Abstract
We evaluated the protective effects of probiotic administration as a prophylaxis treatment and immediately after fever onset in increasing the immune response and decreasing the renal scarring in a rat model of acute pyelonephritis. Twenty-four rats were apportioned to three groups. In GI (n = 8), the rats were injected with direct inoculation of Escherichia coli into the right kidney. In GII (n = 8), the rats received a probiotic regimen 1 month before E. coli injection and the probiotic regimen was continued for the next 2 months. In GIII (n = 8), the probiotic regimen was started just after E. coli injection and was continued for 2 months. Technetium-99m-DMSA renal scan, histopathological evaluations, concentrations of CA19-9, IgA, blood urea nitrogen (BUN), and creatinine were assessed 1 and 2 months post-injection. It took an average of 4.2 ± 1.1 h between the injection and onset of fever in GI and GII. In GIII, this period was longer (7.5 ± 1.4). Probiotic administration resulted in reduction of interstitial fibrosis and tubular and glomerular atrophy in GII in all follow-ups. Technetium-99m-DMSA renal scan showed that the right kidney reached near the normal cortical integrity (47%) in GII compared to GI (32%) after 2 months of injection. However, the renal integrity did not improve significantly in GIII (41%). In GII, CA19-9 was lower (p < 0.05), while the levels of serum and fecal IgA were higher (p < 0.05). Administration of the probiotic regimen in the rat model may decrease renal damage in pyelonephritis. In spite of better results in the prophylactic group compared to the treatment group, no strong evidence was found to prove the advantage of its prophylactic application over the treatment administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
End-stage renal failure occurs in approximately 8–20% of children with acute pyelonephritis (APN) and young adults [1]. APN presents with nonspecific manifestations while specific signs usually commence after a gap of approximately 1–3 days from the initiation of infection. However, postponed diagnosis and management of APN may result in irreversible renal scar formation [2]. It has been documented that the first febrile urinary tract infection (UTI) could result in renal scarring in 5 to 15% of children [3].
Immediate initiation of antibiotic therapy has been considered as the conventional approach for the prevention of scar formation in pediatric population [4]. However, radical scavengers and anti-inflammatory materials such as vitamin E, allopurinol, and prednisolone administration immediately after bacterial inoculation have been the recently applied strategies in different animal models [5,6,7]. Nevertheless, delayed intervention which can happen as a consequence of nonspecific presentations of pyelonephritis in clinical manifestation of patients with underlying disease may result in irreversible renal scar and chronic renal failure. In this regard, modern dialysis techniques and kidney transplantations are the only successful techniques in decreasing the mortality and morbidity rate.
The efficacy of lactobacilli in the prevention of UTI has been assessed for the first time by Reid et al. in which Escherichia coli (E. coli) colonization in urinary tract has been prevented in 84% of experimental rats [8]. Probiotics have been suggested to have preventive effects in colonization of uropathogenic bacteria [9].
On the basis of these data, we decided to administer probiotic treatment in an animal model with pyelonephritis. In the present study, we assessed the efficacy of probiotic administration as a prophylaxis treatment and immediately after fever onset in increasing the immune response and renal integrity in the rat model of APN.
Materials and Methods
Animals and Treatments
A total of 24 female Sprague-Dawley rats weighting 250–300 g were used in this study. The local ethics committee approved the experimental protocol. The principles of laboratory animal care (NIH publication no. 85–23, revised 1985) were respected for animal treatment.
Probiotic Yoghurt Production
After heating milk at 85 °C for 30 min and cooling it to the fermentation temperature (43 °C), inoculation was performed with yoghurt bacteria (Chr. Hansen, Denmark), Lactobacillus acidophilus (LA-5), and Bifidobacterium (BB-12). After incubation at 37 °C, it was cooled and stored at 4–6 °C. No significant reduction in the total bacterial count was expected during the shelf time (2 weeks). The probiotic was administered in a dosage of 0.25 ml/kg from 100 ml yoghurt containing 107 colony-forming unit (CFU)/ml of LA5 and Bifidobacterium animalis subsp. Lactis (Bb12) three times a day in the experimental groups. Considering the fact that shelf life was 2 weeks, different batches were applied in order to avoid administration of variable concentrations.
Bacterial Culture
E. coli ATCC 25922 strain was used for inoculation. This is a pathogenic strain to rats with a reliable production of APN. As we have previously described [7], the bacteria was cultured overnight on blood a gar plates at 35 °C and diluted to a final concentration of approximately 1010 organisms/ml in sterile phosphate-buffered saline (PBS). The solution was used on the day it was prepared.
