Abstract
Coccinellids are important biological control agents and aphid predators in horticultural crops around the world. Neotropical Cycloneda sanguinea, Eriopis connexa and Coleomegilla quadrifasciata octodecimpustulata, and the exotic Harmonia axyridis (all Coleoptera: Coccinellidae), are predators that mainly feed on aphids. In this work we described the abundance of these coccinellid species and their spatial and temporal cooccurrence in agroecological eggplant crop of Argentina. We also estimate the intensity and symmetry of the intraguild predation between C. quadrifasciata octodecimpustulata and the other species in laboratory experiments. Both temporal and spatial segregation was observed. H. axyridis was the strongest intraguild predator in the interaction with C. quadrifasciata octodecimpustulata, and this latter was the strongest intraguild predator in interaction with C. sanguinea and E. connexa. In this context, native coccinellid species would be vulnerable to the exotic and invasive H. axyridis; however, the lack of cooccurrence of this species with the native ones, under conditions of low density of extra-guild prey would indicate that spatial segregation could be the mechanism by which these species coexist in the eggplant crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coccinellids are important aphid predators in horticultural crops around the world and are used in classical (Rondoni et al., 2021) and augmentative (Albajes et al., 2002) biological control programs. In the Neotropical region several species have deserved attention as potential biological control agents mainly in horticultural crops (Dode & Romero Sueldo, 2013; Dos Santos et al., 2020; Fonseca et al., 2017; Zalazar & Salvo, 2007).
The more frequent aphidophagous coccinellids in Argentinian horticultural crops are the natives Cycloneda sanguinea (L.), Eriopis connexa (Germar), and Coleomegilla quadrifasciata octodecimpustulata (Mulsant), and the exotic Harmonia axyridis (Pallas) (Del Pino et al., 2012; Rizzo, 2020). C. sanguinea, E. connexa and C. quadrifasciata octodecimpustulata have Neotropical distribution (González, 2014), and these species are present in several crops such as corn, sunflower, alfalfa, fruit trees, sweet pepper, coriander (Andorno et al., 2015; De la Fuente et al., 2006), recently in eggplant (pers. obs.), and weeds (Castresana & Paz, 2019; Engel et al., 2020; Saini, 2004). The first two coccinellid species can be considered good aphid control agents as they have high consumption rates on aphid species frequently found in horticultural crops (Rocca et al., 2017, 2021). C. quadrifasciata octodecimpustulata is a more generalist predator, feeding on immature and adult prey, including aphids, thrips, scale insects, psyllids, whiteflies, mites, and eggs and immature stages of coleopterans and lepidopterans (Lixa et al., 2010), and its potential as biological control agents have not been studied. On the other hand, H. axyridis, native to Asia, is widely distributed worldwide, and it has been introduced as a biological control agent or accidentally in many countries (Camacho-Cervantes et al., 2017; Roy et al., 2016). Due to several traits―larger size than other coccinellids, high predatory capacity, high fecundity and fertility, superior competitive ability in relation to other coccinellid species by exploitation and interference―H. axyridis became an invasive exotic species and has the potential to displace several native coccinellids (Brown & Roy, 2018; Roy et al., 2016). In South America, there are reports of such displacements of native species in Chile and Brazil (Grez et al., 2016; Martins et al., 2009). In Argentina this species was introduced in 1986 (García et al., 1999) and nowadays it extends from 24° to 43° S and from 71° 30´ to 54° W, having arrived in ten biogeographical regions (Wagner et al., 2017; Werenkraut et al., 2020). Although there are no recent records of relative abundances of H. axyridis in relation to other coccinellids in horticultural crops, Saini (2004) found, in pecan trees, relative percentages between 51 and 74% of H. axyridis in relation to Cycloneda sanguinea, Olla v-nigrum, Eriopis connexa, Coleomegilla quadrifasciata y Adalia bipunctata.
The temporal and spatial cooccurrence of different species of coccinellids creates opportunities for intraguild predation that could negatively affect their coexistence (Burgio et al., 2002; Michaud, 2002; Schellhorn & Andow, 1999). This interaction is usually bidirectional and asymmetric (Michaud & Grant, 2003; Ware & Majerus, 2007), and its outcome is determined by several factors such as specific traits associated with particular species―e.g., physical and chemical defenses―, developmental stages, relative body size, mobility, behavior, and encounter rates of the individuals who participate in the interaction (Lucas, 2012; Lucas et al., 1998). In turn, eggs, small larvae, pupae, and molting stages are especially susceptible to intraguild predation (Hodek & Evans, 2012). It is known, for example, that H. axyridis exerts a strong asymmetric intraguild predation on eggs and larvae of C. sanguinea (Michaud, 2002) and E. connexa (Mirande et al., 2015), and Rocca et al. (2017, 2019) found asymmetric intraguild predation between E. connexa and C. sanguinea, the former being the strongest intraguild predator on eggs and larvae of C. sanguinea. However, the interaction between C. quadrifasciata octodecimpustulata and the other three species is unknown. The knowledge of the cooccurrence of C. sanguinea, E. connexa, C. quadrifasciata octodecimpustulata, and H. axyridis in horticultural crops, as well as the intraguild predation between them, is relevant to develop efficient biological control programs involving native biological control agents in the presence of exotic species.
