Abstract
As planktonic ciliates have been recognized as important players in marine microbial food webs, relevant studies have been conducted in the western coastal waters of the Yellow Sea. However, little is known about ciliate distributions from the eastern coast of the Yellow Sea near the Korean Peninsula. A spring cruise in April 2019 was carried out to investigate vertical and horizontal distributions of ciliate plankton at 18 stations that form three zonal sections in the eastern area. Biological (picoplankton, nanophytoplankton, and mesozooplankton) and hydrological (water temperature and salinity) environments were also analyzed to understand relationships between the ciliate distributions and the environments. High abundance (ca. 2400 cells L−1) of a large ciliate species, Laboea strobila, was observed at the surface water of Stn. 37–5. Abundance peak of large (> 50 μm) ciliates coincided with the peak of mesozooplankton abundance. Spatial distribution of the large ciliates was associated with nanophytoplankton distribution while a smaller (20–50 μm) ciliate group was associated with picoplankton distribution. Therefore, the spring distribution of ciliate plankton in the southeastern coast of the Yellow Sea indicates that food size for the ciliates is one of the important factors controlling ciliate compositions as well as their abundances. Further investigations need to be carried out to understand seasonal differences of ciliate distributions in the eastern Yellow Sea.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nano- and picoplankton have been recognized as dominant contributors of phytoplankton biomass and productivity in the marine pelagic ecosystem (Malone 1980; Stockner 1988; Worden et al. 2004, 2015). Microzooplankton more than mesozooplankton grazers efficiently preys upon nano- and picoplankton. Planktonic ciliates, a major component of microzooplankton, have been focused on understanding marine planktonic food webs, acting as top-down regulators of pico- and nanoplankton and as a food source for mesozooplankton. Thus, the ciliates have been targeted by many ecological studies for decades in coastal and pelagic areas, and reviewed in studies related to seasonal distributions and population dynamics (Pierce and Turner 1992).
The Yellow Sea, a shallow semi-enclosed marginal sea of the western Pacific, is located between the Korean Peninsula and the Chinese continent and possesses typical characteristics of Large Marine Ecosystems. High productivity and trophically linked species in the Yellow Sea support rich biological resources and fishing grounds (Zhang et al. 2019). As a planktonic ecosystem in marine food webs forms the basis for fisheries productivity, a lot of studies on phytoplankton and zooplankton have been carried out in the Yellow Sea (Kang and Kim 2008; Kang et al. 2007; Shi et al. 2020; Wang et al. 2019). Higher contribution, ca. 53%, of small phytoplankton (< 2 μm) to total chlorophyll a was recorded in the Yellow Sea in summer (Jang et al. 2018). A change from microplankton to pico- and nanoplankton dominance was also reported in the surface central southern Yellow Sea, which is considered to be due to the existence of the Yellow Sea Cold Water Mass (Sun et al. 2019). As ciliate plankton are a major consumer of pico- and nanoplankton, it is important to assess ciliate ecology to understand microbial food webs in the Yellow Sea. Distribution of ciliate plankton has been mainly surveyed in the western Yellow Sea where it is bounded by Chinese coastal waters (Chen et al. 2018; Yu et al. 2014; Zhang et al. 2008, 2009, 2018; Zhao et al. 2018). Seasonal variations of ciliate distributions have been well reported on using data collected from the western Yellow Sea. Unfortunately, there is no information about ciliate distributions in the eastern Yellow Sea of the Korean EEZ.
Based on a serial survey of seasonal distributions of ciliate plankton, the spatial distribution of the ciliates was investigated in the southeastern Yellow Sea in spring of 2019, and the data and results are discussed in relation to previous information of ciliate plankton from the southwestern Yellow Sea.
2 Materials and Methods
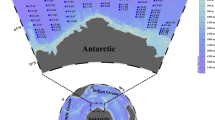
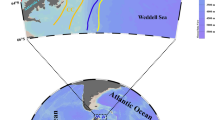
A cruise was conducted in spring (19–26 April) of 2019 in the southeastern Yellow Sea on board R/V “Onnuri”. Sampling for ciliate plankton and associated relevant environmental factors was undertaken at 18 stations that form three zonal sections, Line 35, 36 and 37 (Fig. 1).