Bacterial Inoculation
Intraperitoneal ketamine (40 mg/kg) and xylazine (4 mg/kg) were administered for anesthetization of the rats. The right kidneys were surgically exposed through a standard flank incision, and 109 colony-forming units of the bacteria (0.1 ml) were gradually injected through the cortex down to the medulla, both in the superior and inferior poles. The left kidneys remained intact in all animals. This technique was first described by Kaye [10], in which direct inoculation of E. coli induces a constant and severe APN.
Experimental Groups
The rats were randomly allocated to three groups, each containing eight animals. In GI (control), the rats were given bacterial inoculation in the right kidney. In GII, the probiotic regimen was started 30 days before bacterial inoculation and the treatment was continued for the next 2 months after E. coli injection. In GIII, the probiotic regimen was started after the bacterial inoculation for duration of 2 months. All animal groups were treated in the same manner regarding the caloric intake and were given free access to food and water. The only difference was regarding the supply of probiotics for GII and GIII. A transrectal probe that was connected to a digital rectal thermometer was applied for monitoring the animals’ body temperature at 1 h intervals, beginning 30 min after pyelonephritis induction for 20 h.
Collection of Urine, Fecal, and Blood Samples
Rats were kept in metabolic cages for 24-h urine and fecal collection with 1-week intervals. For the rats in the prophylaxis group (GI), this procedure was also performed during the prophylaxis period (1 month before the operation) as well as after the bacterial inoculation. Urine samples were centrifuged at 3.500 rounds per minute (rpm) for 15 min. Blood samples were obtained from the tail vein and were centrifuged at 4.000 rpm. All samples were maintained in − 80 °C and submitted for duplicate analysis. CA 19-9, serum and fecal IgA, blood urea nitrogen (BUN), and creatinine levels were determined. The CA19-9 level was measured by applying rat gastrointestinal cancer marker CA199 ELISA Kit with the limit of detection defined as 1 U/ml. The IgA level was measured applying rat IgA ELISA Kit (Bioassay Technology Laboratory).
Dimercaptosuccinic Acid Scan Evaluation
In order to evaluate the renal scars after pyelonephritis induction, injection of 37 MBq of 99mTc dimercaptosuccinic acid (DMSA) was performed in the tail vein before bacterial inoculation and 1 week postoperatively. A high-resolution collimator was applied to obtain anterior and posterior static images and grade renal scars 2 hours after injection of the contrast as described previously [11]. DMSA scan was performed before and after bacterial inoculation to make sure that the scars were acute and due to the recent inoculation and normal rats were included in the study. The same procedure was repeated after 1 and 2 months of bacterial inoculation (N = 2 after 1 month and N = 2 after 2 months of inoculation). The following criteria were considered for grading the scar: no damage = 0; less than 33% = 1, 33–66% = 2, more than 66% damage = 3.
Tissue Processing for Histopathology and Enzyme Immunoassay
The rats underwent another surgery 8 weeks later and unilateral nephrectomy was performed. The renal morphological evaluation was performed including the weight, longitudinal size, and renal cortical thickness. An average of four sections (3-μm thick), were obtained for each tissue after routine processing of paraffin blocks. The sections were stained with hematoxylin and eosin (H&E) and examined by light microscopy. General microscopic criteria for pyelonephritis was applied for semi-quantitative comparison of interstitial inflammation and fibrosis (none, 0; mild, 1+; moderate, 2+; severe/focal, 3+; multi-focal, 4+) according to the grading of Chen et al. [12]. In order to reduce the bias, several views with different magnifications were analyzed by two different pathologists who were totally blind to the current study.
Statistical Analysis
Image analysis was performed with Photoshop 10.0 software (Adobe Systems, Inc., Mountain View, CA, USA) and Image Pro (Image Pro Inc., Boston, MA, USA). Five photomicrographs (×100) of each sample were evaluated and the mean scores were obtained as final value for analysis. P < 0.05 was regarded as significant. Data analysis was done by statistical package SPSS 19 (Chicago, IL, USA).
Results
Fever monitoring
It took an average of 4.2 ± 1.1 h between the injection and onset of fever in the control group. However, this period was longer (7.5 ± 1.4 and 6.9 ± 1.2 h in GII and GIII, respectively) with no significant difference. The temperature did not reach as high as GI (39.2 °C) in GII (38.1 °C) and GIII (38.4 °C). However, the differences were not significantly different between the experimental groups.
