Abstract
The Korean rockfish, Sebastes schlegeli, is most commonly farmed in sea cages along the coast of Korea; however, detailed information on intestinal microbiota regarding this fish is not readily available. In this study, comparison of the seasonal changes of microbial communities in the intestine between farmed and wild through the amplicon sequencing approach was conducted. The composition of major species in the intestine of this fish was very simple compared to that of other marine fish species, with members affiliated with the family Vibrionaceae hyper-dominating and comprising on average 97.6% of microbiota. However, the composition at the genus or species level and the pattern of seasonal changes of diversity indices showed significant differences between farmed and wild fish. In the farmed fish, Photobacterium phophoreum was most dominant throughout the year, accounting for 58.8% of the total. Aliivibrio fisherii and/or Aliivibrio finisterrensis also were dominant in the fall to winter but substituted by Photobacterium damselae during spring to summer. In the wild fish, on the other hand, opportunistic pathogens in the genera Aliivibrio or Vibrio were dominant in most of the samples. The analysis of shared species between gut microbiome, feed microbiota, and seawater microbiota indicated that the intestinal microbial diversity of farmed fish was affected more by microbiota of seawater than that of feed in spring and winter seasons. Additionally, the proportion of potential pathogenic Vibrio spp. in the gut showed a negative correlation with plasma glucose levels of the host. This study and following studies will be helpful in understanding the interaction between microbiome hosts and the development of techniques to enhance production of healthy Korean rockfish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The gut microbiota in vertebrates, including humans, play an important role in nutritional provisioning, homeostasis, immune defense, etc. (Gómez and Balcázar 2008; Sullam et al. 2012). In fish, gut microbiota have also a substantial influence on growth promotion, improvement of digestion, improvement of the immune system, resistance against stressful conditions and disease control (Ntranos and Casaccia 2018; Sayes et al. 2018; Wang et al. 2018). The gut microbiota of fish are affected by food, ambient water, season and life stage, and varies between species as well as within species (Ramírez and Romero 2017; Egerton et al. 2018).
Korean rockfish (Sebastes schlegelii, family Scorpaenidae) are distributed in the Northwest Pacific Ocean, and are usually farmed in sea cages in Korea. In nature, the preferred temperature for this species is 8–21.3 °C (average 17 °C), and the suitable temperature for growth is 15–20 °C (Choi et al. 2009). However, the temperature in the Southern coast of Korea is about 7–25 °C year round, and the period suitable for growth is limited to 8 months (Sonh et al. 2007). In particular, Korea is one of the ten countries that are expected to suffer the greatest damage to the marine aquaculture industry due to global warming (Soto et al. 2018), and the period when summer water temperature is maintained above 25 °C was 35 days in 2017 and daily water temperature changes shows a maximum of 6.5 °C/day in 2017. These unfavorable water temperature conditions for the sea cage farming of Korean rockfish have led to an increase in mortality as a result of the outbreak of bacterial disease and have also contributed to retarded growth and poor health (Kim et al. 2019).
Chemicals such as growth enhancers, metabolic agents, and antibiotics for disease prevention and treatment in farmed fish have negative impacts on the environment and food safety. Therefore, it is necessary to promote healthy gut microbiota when conducting fish farming (Llewellyn et al. 2014). Recently, a study has been conducted on the characteristics of the gut microbiota of farmed Korean rockfish in their early life stages (1–95 days after hatching), but sufficient data have not been provided (Jiang et al. 2020). They analyzed trends and the source of core microbiota of rockfish and reported that Proteobacteria and Firmicutes are the most dominant phyla in rockfish (Jiang et al. 2020). Investigations of intestinal microbiota of Korean rockfish have been carried out through culture-dependent methods under aerobic conditions and some novel species were isolated (Hyun et al. 2015; Kang et al. 2016; Tak et al. 2018).
For eco-friendly farming of marine fish with the reduced use of chemicals, it is important to obtain baseline data of the gut microbiota of healthy wild populations and compare them with the farmed population (Egerton et al. 2018). Thus, in this study, we analyzed the changes of gut microbial communities in the grow-out stages of farmed and wild populations of Korean rockfish and the differences between the two populations were compared.
2 Materials and Methods
2.1 Sample Collection and Processing
The farmed Korean rockfish used in the experiment were artificially produced in land-based tanks, and stocked at sea cages installed on the coast of Tongyeong City in June 2018. During the breeding period in sea cages, commercial dry pellets were supplied for the first 3 months, and moist pellets (a mixture of frozen fish and powdered feed containing fishmeal at a weight ratio of 95:5, moisture 75%, crude protein 69%, crude lipid 16%, and ash 15%) were supplied thereafter. The wild fish were collected by fishing boats in the vicinity of Maemuldo Island in Tongyeong City to exclude the effects of artificial feed supplied to sea cages. The intestinal contents were sampled at four water temperatures (14.4 °C, 12.1 °C, 16.8 °C, and 22.1 °C) for the farmed population and three water temperatures, excluding 22.1 °C for the wild population (Table 1). The intestinal microbiome composition of fish may vary depending on the growth stage (Aquilera et al. 2013). Therefore, individuals of the same growth stage (i.e., pre-adult stage) and of the same period after feeding were used in experiments.