Water temperature and salinity were measured using the CTD profiler (SBE 911, Seabird) attached on a rosette sampler. Seawater was collected at several depths from each station. Sample depths (generally 3–7 depths) were chosen depending on the vertical structures of water properties including the subsurface chlorophyll maximum (SCM) layer estimated by CTD down casting.
Two liters of seawater for ciliate samples was fixed with neutral Lugol solution at a final concentration of 2%. Depending on cell density, 5–50 ml aliquots of fixed samples were concentrated by overnight sedimentation. Tintinnid ciliates were identified based on lorica morphology (Alder 1999; Kim and Choi 2016; Kofoid and Campbell 1929). Naked ciliates were divided into four size categories according to cell length (> 20, 20–50, 50–100, and > 100 μm). Ciliate cells were counted using a Sedgwick-Rafter counting chamber at 200 × magnification using a Zeiss Axioscope 2.
For naked ciliates, their cell length and width were measured as similar geometrical shapes (cylinder, sphere, and cone) to obtain the cell volume. For tintinnid ciliates, the lorica volume of the cell was calculated by measuring cell dimensions. Carbon biomass of naked ciliates was calculated based on cell volume with a conversion factor of 0.19 pg μm−3 (Putt and Stoecker 1989). Carbon biomass of tintinnid cells was calculated by the equation: carbon (pg) = 444.5 + 0.053 lorica volume (μm3), formulated by Verity and Langdon (Verity and Langdon 1984).
Water samples for determining abundances of small phytoplankton were preserved in a mixture of paraformaldehyde and glutaraldehyde at final concentrations of 1 and 0.05%, respectively and frozen at −70 °C (Marie et al. 1996). Autotrophic picoeukaryotes and nanophytoplankton were enumerated using flow cytometry after filtering through a 35 μm cell strainer (Falcon). Heterotrophic and autotrophic bacteria (Synechococcus) were counted respectively using flow cytometry after staining with SYBR Green I (Sigma-Aldrich, St. Louis, MO, USA) (Marie et al. 1997).
Mesozooplankton was sampled by vertically towing a conical net (200 μm mesh, 60 cm mouth diameter) equipped with a digital flowmeter (Model 438-110, Hydro-bios, Germany) from near-bottom to surface. The samples were immediately preserved in buffered formalin at 4% final concentration. The mesozooplankton specimens were identified to species or genus level using a stereomicroscope (Stemi 2000-C, Zeiss, Germany).
Principal component analysis was used to analyze relationships among water temperature, salinity, and biological parameters of ciliate-sized groups and possible prey items (heterotrophic and autotrophic bacteria, picoeukaryotes, and nanophytoplankton). Statistical computation was performed using XLSTAT 2010 (AddinSoft™).
3 Results
3.1 Vertical and Horizontal Distribution of Ciliate Plankton
Ciliate plankton was mainly distributed above 20 m depth of an upper water column (Fig. 2). Higher abundances (> 4000 cells L−1) of ciliate plankton were observed at Stns. 35–5, 35–7 and 35–13 in Line 35, Stns. 36–1, 36–2 and 36–7 in Line 36, and Stn. 37–5 in Line 37. Vertical distribution of ciliate abundances in Line 37 was well reflected in vertical changes of carbon biomass. However, there were considerable differences in the vertical distribution between cell abundances and carbon biomass of the ciliates at Stn. 35–13 in Line 35 and Stn. 36–5 in Line 36.
Cell size distributions of ciliate plankton showed that a smaller sized (< 20 μm) group of naked ciliates was abundant at Stn. 35–13 (Fig. 3a). In contrast, size compositions of ciliate cells at Stn. 37–5 where the abundances of ciliate plankton were well reflected in the biomass distribution indicated that larger naked ciliates were relatively abundant in the upper layer (Fig. 3b). Laboea strobila was a dominant species among the larger ciliates over 100 μm.
Horizontal distributions of ciliate abundance and carbon biomass integrated from the surface to subsurface chlorophyll maximum (SCM) layer showed a different pattern. A patchy distribution of ciliate abundances was found at Stn. 35–13 where the smaller cells were abundant (Figs. 3a and 4a) while higher carbon biomass of ciliates appeared at Stn 37–5 and 37–6 where the larger species, L. strobila, was dominantly distributed (Figs. 3b and 4b).