Renal morphological evaluation
The renal longitudinal size was assessed when the kidney was exposed for bacterial inoculation, and after right nephrectomy was performed. However, the cortical thickness and renal weight were measured after the second surgery for nephrectomy. As shown in Table 1, the cortical thickness was decreased in the control group, while it was better preserved in GII and GIII. The cortical thickness was significantly higher in GII as compared to GI (3.75 ± 0.2 vs. 1.45 ± 0.1, p = 0.02). Similar results were obtained while comparing GIII and GI (2.5 ± 0.3 vs. 1.45 ± 0.1, p = 0.04). However, the statistical analysis did not show any significant difference between GII and GIII (p = 0.1).
Similar results were obtained for the renal weight in which a significant difference was observed between GI (4.86 ± 0.6) and GII (1.75 ± 0.3) (p = 0.03). However, the difference between GII and GIII was not statistically significant (p = 0.08). Similarly, the renal longitudinal size was significantly different between GI (32.5 ± 4.2) and GII (25.6 ± 3.7) (p = 0.02); however, there was no significant difference between GII and GIII (p = 0.07).
Histopathological findings
APN and renal scar formations were detected in all rats in the control group that had received neither prophylactic nor therapeutic probiotic administration (Fig. 1). Interstitial inflammation and fibrosis scores of all the experimental and control groups are summarized in Table 1. Accordingly, histological scores for interstitial inflammation of rats in prophylactic and treatment groups were significantly lower than in the control group (p = 0.02 and p = 0.03, respectively). However, no significant difference was detected between the experimental and treatment group in terms of interstitial inflammation (p = 0.25). The grade of interstitial fibrosis was compatible with the inflammation level. Similarly, in spite of the fact that no significant difference was found between experimental groups (p = 0.32), the histological score of fibrosis was significantly higher in the control group as compared with the prophylactic and treatment groups (p = 0.01 and p = 0.02, respectively).
DMSA renal scintigraphy
The cortical integrity of the right kidney was 31 ± 2% in rats of the control group after 2 months of injection. In spite of a significant improvement in rats of GII (46 ± 1%), the renal integrity did not improve significantly in GIII (41 ± 2%) in the same time-point (Fig. 1).
Immunoassay
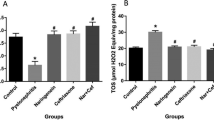
The results of serum and fecal IgA, CA 19-9, BUN, and creatinine level are summarized in Table 1. Accordingly, the level of serum IgA was significantly higher in the prophylaxis group (72.3 ± 2.4) as compared to the control group (29.8 ± 4.6) after 2 months of bacterial inoculation (p = 0.03). Similar results were obtained for fecal IgA (0.41 ± 0.22 vs. 0.09 ± 0.04, p = 0.02). However, no statistically significant difference was detected between the prophylaxis and treatment groups in serum and fecal IgA level (p > 0.05). The level of serum and fecal IgA had a gradual increase in GII during the prophylaxis period (33.8 to 57.9 mg/l for serum IgA) (0.08 to 0.34 mg/g for fecal IgA).
The CA19-9 level was significantly lower in the prophylaxis group (41.3 ± 2.7) as compared to the control group (146.4 ± 3.7) (p = 0.025). Nevertheless, no significant difference was found between the prophylaxis and treatment groups (52.6 ± 4.5) in CA19-9 measurement (p > 0.05). A trend of gradual decrease of urinary CA19-9 and increase in IgA level was observed for the duration of probiotics administration without any significant difference between GII and GIII. The level of CA19-9 had a gradual decrease in GII during the prophylaxis period (24.2–19.3 U/mL).
Discussion
The results of the current study revealed that probiotic administration was effective in preventing renal scarring in the rat model of pyelonephritis. In spite of the fact that the treatment regimen was not as successful as prophylactic application; the differences were not statistically significant.
E. coli and other members of enterobacteriaceae family which are common habitants of human intestines are the major cause of UTI [13]. Conservative approaches for recurrent UTI include antibiotics with anticholinergic agents and increased oral intake of fluids and agents to alkalinise the urine. Conservative management for the bowel and bladder dysfunctions primarily includes voiding behavior modification including timed voiding schedules, effective biofeedback therapy, and treatment of constipation, if present. In addition, animated biofeedback is an effective treatment of dysfunctional voiding in the pediatric population. In spite of wide application of antibiotics in management of acute UTI, there is still a high incidence of recurrence and the efficient preventive method is still unclear [14]. However, introducing an efficient alternative both for treatment and prophylaxis uses is of great value.