The individual fish sample was designated with four letter and first letter means its origin, second and third for season, and the fourth designated individual number (FWi1 designated farmed fish collected in winter). The hind gut of the intestine (from anus up to 5 cm of digestive tract) was removed from anesthetized fish by Tricaine-S (Syndel USA, Ferndale, Washington, USA), slit open with scissors, and the faecal-like contents were washed off with phosphate buffered saline (PBS). Then the intestinal mucus was scrapped off with a spatula or was squeezed out by a pincette. The intestinal mucus was transferred to a 15 ml conical tube filled with PBS and stored at 2–4 °C until analysis. Contents of intestines were suspended in 5 ml of PBS and stored at 4 °C until the DNA extraction process was carried out. To examine the impact of environmental factors on the fish intestinal microbiota, feed and seawater samples were collected. Moist pellets as feed stored at − 20 °C were transported to the laboratory in a frozen state. Surface seawater inside the sea cage was collected in spring and winter and was stored at 4 °C before filtration (Table 1). All the experimental procedures involving fish were performed in accordance with national guidelines for the care and use of animals.
2.2 Total DNA Extraction and DNA Library Construction
The stored intestinal contents were homogenized with a vortex mixer and were precipitated for 3 min using ice to remove impurities (suspended solids). Then, the supernatants were collected and centrifuged for 20 min at 4000×g. Seawater was filtered through a PCTE membrane filter (d = 47 mm, ϕ = 0.2 μm, GVS, USA) and cells collected on membrane were separated by rinsing with PBS bufferand concentrated by centrifugation. The DNA from moist pellets was extracted using QIAamp® Fast DNA Stool Mini Kit (QIAGEN, Germany) following the manufacturer’s instructions. DNA from moist pellets was extracted manually using PCI solution (phenol:chloroform:isoamyl alcohol 25:24:1, Saturated with 10 mM Tris, pH 8.0, 1 mM EDTA, Sigma-Aldrich, USA) from a 100 mg sample.
The extracted DNAs were used as templates for amplification of the16S rRNA gene sequence through polymerase chain reaction (PCR). Universal primer set 341F (5′-CCTACGGGNBGCASCAG-3′) and 805R (5′-GACTACNVGGGTATCTAAT-3′) targeting the V3 and V4 regions of the 16S rRNA gene were used for amplification (Takahashi et al. 2014). PCR products were purified using QIAquick® PCR Purification Kit (QIAGEN, Germany) and confirmed with 1% agarose gel electrophoresis and spectrophotometer (NanoDrop2000, Thermo-Scientific, Korea).
2.3 Sequencing and Data Analysis
The high throughput sequencing was performed through Illumina Miseq platform commercially at ChunLab Co. Ltd. (Seoul, Korea). After the sequencing was completed, the short or low-quality reads were removed by Trimmomatic software, sorted out by tags, and primer sequences were removed. Finally, all reads were identified via BLAST analysis against the EzBioCloud database (Yoon et al. 2017) and visualized using CLCommunity™ (Ver3.46) browser. Operational taxonomic units (OTUs) by CD-HIT algorithm were extracted with a cut-off value of 97% similarity and various alpha diversity indices were calculated such as Shannon, Chao1, etc. through the Mothur package of CLCommunity™. Hierarchical cluster analysis (HCA) and principal coordinate analysis (PcoA) were conducted on farmed and wild catch rockfish during four seasons using the Fast UniFrac distance metrics (Lozupone et al. 2011). For HCA, the unweighted pair group method with arithmetic mean (UPMGA) was adopted.
2.4 Plasma Glucose and Statistical Analysis
The level of plasma glucose (mg/dl) of the fish was determined in duplicate using an automatic analyzer (FUJI DRI-CHEM 4000i, Fujifilm Co., Tokyo, Japan). Operation of the automatic analyzer was conducted according to the manufacturer's protocol using multi‐layered slides (GLU-PIII; Fujifilm Co., Tokyo, Japan).
2.5 Statistical Analysis
Results were expressed as mean ± standard error (SE). Significant differences in the diversity index and glucose levels between wild and farmed fish of each season were determined by the one-way analysis of variance (ANOVA) followed by the Tukey–Kramer tests. Statistical significance was defined as P < 0.05. Statistical analysis was conducted using SPSS Statistics 21 (IBM SPSS Statistics Version 21 program, SPSS Inc., Chicago, IL, USA).