3.2 Hydrological Environments on Ciliate Distribution
The vertical profile of water temperature showed a rapid decrease in the upper depth (< 20 m) at most sampling stations except at five stations (37–2, 36–1, 36–2, 35–1, and 35–3) which were located close to the western coast of the Korean Peninsula (Fig. 5). The thermocline was relatively well developed at 10–25 m depth. There was no clear difference in the vertical changes of salinity and it tended to increase further from the coast.
It is noticeable that the temperature displayed a considerable drop, 11.3–6.7 °C, between the surface and 25 m depth at the northern hot pot (Stn. 37–5) of ciliate distribution. A relatively high temperature of ca. 12 °C was recorded at the upper layer (surface to 10 m depth) of Stn. 35–13 where the small ciliates were abundant.
3.3 Biological Environments on Ciliate Distribution
The highest biomass of ciliate plankton was recorded at Stn. 37–5 in Line 37 (Fig. 6a). Among the size categories of ciliate cells, the larger ciliates over 50 μm were abundant in ca. 60% of total carbon biomass at the hot spot. The maximum abundance of mesozooplankton also occurred at the same site, Stn. 37–5. A copepod species, Acatia hongi, dominated at the site (Fig. 6b).
Principal component analysis of ciliate plankton and environmental variables showed close relationships among the ciliate-sized groups and the biological environments. In detail, the larger ciliates over 50 μm were closely related with nanophytoplankton distribution while the smaller ciliate group (20–50 μm) was plotted in closer distribution with picoplankton groups of heterotrophic and autotrophic bacteria as well as autotrophic picoeukaryotes (Fig. 7).
Principle component analyses of naked ciliate-sized groups (NC < 20, NC 20–50, NC 50–100, and NC > 100), possible items [heterotrophic bacteria (HB), autotrophic bacteria (AB), autotrophic picoeukaryotes (APE), nanoplankton (NP)] for prey, and hydrological factors [water temperature (WT), salinity (SAL)]
4 Discussion
4.1 Size Distribution of Ciliate Plankton
Different size species of ciliate assemblages feed on different size categories of prey. Therefore, information regarding cell size distribution in ciliate communities is important to understand the amount of food available to them under certain circumstances. Partitioning of pico- and nanoplankton consumed by marine ciliates was estimated (Rassoulzadegan et al. 1988). Ciliates smaller than 30 μm fed on 72% picoplankton and 28% nanoplankton whereas for 30–50 μm ciliates, the proportions were reversed—30% picoplankton and 70% nanoplankton. Larger ciliates (> 50 μm) consumed nanoplankton almost exclusively—at a level of 95%. Subsequently, based on the ciliate size-dependent consumption, total ciliate production was estimated in the coastal N–W Mediterranean.
Ciliate community, a major component of microzooplankton, was suggested as an important factor to regulate picoplankton distribution in the East China Sea and in northern Yellow Sea (Guo et al. 2014; Yang et al. 2020; Zhao et al. 2018). Cell abundances of three functional groups in picoplankton, heterotrophic bacteria, autotrophic bacteria (Synechococcus), and autotrophic picoeukaryotes, were separately analyzed in this study. These picoplankton groups were associated with 20–50-μm-sized ciliates (Fig. 7). Especially, a very close relationship was shown between autotrophic picoeukaryotes and 20–50-μm-sized ciliates. The result suggests that the picoeukaryotes can serve as an effective food source for the small ciliates that occur in spring in the southeastern Yellow Sea. It has been generally understood that nano sized (≤ 20 μm) planktonic ciliates ingest bacterioplankton (Pierce and Turner 1992). Although there were no significant relationships in spatial distributions between the nanociliates and the bacterioplankton in the spring study, bacterioplankton potential as an available food source for the nanociliates should be carefully reassessed through further seasonal studies in the southeastern Yellow Sea.
Picoeukaryotes contribute an important fraction to primary production in the photic ocean seawaters (Worden et al. 2015). It seems likely that protist grazers like ciliates and dinoflagellates serve more generally as consumers of picoeukaryotic phytoplankton in surface ocean environments (Landry et al. 2011; Pasulka et al. 2015). Especially, the mixotrophic Strombidium species of naked ciliates prefer picoeukaryotic prey like Micromonas pusilla (Orsi et al. 2018). 20–50-μm-sized ciliates with a tail, which was a taxonomical group in the former genus Tontonia, were abundant in this study area of the Yellow Sea. Considering most species among tailed ciliates are mixotrophic (Pierce and Turner 1992), the result suggests that a close relationship between the ciliates in the size class of 20–50 μm and the picoeukaryotes seems reasonable in terms of a prey and predator coupling. It was reported that community patterns and temporal variation of picoeukaryotes differ in response to changes in the Yellow Sea Warm Current (Xu et al. 2017). Therefore, further analyses need to determine distribution dynamics among ciliates, picoeukatyotes, and warm currents in the southeastern Yellow Sea.