Loss of the normal genital microbiota, mainly Lactobacillus species, is highly associated with an increased incidence of both genital and urinary infections [15]. The release of reactive oxygen species from invasive neutrophils that will induce cell death through necrosis might be the fundamental mechanism for cellular damage during APN. Inhibition of cell proliferation has been considered as a contributing factor in renal damage in children with APN [16].
Recent studies have suggested that DNA extracted from probiotic organisms is capable of mediating anti-inflammatory activity through toll-like receptor 9 signaling [17, 18]. Moreover, immunomodulatory aspects and host immune system stimulation, anti-pathogenic features by producing antimicrobial factors, competition with pathogens for nutrients or host binding sites, cell proliferation regulation, promoting normal physiologic development of the mucosal epithelium, and improvement of nutrition are among the other roles attributed to probiotics [19].
Intraurethral administration of probiotic Lactobacillus casei has shown promising results in a murine model of Escherichia coli UTI [20]. The effect of probiotic in gynecology field has been well documented. It has been demonstrated that vaginal suppositories of Lactobacillus crispatus GAI 98322 was significantly associated with significant reduction of UTI recurrence [21]. In a pilot study, it was suggested that regular consumption of L. acidophilus vaginal suppositories is safe and effective in reducing the episodes of UTI breakthrough [22]. In the study of Lee et al., it has been demonstrated that probiotics consumption was as effective as antibiotic prophylaxis in children with resistant primary VUR [23]. Similarly, the results of our previous study revealed that probiotic and antibiotics consumption in children with recurrent UTI is more effectual as compared to prophylactic antibiotics alone [24]. However, more studies are needed to find the most appropriate vehicle. In the current study, we aimed to compare the results of probiotic administration as treatment or prophylactic regimen. In spite of the fact that prophylactic administration showed better results, the differences were not statistically significant while similar dosage of probiotics was administered in both the prophylactic and treatment group. This may be due to the fact that the same dosage of probiotic was the same in both groups. Higher concentrations of probiotics may have more satisfactory results for treatment purposes. On the other hand, yogurt may not be the most suitable vehicle for treatment of acute urinary infection. As a result, more studies are required to find the exact dose of probiotic administration and the most appropriate way for treating acute UTI.
The results of a meta-analysis by Grin et al. revealed the safeness and effectiveness of probiotic strains of Lactobacillus in preventing recurrent UTI in adult women [25]. In contrast, Schwenger et al. demonstrated that the therapeutic advantage of probiotic administration when used to prevent UTIs in susceptible patient populations showed no significant benefits in terms of morbidity and mortality rates [26]. However, establishment of an animal model for comparison of probiotic administration, before and after bacterial inoculation may be important to draw more firm conclusions. The result of the present study showed a significant decrease in CA 19-9 and increase in the IgA level in the prophylaxis group that are compatible with the results obtained by the study conducted by Grin et al., confirming the preventing properties of probiotics.
CA 19-9 is a glycolipid which is applied in the diagnosis and management of various types of cancers and is rarely increased in healthy patients [27]. The correlation of hydronephrosis and urinary/serum CA19-9 was shown in other clinical studies [28, 29]. The present study showed that the prophylactic application of probiotics may have better results in decreasing the CA19-9 level, increasing the immune response and prevention of renal scar, as well as ameliorating the pathophysiological aspects of renal tissue in the rat model of pyelonephritis.
Neither clinical findings nor laboratory studies are accurate enough for distinguishing pyelonephritis from cystitis [30]. DMSA renal scintigraphy has been considered as a method of choice for localizing the UTI site in adult patients [11]. However, it is an invasive method especially in infants and several concerns exist about its accuracy [31].
Considering the results of the current study, it can be assumed that probiotic administration may not prevent renal scars from occurring. As a result, probiotic application could be commenced in cases at high risk of APN to prevent further recurrences and damage. In spite of the promising results of the present study, several limitations exist such as loss of validated histochemical scoring, application of semi-quantitative analysis, and small sample size of the experimental groups. Additionally, the effectiveness of this regimen should be elucidated in future long-term studies in cases with different grades of pyelonephritis.