3 Results and Discussion
3.1 Taxonomic Composition of Microflora in the Wild and Farmed Rockfish Intestines
Clean reads were obtained from 39 samples including 36 gut contents, 2 seawaters, and 1 feed by Illumina Miseq system. The sequencing yielded a total of 10,702,066 reads from the 37 intestinal contents, 529,633 reads from two seasons of seawater samples and 431,549 reads for the feed sample. After trimming of the low-quality sequences and adaptor sequence, OTUs were extracted. We obtained 446 OTUs from the entire intestinal content samples, 1147 OTUs from the seawater samples, and 1127 OTUs from the feed sample. Detailed information on the sequencing results is presented in Table S1 of the supplementary material. The microbial composition at the genus level in the intestine of sampled fish was determined based on OTUs analysis, which revealed a taxonomic composition distinguished by each seasonal samples of farmed and wild fish. At the phylum level, the majority of all the intestine of sampled fish were dominated by Proteobacteria with the relative abundance about 75.7–99.9% (mean = 99.0%). Most of the Proteobacteria reads were designated as γ-Proteobacteria (mean = 99.0%), of which Vibrionaceae was the most dominant family accounting for 97.6% of the whole fish gut sample. At the genus level, Photobacterium (mean = 60.9%), Aliivibrio (mean = 23.4%), and Vibrio (mean = 12.3%) were the dominant genera. Few samples contained more than 1% of other phylum other than Proteobacteria, and those were FSu1 (farmed fish sample in the summer), WSp4, WSp3, and WSp5 with Firmicutes (Clostridiaceae) accounting for 23.6%, Tenericutes (Mycoplasmataceae) accounting for 4.5%, and Fusobacteria (Fusobacteriaceae) accounting for 2.4% and 1.9%, respectively (Fig. 1).
The composition of the intestinal microbiota showed significant differences at the species level with seasonal changes between wild and farmed fish. Photobacterium phosphoreum was the most abundant species included in the intestine of both farmed (mean = 58.8%) and wild (mean = 44.0%) fish. P. phosphoreum is known for symbiont of various kinds of marine organismsand widespread in marine environments including the intestine of fish (Haygood 1993). In the farmed fish samples in the summer, the Photobacterium phophoreum group (mean = 64.49%) was still the most dominant, but the Photobacterium damselae group (mean = 27.89%) arose as a new major species. The P. damselae group comprises two subspecies, P. damselae subsp. damselae (Pdd) and P. damselae subsp. piscicida (Pdp) (Gauthier et al. 1995). Pdd and Pdp were reported to cause disease in many kinds of aquatic animals including economically important maricultural fish species (Rivas et al. 2013; Romalde 2002). Typical symptoms of Pdd infection are hemorrhages and ulcers overall the body surface and Pdp causes bacterial septicaemia named pasteurellosis or pseudotuberculosis (Rivas et al. 2013; Romalde 2002). According to the taxonomic composition, the P. damselae group appeared only in spring (16.8 °C) and summer (22.3 °C) seasons when the seawater temperature matched their required growth temperature. Previous reported cases indicate that the outbreak of disease by the P. damselae group in sea cage farms is associated with rising seawater temperatures in the summer season (Matanza and Osorio 2018); fortunately in the study area, no outbreaks occurred. Members affiliated with the genus Aliivibrio were the dominant genus in fall (mean = 33.0%) and winter (39.6%), and most of the reads were assigned as non-phathogenic Aliivibrio fischeri and Aliivibrio finisterrensis. Appearance of these species at low water temperature levels is in accordance with previous reports (Beaz-Hidalgo et al. 2010; Ruby et al. 2005).
In the wild fish samples, however, the P. phophoreum group as well as the potential pathogenic Aliivibrio or Vibrio groups dominated overall in most of the samples (Fig. 1). A. salmonicida dominated in winter samples (mean = 32.7%) and appeared in the fall samples at relatively lower levels (mean = 4.9%). A. salmonicida is known as a causative bacterium of cold-water vibriosis (CWV) in various aquaculture species (Egidius et al. 1981, 1986) and this CWV mainly occurs at low water temperatures and is characterized by anemia and extended hemorrhages at the epidermis of intestinal organs of fish (Egidius et al. 1981, 1986). This is attributed to the fact that the growth of A. salmonicida occurs in the temperature range of 1–22 °C with the optimum growth temperature being 15 °C (Egidius et al. 1981). In the spring and fall samples, microbial composition reveals a more diverse group of the genus Vibrio such as V. scophthalmi (11.4%), V. splendidus (5.5%), V. lentus (4.5%), V. atypicus (1.4%), V. parahaemolyticus (1.0%) and uncultured Vibrio spp. (2.0%). Among them, V. scophthalmi, V. splendidus, V. lentus, and V. parahaemolyticus are known as potential pathogenic bacteria for marine life (Baross and Liston 1970; Baticados et al. 1990; Farto et al. 2003, 2006; Qiao et al. 2012).