In the southeastern Yellow Sea, spatial distributions of larger ciliate groups (50–100 and > 100 μm) and nanophytoplankton abundances were significantly correlated. High consumption of nanophytoplankton by ciliate plankton may be a function of the oral diameter of the ciliate species. Optimal food particles of three naked ciliates, Strombidium vestinum, S. reticulatum, and Lohmaniella spiralis, with a cell size range of 20–70 μm (ESD) varied from 2 to 10 μm in the food particle size (Jonsson 1986). A linear relationship between cell volume of planktonic ciliates and their natural food sizes was demonstrated by Bernard and Rassoulzadegan (Bernard and Rassoulzadegan 1990). Therefore, it is naturally accepted that the distribution of larger ciliate abundances in this study was more closely related to changes in nanophytoplankton distribution than the distribution of the smaller prey of three picoplankton groups.
4.2 Ecological Significance of Laboea strobila Bloom
Approximately 42% of planktonic ciliates have chloroplasts in surface waters during spring and summer (Stoecker et al. 1987). A larger oligotrich species among the plastidic ciliates, Laboea strobila, is well known as an obligate mixotroph and accounts for 46% of the biomass of plastidic ciliates (Stoecker et al. 1988). Its distribution has been reported around the world in temperate coastal waters (Dolan 1991; Dolan and Marrasé 1995; Modigh 2001). Over 600 cells L−1 of L. strobila was observed in the temperate coastal Long Island Sound USA (McManus and Fuhrman 1986). The dominant distribution of L. strobila was also reported in the Yellow Sea. Maximum abundance of L. strobila was recorded in the range of 640 and 10,000 cells L−1 in April, 2000 and in June, 2006 in the southwestern Yellow Sea, respectively (Zhang et al. 2002, 2018). In this study of April in 2019, high abundance (ca. 2400 cells L−1) of L. strobila in the larger size (> 100 μm) ciliates was observed at the surface water of Stn. 37–5. Therefore, it is assumed that L. strobila in the Yellow Sea displays a clear seasonality in terms of its population growth in spring.
Chlorophyll a concentration of L. strobila had an average value of 82 pg cell−1 (Putt 1990). Seven percent of total chlorophyll concentration was contributed by L. strobila cells when the abundance peaked in spring from Chinese coastal waters of the southwestern Yellow Sea (Zhang et al. 2018). If the cellular concentration was used to estimate the chlorophyll content of the plastidic ciliate, L. strobila, the chlorophyll concentration of 0.2 μg L−1 could have been contributed when the bloom of L. strobila occurred at the surface of Stn. 37–5. This value of maximum contribution was 8% of total chlorophyll a (2.5 μg L−1, data from personal communication with D.H. Choi) and is a similar value compared to the chlorophyll contribution in the Chinese coastal waters of the Yellow Sea. Laboea strobila has photosynthetically functional chloroplasts and its photosynthesis can make an important contribution to the carbon budget (Stoecker et al. 1988). It was concluded that L. strobila may have higher trophic efficiencies than heterotrophic ones because respiratory and excretory requirements are augmented by photosynthesis (Putt 1990). Therefore, the trophic position of ciliates in planktonic food webs needs to be reconsidered in relation to mixotrophic ciliates in the Yellow Sea. Further studies on ciliate plankton should be expanded using technical approaches for understanding major trophic pathways that are particular to the Yellow Sea.