Conclusion
Increasing antibiotics resistance and failure of antibiotics therapy in genitourinary infections has led to a gap for health care system. The probiotic regimen may be a promising alternative in susceptible children with recurrent UTI and pyelonephritis. This experimental study might be clinically applicable by reducing the UTI episodes and subsequent renal scaring in this subgroup of patients, end-stage renal disease and the potential need for a surgery may be decreased.
Abbreviations
- APN:
-
Acute pyelonephritis
- UTI:
-
Urinary tract infection
- E. coli :
-
Escherichia coli
- IgA:
-
Immunoglobulin A
- BUN:
-
Blood urea nitrogen
References
Wassner S, Baum M (1999) Physiology and management. Pediatric Nephrology Lippincott Williams Wilkins, Baltimore, pp 1155–1182
LeBrun M, Grenier L, Gourde P, Bergeron MG, Labrecque G, Beauchamp D (1999) Effectiveness and toxicity of gentamicin in an experimental model of pyelonephritis: effect of the time of administration. Antimicrob Agents Chemother 43(5):1020–1026
Koch VH, Zuccolotto SM (2003) Urinary tract infection: a search for evidence. J Pediatr 79(Suppl 1):S97–106. https://doi.org/10.1590/S0021-75572003000700011
Tekgül S (2015) A snapshot of the guidelines on vesicoureteral reflux in children. Eur Urol Suppl 14(1):9–11. https://doi.org/10.1016/j.eursup.2015.01.005
Celik S, Gorur S, Aslantas O, Erdogan S, Ocak S, Hakverdi S (2007) Caffeic acid phenethyl ester suppresses oxidative stress in Escherichia coli-induced pyelonephritis in rats. Mol Cell Biochem 297(1–2):131–138. https://doi.org/10.1007/s11010-006-9337-x
Kaur A, Garg U, Sethi A, Gorowara S, Sharma S, Ganguly N (1988) Effect of various oxygen free radical scavengers in preventing tissue injury caused by Escherichia coli in pyelonephritic mice. Biochem Int 16(6):1083–1093
Sadeghi Z, Kajbafzadeh AM, Tajik P, Monajemzadeh M, Payabvash S, Elmi A (2008) Vitamin E administration at the onset of fever prevents renal scarring in acute pyelonephritis. Pediatr Nephrol 23(9):1503–1510. https://doi.org/10.1007/s00467-008-0853-7
Reid G, Chan RC, Bruce AW, Costerton JW (1985) Prevention of urinary tract infection in rats with an indigenous Lactobacillus casei strain. Infect Immun 49(2):320–324
Cadieux PA, Burton J, Devillard E, Reid G (2009) Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J Physiol Pharmacol 60 Suppl 6(Suppl 6):13–18
Kaye D (1971) The effect of water diuresis on spread of bacteria through the urinary tract. J Infect Dis 124(3):297–305. https://doi.org/10.1093/infdis/124.3.297
Rushton HG (1997) The evaluation of acute pyelonephritis and renal scarring with technetium 99m-dimercaptosuccinic acid renal scintigraphy: evolving concepts and future directions. Pediatr Nephrol 11(1):108–120. https://doi.org/10.1007/s004670050243
Chen SM, Mukoyama T, Sato N, Yamagata S, Arai Y, Satoh N, Ueda S (2002) Induction of nephrotoxic serum nephritis in inbred mice and suppressive effect of colchicine on the development of this nephritis. Pharmacol Res 45(4):319–324. https://doi.org/10.1006/phrs.2002.0948
Katouli M (2010) Population structure of gut Escherichia coli and its role in development of extra-intestinal infections. Iran J Microbiol 2(2):59–72
Montini G, Tullus K, Hewitt I (2011) Febrile urinary tract infections in children. N Engl J Med 365(3):239–250. https://doi.org/10.1056/NEJMra1007755
Amdekar S, Singh V, Singh DD (2011) Probiotic therapy: immunomodulating approach toward urinary tract infection. Curr Microbiol 63(5):484–490. https://doi.org/10.1007/s00284-011-0006-2
Serlachius E, Sundelin B, Eklof AC, Jahnke M, Laestadius A, Aperia A (1997) Pyelonephritis provokes growth retardation and apoptosis in infant rat renal cortex. Kidney Int 51(6):1855–1862. https://doi.org/10.1038/ki.1997.253
Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E (2004) Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126(2):520–528. https://doi.org/10.1053/j.gastro.2003.11.019