Some studies have concluded that shared bacteria communities are specific for their host taxa and might play some type of important symbiotic role because of metabolic benefits derived from the relationship between the bacteria and its host (Lee et al. 2018; Tinta et al. 2019). Therefore, shared bacterial species from the intestine of fish based on species pool at each season were determined (Fig. 2). Out of the 283 OTUs identified in the wild fish through spring, fall, and winter, 42 OTUs (14.8%) affiliated with the genera Photobacterium, Aliivibrio, Vibrio, Catenococcus, Shewanella, and Moritella were shared (Fig. 2a). In the farmed fish samples, 22 OTUs (9.3%) affiliated with the genera Photobacterium, Aliivibrio, Vibrio, Enterovibrio, and Shewanella were shared out of the 236 OTUs identified throughout all the seasons (Fig. 2b). However, any shared species was found on the individual level; although, it did appear that P. phosphoreum and Aliivibrio fisherii compensated each other. The exceptions were fish dominated by potential pathogens such as P. damselae and V. scolphthalmi (Fig. 1). The result was markedly different from a previous report that found that 14 genera were shared in the healthy larval guts of Korean rockfish (Jiang et al. 2020), and only the genus Vibrio was found to be shared with this study. The difference of gut microbiome profiles between two studies seems to stem from variations in environmental conditions, provided food, and the growth stages.
3.2 Diversity of Microflora in the Wild and Farmed Rockfish Intestines
Alpha diversity for each species in the intestine of wild and farmed fish was analyzed with various diversity indices (Table S1). Both Shannon and Chao 1 indexes were higher in the wild than in farmed fish (Fig. 3a, b). Interestingly, there were significant differences in the seasonal intestinal microbial diversity between wild and farmed fish. In the wild fish samples, the Shannon value was highest in spring and lowest in fall. Although summer samples were lacking, biodiversity showed an inverted U-shaped pattern that increased from winter to spring and then decreased again in fall (Fig. 3c). The reason for this is that the suitable temperature for growth of rockfish is 15–20 °C, and the spring temperature (16.8 °C) falls within this range (Table 1). In addition, the cause of the low diversity regarding the winter and fall samples is believed to have been influenced by feed sources along with seawater temperature. Unlike farmed rockfish, wild rockfish are known to eat primarily fish (Acanthopagrus schlegeli, Sebastes inermis, Engrau japonicas, Sillago spp. etc.) and additionally shrimps, crabs, amphipods and polychaetes (Park et al. 2007), however, in winter and fall, wild rockfish can experience starvation or food source limitations. On the other hand, the Shannon value of farmed fish samples was lowest in winter and increased with spring, summer, and fall (Fig. 3d). These results suggest that the biodiversity of farmed fish samples is more affected by seawater conditions than the feed source. Details will be provided in the next section which is concerned with the relationship between intestinal microbiomes and environmental factors.
Comparison of intestinal microbial diversity showed the difference between wild and farmed rockfish. Shannon (a) and Chao 1 (b) indices for overall samples and seasonal changes of Shannon index of wild (c) and farmed (d) rockfish. There is significant difference between groups marked in different lowercase alphabets (P < 0.05)
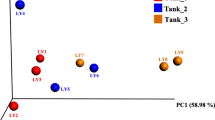
Clustering and outliers between individual samples were analyzed by hierarchical cluster analysis (HCA) (Fig. 4a) and principal coordinate analysis (PcoA) (Fig. 4b) using the Fast UniFrac distances. The HCA showed that spring (WSp1, 2, 3, and 5) and winter (WWi3, 5, and 6; WWi 2 and 4) samples of wild fish were clustered, and summer (FSu2, 5, and 7; FSu 3 and 6), fall (FFa1-5), and winter (FWi1, 2, 6 and 7; FWi3, 4, 5 and 9) samples of farmed fish were clustered (Fig. 4a). Unusually, winter samples of farmed/wild fish and the summer samples of farmed fish formed two distinct clusters (Fig. 4a). The PcoA results exhibited that samples of wild fish showed more diverse distributions than that of farmed fish (Fig. 4b). In addition, winter samples of farmed and wild fish formed two distinct clusters, and these patterns were consistent with the HCA results (Fig. 4a). There was no significant variation among individuals within each sample groups, and they were well clustered into wild or farm seasonal samples, respectively.