4.3 Planktonic Ecosystem in the Yellow Sea in Spring
Ciliate plankton play important roles as alternative food sources for copepods and first-feeding fish larvae (Pierce and Turner 1992). Relatively large ciliate, such as L. strobila, can be efficiently preyed upon by a variety of mesozooplankton. A study in the Yellow Sea in spring was carried out to learn about the spatial distribution of ciliates as a prey source for higher trophic levels like mesozooplankton and fish larvae (Zhang et al. 2002). The abundance peaks of L. strobila and available predators like anchovy larvae coincided in that study. Similarly, in the present study, higher abundances of the large (> 50 μm) ciliates and the mesozooplankton were detected at the same place (Fig. 6). It is assumed that the concurrence of abundance in both communities of ciliates and mesozooplankton can be understood on the basis of a prey-predator relationship in the planktonic ecosystem of the Yellow Sea in spring. Acartia hongi, a dominant species of mesozooplankton at the peak site, was reported as an effective grazer on ciliates rather than phytoplankton in Gyeonggi Bay of the Yellow Sea (Yang et al. 2010). Therefore, the bottom-up control of ciliate plankton, especially large naked ciliates, is effective for mesozooplankton distribution in spring of the bay. Zooplankton distribution in autumn was reported from the same study area in this study (Kim and Kang 2019). Compared to the results of this study in spring, the dominant species of zooplankton was clearly different in fall. There was no significant relationship between the abundance of dominant species and chlorophyll concentration. This indicates that other alternative food items like ciliates may be considered. Further studies need to be conducted to understand seasonal differences in the ecological roles that ciliate plankton play in the Yellow Sea.
Ciliate plankton can be also controlled by available food sources such as pico- and nanoplankton (Pierce and Turner 1992). Ciliates were distributed as the most major component of nano- and micro-zooplankton communities in Gyeonggi Bay of the Yellow Sea (Yang et al. 2008). Their size distribution was positively correlated with size-fractioned phytoplankton. In this work in the southeastern coastal waters of the Yellow Sea, the spatial distributions of prey communities and ciliates showed reasonable results from the viewpoint of trophic relationships. In detail, the distribution of picoeukaryotes and medium-sized (20–50 μm) ciliates displayed a positive relationship while a significant correlation was shown between the abundances of nanophytoplankton and the large (> 50 μm) ciliates. High consumptions of edible sized prey by ciliates can be understood based on the knowledge that optimal prey size can be determined by the oral size of ciliate cell (Pierce and Turner 1992; Spittler 1973). Therefore, the ciliate distribution in the Yellow Sea suggests that food size is one of the important factors controlling ciliate compositions as well as their abundances. Concentrations of pico- and nano-sized chlorophyll a accounted for 25 ± 16–52 ± 21% of the total chlorophyll a, 1.4 ± 1.2 µg L−1, respectively (data from personal communication with D.H. Choi). Accordingly, the feeding potential of the ciliate plankton on these small phytoplankton groups might be considerable in the southeastern Yellow Sea. To better understand top-down control of ciliate plankton, distributions of picoplankton and nanophytoplankton should be investigated in detail in subsequent works.
In conclusion, Laboea strobilia, which is a large mixotrophic species among naked ciliate plankton, can act as important prey source for mesozooplankton growth in spring of the Yellow Sea. Ciliate plankton can consume proper sized algal preys: nanophytoplankton for large-sized (> 50 μm) ciliates and picoplankton for medium-sized (20–50 μm) ciliates (Fig. 8).
References
Alder VA (1999) Tintinnoinea. In: Boltovskoy D (ed) South Atlantic zooplankton. Backhuys Publishers, Leiden, Netherlands, pp 321–384
