermentation of plant raw materials. Probiotics Antimicro 9:435–443
Pryor SW, Gibson DM, Hay AG, Gossett JM, Walker LP (2007) Optimization of spore and antifungal lipopeptide production during the solid-state fermentation of Bacillus subtilis. Appl Biochem Biotechnol 143:63–79
Zhao S, Hu N, Huang J, Liang Y, Zhao B (2008) High-yield spore production from Bacillus licheniformis by solid state fermentation. Biotechnol Lett 30:295–297
Jian X, Shouwen C, Ziniu Y (2005) Optimization of process parameters for poly γ-glutamate production under solid state fermentation from Bacillus subtilis CCTCC202048. Process Biochem 40:3075–3081
Prakasham RS, Subba Rao C, Sreenivas Rao R, Sarma PN (2005) Alkaline protease production by an isolated Bacillus circulans under solid-state fermentation using agroindustrial waste: process parameters optimization. Biotechnol Prog 21:1380–1388
Zhang YR, Xiong HR, Guo XH (2014) Enhanced viability of Lactobacillus reuteri for probiotics production in mixed solid-state fermentation in the presence of Bacillus subtilis. Folia Microbiol 59:31–36
Rai AK, Sanjukta S, Chourasia R, Bhat I, Bhardwaj PK, Sahoo D (2017) Production of bioactive hydrolysate using protease, beta-glucosidase and alpha-amylase of Bacillus spp. isolated from kinema. Bioresour Technol 235:358–365
Hotz C, Gibson RS (2007) Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J Nutr 137:1097–1100
Song YS, Pérez VG, Pettigrew JE, Martinez-Villaluenga C, de Mejia EG (2010) Fermentation of soybean meal and its inclusion in diets for newly weaned pigs reduced diarrhea and measures of immunoreactivity in the plasma. Anim Feed Sci Tech 159:41–49
Choi JY, Shinde PL, Ingale SL, Kim JS, Kim YW, Kim KH, Kwon IK, Chae BJ (2011) Evaluation of multi-microbe probiotics prepared by submerged liquid or solid substrate fermentation and antibiotics in weaning pigs. Livest Sci 138:144–151
Shim YH, Shinde PL, Choi JY, Kim JS, Seo DK, Pak JI, Chae BJ, Kwon IK (2010) Evaluation of multi-microbial probiotics produced by submerged liquid and solid substrate fermentation methods in broilers. Asian Austral J Anim Sci 23:521–529
Ren H, Su YT, Guo XH (2018) Rapid optimization of spore production from Bacillus amyloliquefaciens in submerged cultures based on dipicolinic acid fluorimetry assay. AMB Express 8:21
Liang XS, Liu C, Long Z, Guo XH (2018) Rapid and simple detection of endospore counts in probiotic Bacillus cultures using dipicolinic acid (DPA) as a marker. AMB Express 8:101
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Chen ZM, Li Q, Liu HM, Yu N, Xie TJ, Yang MY, Shen P, Chen XD (2010) Greaterenhancement of Bacillus subtilis spore yields in submerged cultures byoptimization of medium composition through statistical experimental designs. Appl Microbiol Biotechnol 85:1353–1360
Hazan R, Que YA, Maura D, Rahme LG (2012) A method for high throughput determination of viable bacteria cell counts in 96-well plates. BMC Microbiol 12:259
Raghavarao KSMS, Ranganathan TV, Karanth NG (2003) Some engineering aspects of solid-state fermentation. Biochem Eng J 13:127–135
Baysal Z, Uyar F, Aytekin Ç (2003) Solid state fermentation for production of α-amylase by a thermotolerant Bacillus subtilis from hot-spring water. Process Biochem 38:1665–1668
Ellaiah P, Adinarayana K, Bhavani Y, Padmaja P, Srinivasulu B (2002) Optimization of process parameters for glucoamylase production under solid state fermentation by a newly isolated Aspergillus species. Process Biochem 38:615–620
Granger AC, Gaidamakova EK, Matrosova VY, Daly MJ, Setlow P (2011) Effects of Mn and Fe levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl Environ Microbiol 77:32–40
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 31672455), the Outstanding Young Talent Fund from Key Projects in Hubei Province Natural Science Foundation (NSF; 2018CFA077), and the Fundamental Research Funds for the Central Universities (CZT18002, CZT18003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Su, Yt., Liu, C., Long, Z. et al. Improved Production of Spores and Bioactive Metabolites from Bacillus amyloliquefaciens in Solid-state Fermentation by a Rapid Optimization Process. Probiotics & Antimicro. Prot. 11, 921–930 (2019). https://doi.org/10.1007/s12602-018-9474-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9474-z