3.3 The Relationship Between Intestinal Microbiota and Environmental Microbiota
We conducted microbial composition analysis on environmental factors including feed and seawater. In the microbial composition of feed, Proteobacteria were the most dominant phylum accounting for 75.7% of the total reads and most of them were assigned to γ-Proteobacteria. At the genus level, Psychrobacter and Photobacterium accounted for a majority of microbiota—46.5% and 14.6%, respectively (Fig. 5). Feed samples were collected in the summer season but were stored in the freezer of fish farm. The storage environment of the feed may have resulted in the proliferation of the genus Psychrobacter, which can be reproduced at low temperatures (Bowman 2006). At the species level, 12.9% of the total reads were allocated to Photobacterium phosphoreum which is commonly found to be dominant in both wild and farmed fish microbiota. The existence of Photobacterium damselae and Aliivrio salmonisida was also confirmed to be 1.2% and 1.2% of total reads, respectively (Fig. 5). They were representative pathogens found in the intestinal microbiota in summer and winter seasons. The microbial composition of seawater samples collected in winter and spring was analyzed. The most dominant phyla in the majority of seawater samples were Proteobacteria and Bacteroidetes. In winter seawater, the most dominant phylum was Proteobacteria which accounted for 75.7% (46.6% of γ-Proteobacteria and 29.8% of α-Proteobacteria) of the total reads followed by Bacteroidetes (14.4%) and Verrucomicrobia (1.2%) (Fig. 5). In spring seawater, Alpaproteobacteria increased compared to the winter season, accounting for 55.0% of the total reads, with γ-Proteobacteria (19.2%), Bacteroidetes (13.6%), Actinobacteria (3.4%), and Verrucomicrobia (2.2%) also found to be present (Fig. 5).
To analyze the relationship between environmental microbiota and intestinal microbiome of farmed fish, the shared OTUs of intestinal microbiome, feed microbiota, and seawater microbiota were analyzed. Many types of factors, such as nutritional condition, feeding habits of the host, and environmental factors, are known to affect microbial communities in the fish (Banerjee and Ray 2017 and references therein). Analysis of the shared species among the intestine of farmed fish, feed, and seawater samples is shown in Fig. 6. In spring, 15 shared OTUs appeared between fish gut and feed, and 39 shared OTUs appeared between fish gut and seawater, accounting for 24.2% and 62.9% of total species of fish intestine, respectively (Fig. 6a). Similar to the spring sample, 13 shared OTUs appeared between fish intestine and feed in winter, and 29 shared OTUs appeared between fish intestine and seawater, accounting for 13.4% and 29.9% of total species of fish gut, respectively (Fig. 6b). These results indicate that the intestinal microbial diversity of farmed fish is more affected by seawater than by feed in spring and winter seasons, and this is consistent with the results of the diverse index analysis of previous seasonal samples of farmed fish. This is probably because the feed source was always provided in a consistent and constant manner, but microbiota of seawater changes by season. In general, it has been reported that feed microbiota has a more significant impact on the microbial structure of fish intestines than that in seawater (Jiang et al. 2020; Walburn et al. 2018). Most of the reported studies have been performed in closed aquaculture systems (Jiang et al. 2020; Walburn et al. 2018). However, this study was not conducted in a closed system, but was conducted in a cage farm, where seawater can reciprocate. Common in spring and winter samples, shared species of seawater, feed, and fish gut included P. phosphoreum, A. salmonicida, V. lentus, and V. splendidus. P. phosphoreum was a dominant group accounting for more than 90% of farmed spring and winter samples, except for a few individuals. On the other hand, A. salmonicida, V. lentus, and V. splendidus were a minority group with less than 3% (Fig. 1). Interestingly, A. salmonicida dominated in winter samples of wild fish, and V. lentus and V. splendidus dominated in several spring samples of wild fish (Fig. 1). These groups were known as pathogenic microorganisms (Baticados et al. 1990; Egidius et al. 1986; Farto et al. 2003, 2006).
3.4 Interaction of Host and Intestinal Microbiota
To understand the interaction between the intestinal microbiome and the host, components of blood samples of farmed or wild fish in spring and winter, respectively, and the correlation with intestinal microbiota were analyzed. The level of plasma glucose is used as an indicator to evaluate the stress of fish caused by physiological factors such as changes in food and water temperature (Islam et al. 2020). Bacterial disease in fish commonly results in major alterations in blood biochemical composition (Iwama and Ashida 1986; Møyner et al. 1993). Pathogenic Vibrio groups have been reported to have a positive or negative correlation with plasma glucose levels (Li and Woo 2003; Pan et al. 2019). In sea bream infected by pathogenic Vibrio species, a significantly lower level of plasma glucose was demonstrated, regardless of whether the infection was induced naturally or experimentally (Li and Woo 2003). On the other hand, in intestine of grass carp, the potential pathogenic Vibrio was reported to be positively correlated to plasma glucose levels (Pan et al. 2019). Interestingly, potential pathogenic species in the genus Vibrio were significantly correlated with plasma glucose level. Pathogenic Vibrio groups such as Vibrio scophthalmi, Vibrio splendidus, and Vibrio lentus dominated at an average level of 10.2%, 13.0%, 6.7%, respectively, in spring samples of wild fish (Fig. 1), and plasma glucose levels in these individuals were remarkably low and averaged 35.10 ± 1.55 mg/dl (Figs. 1, 7). On the other hand, in the spring samples of farmed fish, these pathogenic Vibrio groups accounted for less than 3%, and the plasma glucose level of these individuals were the highest at an average of 283.75 ± 37.75 mg/dl (Figs. 1, 7). These results indicate that the potential pathogenic Vibrio group in rockfish gut negatively correlated with plasma glucose levels. Pathogen-induced fish infection changes the biochemical composition of blood, which can affect the health of the host. This study will help to understand the interaction between microbiome hosts, and furthermore, develop the techniques for the production of healthy Korean rockfish.