Bernard C, Rassoulzadegan F (1990) Bacteria or microflagellates as a major food source for marine ciliates: possible implications for the microzooplankton. Mar Ecol-Prog Ser 64:147–155
Chen X, Li H, Zhao Y, Zhao L, Dong Y, Zhang W, Xiao T (2018) Distribution of different biogeographical tintinnids in Yellow Sea and Bohai Sea. J Ocean Univ China 17:371–384
Dolan JR (1991) Guilds of ciliate microzooplankton in the Chesapeake Bay. Estuar Coast Shelf S 33:137–152
Dolan JR, Marrasé C (1995) Planktonic ciliate distribution relative to a deep chlorophyll maximum: Catalan Sea, N.W. Mediterranean, June 1993. Deep-Sea Res Pt I 42:1965–1987
Guo C, Liu H, Zheng L, Song S, Chen B, Huang B (2014) Seasonal and spatial patterns of picophytoplankton growth, grazing and distribution in the East China Sea. Biogeosciences 11:1847–1862
Jang HK, Kang JJ, Lee JH, Kim M, Ahn SH, Jeong JY, Yun MS, Han IS, Lee SH (2018) Recent primary production and small phytoplankton contribution in the Yellow Sea during the summer in 2016. Ocean Sci J 53:509–519
Jonsson PR (1986) Particle size selection, feeding rates and growth dynamics of marine planktonic oligotrichous ciliates (Ciliophora: Oligotrichina). Mar Ecol-Prog Ser 33:265–277
Kang JH, Kim WS (2008) Spring dominant copepods and their distribution pattern in the Yellow Sea. Ocean Sci J 43:67–79
Kang JH, Kim WS, Jeong HJ, Shin K, Chang M (2007) Why did the copepod Calanus sinicus increase during the 1990s in the Yellow Sea? Mar Environ Res 63:82–90
Kim YO, Choi JM (2016) Tintinnids. In: Choi JK (ed) Protists of Korea. The Korean Society of Protistologists, vol 3, pp 2–68 (in Korean)
Kim G, Kang HK (2019) Mesozooplankton distribution in the southern Yellow Sea in autumn. Ocean Polar Res 41:251–263 (in Korean)
Kofoid CA, Campbell AS (1929) A conspectus of the marine and fresh-water ciliata belonging to the suborder tintinnoinea, with descriptions of new species principally from the Agassiz expedition to the eastern tropical Pacific 1904–1905. Univ Calif Pub Zool 34:1–403
Landry MR, Selph KE, Taylor AG, Décima M, Balch WM, Bidigare RR (2011) Phytoplankton growth, grazing and production balances in the HNLC equatorial Pacific. Deep Sea Res Pt II 58:524–535
Malone TC (1980) Size-fractionated primary productivity of marine phytoplankton. In: Falkowski PG (ed) Primary productivity in the sea. Plenum, New York, pp 301–319
Marie D, Vaulot D, Partensky F (1996) Application of the novel nucleic acid dyes YOYO-1, YO-PRO-1, and PicoGreen for flow cytometric analysis of marine prokaryotes. Appl Environ Microbiol 62:1649–1655
Marie D, Partensky F, Jacquet S, Vaulot D (1997) Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl Environ Microbiol 63:186–193
McManus GB, Fuhrman JA (1986) Photosynthetic pigments in the ciliate Laboea strobila from Long Island Sound, USA. J Plankton Res 8:317–327
Modigh M (2001) Seasonal variations of photosynthetic ciliates at a Mediterranean coastal site. Aquat Microb Ecol 23:163–175
Orsi WD, Wilken S, del Campo J, Heger T, James E, Richards TA, Keeling PJ, Worden AZ, Santoro AE (2018) Identifying protist consumers of photosynthetic picoeukaryotes in the surface ocean using stable isotope probing. Environ Microbiol 20:815–827
Pasulka AL, Samo TJ, Landry MR (2015) Grazer and viral impacts on microbial growth and mortality in the southern California current ecosystem. J Plankton Res 37:320–336
Pierce RW, Turner JT (1992) Ecology of planktonic ciliates in marine food webs. Rev Aquat Sci 6:139–181
Putt M (1990) Metabolism of photosynthate in the chloroplast-retaining ciliate Laboea strobila. Mar Ecol-Prog Ser 60:271–282
Putt M, Stoecker DK (1989) An experimentally determined carbon: volume ratio for marine “oligotrichous” ciliates from estuarine and coastal waters. Limnol Oceanogr 34:1097–1103
Rassoulzadegan F, Laval-Peuto M, Sheldon RW (1988) Partitioning of the food ration of marine ciliates between pico- and nanoplankton. Hydrobiologia 159:75–88
Shi Y, Niu M, Zuo T, Wang J, Luan Q, Sun J, Yuan W, Shan X, Pakhomov EA (2020) Inter-annual and seasonal variations in zooplankton community structure in the Yellow Sea with possible influence of climatic variability. Prog Oceanogr 185:102349