Hopkin M (2004) Probiotic bacteria health boon. Nature 432:427
Hsieh MH, Versalovic J (2008) The human microbiome and probiotics: implications for pediatrics. Curr Probl Pediatr Adolesc Health Care 38(10):309–327. https://doi.org/10.1016/j.cppeds.2008.09.001
Asahara T, Nomoto K, Watanuki M, Yokokura T (2001) Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother 45(6):1751–1760. https://doi.org/10.1128/AAC.45.6.1751-1760.2001
Uehara S, Monden K, Nomoto K, Seno Y, Kariyama R, Kumon H (2006) A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int J Antimicrob Agents 28:30–34. https://doi.org/10.1016/j.ijantimicag.2006.05.008
Kontiokari T, Laitinen J, Jarvi L, Pokka T, Sundqvist K, Uhari M (2003) Dietary factors protecting women from urinary tract infection. Am J Clin Nutr 77(3):600–604
Lee SJ, Shim YH, Cho SJ, Lee JW (2007) Probiotics prophylaxis in children with persistent primary vesicoureteral reflux. Pediatr Nephrol 22(9):1315–1320. https://doi.org/10.1007/s00467-007-0507-1
Mohseni MJ, Aryan Z, Emamzadeh-Fard S, Paydary K, Mofid V, Joudaki H, Kajbafzadeh AM (2013) Combination of probiotics and antibiotics in the prevention of recurrent urinary tract infection in children. Iran J Pediatr 23(4):430–438
Grin PM, Kowalewska PM, Alhazzan W, Fox-Robichaud AE (2013) Lactobacillus for preventing recurrent urinary tract infections in women: meta-analysis. Can J Urol 20(1):6607–6614
Schwenger EM, Tejani AM, Loewen PS (2015) Probiotics for preventing urinary tract infections in adults and children. Cochrane Database Syst Rev 12:CD008772. https://doi.org/10.1002/14651858.CD008772.pub2
Bedi M, Gandhi M, Jacob G, Lekha V, Venugopal A, Ramesh H (2009) CA 19-9 to differentiate benign and malignant masses in chronic pancreatitis: is there any benefit? Indian J Gastroenterol 28(1):24–27. https://doi.org/10.1007/s12664-009-0005-4
Aybek H, Aybek Z, Sinik Z, Demir S, Sancak B, Tuncay L (2006) Elevation of serum and urinary carbohydrate antigen 19-9 in benign hydronephrosis. Int J Urol 13(11):1380–1384. https://doi.org/10.1111/j.1442-2042.2006.01593.x
Kajbafzadeh AM, Elmi A, Talab SS, Emami H, Esfahani SA, Saeedi P (2010) Urinary and serum carbohydrate antigen 19-9 as a biomarker in ureteropelvic junction obstruction in children. J Urol 183(6):2353–2358. https://doi.org/10.1016/j.juro.2010.02.031
Majd M, Rushton HG, Jantausch B, Wiedermann BL (1991) Relationship among vesicoureteral reflux, P-fimbriated Escherichia coli, and acute pyelonephritis in children with febrile urinary tract infection. J Pediatr 119(4):578–585. https://doi.org/10.1016/S0022-3476(05)82407-2
Linné T, Fituri O, Escobar-Billing R, Karlsson A, Wikstad I, Aperia A, Tullus K (1994) Functional parameters and99mtechnetium-dimercaptosuccinic acid scan in acute pyelonephritis. Pediatr Nephrol 8(6):694–699. https://doi.org/10.1007/BF00869092
Acknowledgements
The authors acknowledge Miss Shahnaz Halimi of the Department of Microbiology, School of Medicine, Tehran University of Medical Sciences for bacterial culture. The authors thank the authorities in charge of R&D department of Iran Dairy Industries Co. (Pegah).
Funding
The authors would like to thank Tehran University of Medical Sciences for funding this study (Grant Number 29548).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The local ethics committee approved the experimental protocol. The principles of laboratory animal care (NIH publication no. 85–23, revised 1985) were respected for animal treatment.
Rights and permissions
About this article
Cite this article
Sabetkish, N., Sabetkish, S., Mohseni, M.J. et al. Prevention of Renal Scarring in Acute Pyelonephritis by Probiotic Therapy: an Experimental Study. Probiotics & Antimicro. Prot. 11, 158–164 (2019). https://doi.org/10.1007/s12602-017-9363-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9363-x