4 Conclusion
In the present study, seasonal changes of intestinal microbiota of wild and farmed Korean rockfish and comparison between the diversity of wild and farmed fish samples were investigated through the amplicon sequencing method. The microbial diversity of both samples (wild and farmed) showed a relative simplicity and almost all reads were affiliated with the family Vibrionaceae in the class γ-Proteobacteria. However, composition of species and patterns of seasonal changes were clearly different between wild and farmed fish and the diversity was higher among the wild fish compared with the farmed fish. In the intestine of farmed fish, the P. phophoreum group, A. fisherii, and A. finisterrensis were hyper-dominant and the P. damselae group, one of the opportunistic pathogens, dominated in some samples from the summer season. On the other hand, in the wild fish samples, opportunistic pathogens such as A. salmonicida, V. parahaemolyticus, V. scophthalmi, Vibrio splendidus, and V. lentus were widespread not only in spring but also in fall and winter. In the spring samples the proportion of pathogenic Vibrio spp. showed a negative relationship with the plasma glucose levels of the host. In addition, the results of the shared species analysis of intestinal microbiome, feed microbiota, and seawater microbiota indicate that the intestinal microbial diversity of farmed fish was affected more by seawater than by feed. From these results, we can tentatively conclude that (1) the plasma glucose level of the host reflects the gut microbial community composition and (2) the composition of gut microbiota in Korean rockfish was very simple and affected more by seawater than feed for farmed fish in spring and winter seasons. From these results, the management process of the fish farm could benefit from the following suggestions: possible infection by pathogenic bacteria could be monitored via plasma glucose level analysis and the intestinal microbiota could be manipulated with the introduction of helpful microorganisms into seawater. These suggestions will be tested in the near future and it is hoped that they will contribute to the improvement of Korean rockfish aquaculture management techniques.
References
Aquilera E, Yany G, Romero J (2013) Cultivable intestinal microbiota of yellowtail juveniles (Seriola lalandi) in an aquaculture system. Lat Am J Aquat Res 41:395–403. https://doi.org/10.3856/vol41-issue3-fulltext-3
Banerjee G, Ray AK (2017) Bacterial symbiosis in the fish gut and its role in health and metabolism. Symbiosis 72:1–11
Baross J, Liston J (1970) Occurrence of Vibrio parahaemolyticus and related hemolytic vibrios in marine environments of Washington State. Appl Microbiol 20:179–186
Baticados M, Lavilla-Pitogo C, Cruz-Lacierda E, De La Pena L, Sunaz N (1990) Studies on the chemical control of luminous bacteria Vibrio harveyi and V. splendidus isolated from diseased Penaeus monodon larvae and rearing water. Dis Aquat Org 9:133–139. https://doi.org/10.3354/dao009133
Beaz-Hidalgo R, Doce A, Balboa S, Barja JL, Romalde JL (2010) Aliivibrio finisterrensis sp. nov., isolated from Manila clam, Ruditapes philippinarum and emended description of the genus Aliivibrio. Int J Syst Evol Microbiol 60:223–228. https://doi.org/10.1099/ijs.0.010710-0
Bowman JP (2006) The genus Psychrobacter. In: Dworkin M (ed) The prokaryotes: an evolving electronic resource for the microbiological community, 3rd edn. Springer, New York, pp 920–930
Choi H-S, Myoung J-I, Park M, Cho M-Y (2009) A Study on the summer mortality of Korean rockfish Sebastes schlegelii in Korea. J Fish Pathol 22:155–162 (in Korean)
Egerton S, Culloty S, Whooley J, Stanton C, Ross RP (2018) The gut microbiota of marine fish. Front Microbiol 9:873. https://doi.org/10.3389/fmicb.2018.00873
Egidius E, Andersen K, Clausen E, Raa J (1981) Cold-water vibriosis or “Hitra disease” in Norwegian salmonid farming. J Fish Dis 4:353–354. https://doi.org/10.1111/j.1365-2761.1981.tb01143.x
Egidius E, Wiik R, Andersen K, Hoff K, Hjeltnes B (1986) Vibrio salmonicida sp. nov., a new fish pathogen. Int J Syst Evol Microbiol 36:518–520. https://doi.org/10.1099/00207713-36-4-518