Spittler P (1973) Feeding experiments with tintinnids. Oikos Suppl 15:128–132
Stockner JG (1988) Phototrophic picoplankton: an overview from marine and freshwater ecosystems. Limnol Oceanogr 33:765–775
Stoecker DK, Michaels AE, Davis LH (1987) Large proportion of marine planktonic ciliates found to contain functional chloroplasts. Nature 326:790–792
Stoecker DK, Silver MW, Michaels AE, Davis LH (1988) Obligate mixotrophy in Laboea strobila, a ciliate which retains chloropalsts. Mar Biol 99:415–423
Sun X, Shen F, Brewin RJW, Liu D, Tang R (2019) Twenty-year variations in satellite-derived chlorophyll-a and phytoplankton size in the Bohai Sea and Yellow Sea. J Geophys Res-Oceans 124:8887–8912
Verity PG, Langdon C (1984) Relationships between lorica volume, carbon, nitrogen, and ATP content of tintinnids in Narragansett Bay. J Plankton Res 6:859–868
Wang X, Xu Q, Jiang M, Liu P, Wang Z (2019) Zooplankton distribution and influencing factors in the South Yellow Sea in spring. Mar Pollut Bull 146:145–154
Worden AZ, Nolan JK, Palenik B (2004) Assessing the dynamics and ecology of marine picophytoplankton: the importance of the eukaryotic component. Limnol Oceanogr 49:168–179
Worden AZ, Follows MJ, Giovannoni SJ, Wilken S, Zimmerman AE, Keeling PJ (2015) Rethinking the marine carbon cycle: factoring in the multifarious lifestyles of microbes. Science 347:1257594
Xu Z, Song X, Wang M, Liu Q, Jiang Y, Shao H, Liu H, Shi K, Yu Y (2017) Community patterns and temporal variation of picoeukaryotes in response to changes in the Yellow Sea Warm current. J Oceanogr 73:687–699
Yang EJ, Choi JK, Hyun JH (2008) Seasonal variation in the community and size structure of nano- and microzooplankton in Gyeonggi Bay, Yellow Sea. Estuar Coast Shelf S 77:320–330
Yang EJ, Ju SJ, Choi JK (2010) Feeding activity of the copepod Acartia hongi on phytoplankton and micro-zooplankton in Gyeonggi Bay, Yellow Sea. Estuar Coast Shelf S 88:292–301
Yang J, Huang S, Fan W, Warren A, Jiao N, Xu D (2020) Spatial distribution patterns of planktonic ciliate communities in the East China Sea: potential indicators of water masses. Mar Pollut Bull 156:111253
Yu Y, Zhang W, Zhang C, Zhou F, Zhao N, Xiao T (2014) Basin-scale variation in planktonic ciliate distribution: a detailed temporal and spatial study of the Yellow Sea. Mar Biol Res 10:641–654
Zhang W, Xu K, Wan R, Zhang G, Meng T, Xiao T, Wang R, Sun S, Choi JK (2002) Spatial distribution of ciliates, copepod nauplii and eggs, Engraulis japonicus post-larvae and microzooplankton herbivorous activity in the Yellow Sea, China. Aquat Microb Ecol 27:249–259
Zhang C, Zhang W, Xiao T, Lü R, Sun S, Song W (2008) Meso-scale spatial distribution of large tintinnids in early summer in southern Yellow Sea. Chin J Oceanol Limn 26:81–90
Zhang C, Zhang W, Xiao T, Lü R, Sun S, Song W (2009) Wintertime meso-scale horizontal distribution of large tintinnids in the southern Yellow Sea. Chin J Oceanol Limn 27:31–37
Zhang S, Li H, Chen X, Dong Y, Zhang F, Xiao T, Zhang W, Zhao Y (2018) Differences in planktonic ciliate spatial distribution in spring and autumn in the southern Yellow Sea. Acta Oceanol Sin 37:48–57
Zhang Z, Qu F, Wang S (2019) Sustainable development of the Yellow Sea large marine ecosystem. Deep-Sea Res Pt II 163:102–107
Zhao L, Zhao Y, Dong Y, Zhao Y, Zhang W, Xu J, Yu Y, Zhang G, Xiao T (2018) Influence of the northern Yellow Sea Cold Water Mass on picoplankton distribution around the Zhangzi Island, northern Yellow Sea. Acta OceanolSin 37:96–106
Acknowledgements
We are grateful to the scientists and crew members of R/V “Onnuri” in the Korea Institute of Ocean Science and Technology. This study was conducted by the support of the research project of KIOST (PE 99813). The authors appreciate the kind comments from anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, Y.O., Choi, J., Kang, HK. et al. Spring Distribution of Ciliate Plankton in the Southeastern Yellow Sea in 2019. Ocean Sci. J. 56, 69–77 (2021). https://doi.org/10.1007/s12601-021-00004-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12601-021-00004-4