Farto R, Armada S, Montes M, Guisande J, Pérez M, Nieto T (2003) Vibrio lentus associated with diseased wild octopus (Octopus vulgaris). J Invertebr Pathol 83:149–156. https://doi.org/10.1016/s0022-2011(03)00067-3
Farto R, Armada S, Montes M, Perez M, Nieto T (2006) Presence of a lethal protease in the extracellular products of Vibrio splendidus–Vibrio lentus related strains. J Fish Dis 29:701–707. https://doi.org/10.1111/j.1365-2761.2006.00746.x
Gauthier G, Lafay B, Ruimy R, Breittmayer V, Nicolas J-L, Gauthier M, Christen R (1995) Small- subunit rRNA sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (Snieszko et al.) Janssen and Surgalla to the genus Photobacterium as Photobacterium damsela subsp. piscicida comb. nov. Int J Syst Evol Microbiol 45:139–144. https://doi.org/10.1099/00207713-45-1-139
Gómez GD, Balcázar JL (2008) A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol 52:145–154. https://doi.org/10.1111/j.1574-695X.2007.00343.x
Haygood MG (1993) Light organ symbioses in fishes. Crit Rev Microbiol 19:191–216. https://doi.org/10.3109/10408419309113529
Hyun D-W, Oh SJ, Kim M-S, Whon TW, Jung M-J, Shin N-R, Kim PS, Kim HS, Lee J-Y, Kang W, Bae JW (2015) Simplicispira piscis sp. nov., isolated from the gut of a Korean rockfish, Sebastes schlegelii. Int J Syst Evol Microbiol 65:4689–4694. https://doi.org/10.1099/ijsem.0.000635
Islam MJ, Slater MJ, Kunzmann A (2020) What metabolic, osmotic and molecular stress responses tell us about extreme ambient heatwave impacts in fish at low salinities: the case of European seabass Dicentrarchus Labrax. Sci Total Environ 749:141458. https://doi.org/10.1016/j.scitotenv.2020.141458
Iwama R, Ashida M (1986) Biosynthesis of prophenoloxidase in hemocytes of larval hemolymph of the silkworm, Bombyx mori. Insect Biochem 16:547–555. https://doi.org/10.1016/0020-1790(86)90032-6
Jiang Y, Liu X, Xu Y, Shi B, Wang B (2020) Microbiota characteristics in Sebastes schlegelii intestine in early life stages. J Oceanol Limnol 38:275–287. https://doi.org/10.1007/s00343-019-9011-2
Kang W, Kim PS, Hyun D-W, Lee J-Y, Kim HS, Oh SJ, Shin N-R, Bae J-W (2016) Comamonas piscis sp. nov., isolated from the intestine of a Korean rockfish, Sebastes schlegelii. Int J Syst Evol Microbiol 66:780–785. https://doi.org/10.1099/ijsem.0.000790
Kim B-T, Brown CL, Kim D-H (2019) Assessment on the vulnerability of Korean aquaculture to climate change. Mar Policy 99:111–122. https://doi.org/10.1016/j.marpol.2018.10.009
Lee MD, Kling JD, Araya R, Ceh J (2018) Jellyfish life stages shape associated microbial communities, while a core microbiome is maintained across all. Front Microbiol 9:1534. https://doi.org/10.3389/fmicb.2018.01534
Li J, Woo NYS (2003) Pathogenicity of vibrios in fish: An overview. J Ocean Univ Qingdao 2:117–128. https://doi.org/10.1007/s11802-003-0039-7
Llewellyn MS, Boutin S, Hoseinifar SH, Derome N (2014) Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front Microbiol 5:207. https://doi.org/10.3389/fmicb.2014.00207
Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5:169–172. https://doi.org/10.1038/ismej.2010.133
Matanza XM, Osorio CR (2018) Transcriptome changes in response to temperature in the fish pathogen Photobacterium damselae subsp. damselae: clues to understand the emergence of disease outbreaks at increased seawater temperatures. PLoS ONE 13:e0210118. https://doi.org/10.1371/journal.pone.0210118
Møyner K, Røed KH, Sevatdal S, Heum M (1993) Changes in non-specific immune parameters in Atlantic salmon, Salmo salar L., induced by Aeromonas salmonicida infection. Fish Shellf Immun 3:253–265. https://doi.org/10.1006/FSIM.1993.1025
Ntranos A, Casaccia P (2018) The microbiome–gut–behavior axis: crosstalk between the gut microbiome and oligodendrocytes modulates behavioral responses. Neurotherapeutics 15:31–35. https://doi.org/10.1007/s13311-017-0597-9
Pan H, Li Z, Xie J, Liu D, Wang H, Yu D, Zhang Q, Hu Z, Shi C (2019) Berberine influences blood glucose via modulating the gut microbiome in Grass Carp. Front Microbiol 10:1066. https://doi.org/10.3389/fmicb.2019.01066
Park K-D, Kang Y-J, Huh S-H, Kwak S-N, Kim H-W, Lee H-W (2007) Feeding ecology of Sebastes schlegelii in the Tongyeong marine ranching area. Korean J Fish Aquat Sci 40:308–314. https://doi.org/10.5657/kfas.2007.40.5.308(inKorean)
Qiao G, Lee DC, Woo SH, Li H, Xu D-H, Park SI (2012) Microbiological characteristics of Vibrio scophthalmi isolates from diseased olive flounder Paralichthys olivaceus. Fisheries Sci 78:853–863. https://doi.org/10.1007/s12562-012-0502-8
Ramírez C, Romero J (2017) Fine flounder (Paralichthys adspersus) microbiome showed important differences between wild and reared specimens. Front Microbiol 8:271. https://doi.org/10.3389/fmicb.2017.00271
Rivas AJ, Lemos ML, Osorio CR (2013) Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front Microbiol 4:283. https://doi.org/10.3389/fmicb.2013.00283
Romalde JL (2002) Photobacterium damselae subsp. piscicida: an integrated view of a bacterial fish pathogen. Int Microbiol 5:3–9. https://doi.org/10.1007/s10123-002-0051-6
Ruby EG, Urbanowski M, Campbell J, Dunn A, Faini M, Gunsalus R, Lostroh P, Lupp C, McCann J, Millikan D, Schaefer A, Stabb E, Stevens A, Visick K, Whistler C, Freenberg EP (2005) Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc Natl Acad Sci USA 102:3004–3009. https://doi.org/10.1073/pnas.0409900102
Sayes C, Leyton Y, Riquelme C (2018) Probiotic bacteria as an healthy alternative for fish aquaculture. In: Savic S (ed) Antibiotics use in animals. InTech Publishers, London, pp 115–132. https://doi.org/10.5772/intechopen.71206
Sonh MH, Park MA, Kim JW, Kim KW, Kang YJ, Park YB, Eo YY (2007) Standard manual of black rockfish culture. National Fisheries Research and Development Institute, Busan, p 184 (in Korean)
Soto D, Ross LG, Handisyde N, Bueno PB, Beveridge MCM, Dabbadie L, Aguilar-Manjarrez J, Cai J, Pongthanapanich T (2018) Climate change and aquaculture: vulnerability and adaptation options. In: Barange M, Bahri T, Beveridge MCM, Cochrane KL, Funge-Smith S, Poulain F (eds) Impacts of climate change on fisheries and aquaculture, synthesis of current knowledge, adaptation and mitigation options. FAO, Rome, pp 465–490
Sullam KE, Essinger SD, Lozupone CA, O’CONNOR MP, Rosen GL, Knight R, Kilham SS, Russell JA, (2012) Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol Ecol 21:3363–3378. https://doi.org/10.1111/j.1365-294X.2012.05552.x
Tak EJ, Kim HS, Lee J-Y, Kang W, Hyun D-W, Kim PS, Shin N-R, Bae J-W (2018) Tessaracoccus aquimaris sp. nov., isolated from the intestine of a Korean rockfish, Sebastes schlegelii, from a marine aquaculture pond. Int J Syst Evol Microbiol 68:1065–1072. https://doi.org/10.1099/ijsem.0.002626
Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M (2014) Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 9:e105592. https://doi.org/10.1371/journal.pone.0105592
Tinta T, Kogovšek T, Klun K, Malej A, Herndl GJ, Turk V (2019) Jellyfish-associated microbiome in the marine environment: exploring its biotechnological potential. Mar Drugs 17:94. https://doi.org/10.3390/md17020094
Walburn JW, Wemheuer B, Thomas T, Copeland E, O’Connor W, Booth M, Fielder S, Egan S (2018) Diet and diet-associated bacteria shape early microbiome development in Yellowtail Kingfish (Seriola lalandi). Microb Biotechnol 12:275–288. https://doi.org/10.1111/1751-7915.13323
Wang AR, Ran C, Ringø E, Zhou ZG (2018) Progress in fish gastrointestinal microbiota research. Rev Aquacult 10:626–640. https://doi.org/10.1111/raq.12191
Yoon S-H, Ha S-M, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67:1613–1617. https://doi.org/10.1099/ijsem.0.001755
Acknowledgements
This work was supported by the grants of KIOST In-house Program (PE99822), Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) through the Golden Seed Project (213008-05-4-SB420) of the Republic of Korea and MarineBiotics Project (20210469) funded by the Ministry of Ocean and Fisheris of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, J., Kang, M.J., Kim, Y.J. et al. Comparison of Intestine Microbiota Between Wild and Farmed Korean Rockfish, Sebastes schlegelii. Ocean Sci. J. 56, 297–306 (2021). https://doi.org/10.1007/s12601-021-00022-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12601-021-00022-2