We studied the spatial and temporal cooccurrence of these coccinellid species in eggplant crop, Solanum melongena L. (Solanaceae), which is economically important in many countries of the world and in Argentina (CHBA, 2005). This crop, like many other horticultural crops, is affected by the aphids Aphis gossypii Glover and Myzus persicae (Sulzer) (Del Pino et al., 2012; Rizzo, 2020), that extract the sap from the plants and reduce their photosynthetic capacity, deform the tender shoots, produce honeydew leading to the development of sooty mold, and most important, they are vectors of viruses (Srinivasan, 2009). The aims of this work were: 1) to describe the abundance of H. axyridis, C. sanguinea, E. connexa, and C. quadrifasciata octodecimpustulata, and the spatial and temporal cooccurrence of these species in the eggplant crop, and 2) to estimate, in the laboratory, the intensity and symmetry of the IGP between C. quadrifasciata octodecimpustulata and the other coccinellid species. Based on the aforementioned background, we predict that H. axyridis will have greater abundances in relation to the other coccinellid species, and in interaction with C. quadrifasciata octodecimpustulata it will be the strongest intraguild predator.
Methodology
Field sampling
Biweekly samplings were carried out in four eggplant crops (250 m2 each) located in agroecological farms of the Horticultural Belt of La Plata (34º 8’ S, 57º 54’ W), Buenos Aires, Argentina, during two production cycles from November to May (2015–2016 and 2016–2017).
On each sampling date, the phenological stage of the crop was recorded: vegetative (V), flowering (F), flowering and fruiting (FF), fruiting (FR) and postharvest (P). The sample unit consisted of a plant that was visually divided into upper, middle, and lower stratum, all of them of equal size. On each sampling date, a visual inspection of 30 plants randomly selected was carried out, and the frequency of each coccinellid species (larvae and adults) by plant stratum was recorded. The relative abundance of the coccinellid species in each phenological stage (number of individuals of one species / total number of individuals in a certain phenological stage × 100), and each plant stratum (number of individuals of one species / total number of individuals in a given stratum × 100), was calculated. In turn, on each sampling date, 30 leaves per stratum were taken at random from different plants (n = 90 from each plant) and the number of aphids was recorded. The mean number of aphids in each phenological stage was estimated taking into account the sample units of all sampling dates corresponding to the same phenological stage and all sites. The mean number of aphids per stratum was estimated in the same way. Kruskal–Wallis test was used to compare, separately, the mean number of aphids among vegetative stages and strata.
The cooccurrence of the coccinellid species were determined at spatial (plant) and temporal (phenological stage) level using the co-occur package (Griffith et al., 2016) with R software (version 3.5. 1). The probabilistic model uses combinatorics to determine the probability that the observed frequency of cooccurrence is significantly equal to that expected (random association α = 0.05), greater than expected: P (gt) ≤ 0.05 (positive association), or significantly lower than expected: P (lt) ≤ 0.05 (negative association) of a pair of species (Veech, 2014).
For the cooccurrence analysis at the plant scale, all sample units were considered, from all sampling dates and sites, in which at least one of the coccinellid species was present. Regarding the temporal analysis, all sample units were considered, from all dates and sites, of the same phenological stage in which at least one of the species was present.
Laboratory assays
The insect rearing and all laboratory experiments were carried out under controlled environmental conditions (25 ± 2ºC, 70 ± 5% HR and 16:8 L:D). Coccinellids (H. axyridis, C. sanguinea, E. connexa, and C. quadrifasciata octodecimpustulata) were collected from agroecological eggplant crops in La Plata, Argentina (34°56′04″S 58°10′14″W). The progeny of each species was used to start the laboratory colonies. The adults and larvae were reared in separate plastic cages (15 cm high × 15 cm long × 25 cm wide) containing water provided on a sponge inserted into an Eppendorf tube, Triticum aestivum L. seedlings infested with Rhopalosiphum padi (L.) (Hemiptera: Aphididae), a paper towel to provide refuge for larvae and oviposition substrate for females and covered with a fine mesh material. Seedlings were previously germinated in plastic pots (6 cm high and 4 cm diameter) with standard substrate (fertile soil and perlite 1:1 v/v) and infested with R. padi at germination and maintained in ventilated plastic boxes (13 cm high × 30 cm long × 23 cm wide) until more than 80% of each were infested with aphids. Twice a week, new seedlings with aphids were added and the paper towel with eggs from the adult cages was transferred to a new plastic box until the larvae hatched. Individuals were randomly selected from the colonies and starved for 24 h prior to the experiments.
The experimental unit consisted of plastic cylinders (6 cm diameter × 5 cm high), containing an eggplant leaf embedded in water agar (1%), sealed with voile. On the leaf, 5 M. persicae adults were placed as extra-guild prey, simulating a situation of scarcity of prey.
As was mentioned, H. axyridis-C. sanguinea, H. axyridis-E. connexa and C. sanguinea-E. connexa interactions are already known, so in this study we tested C. quadrifasciata octodecimpustulata-H. axyridis, C. quadrifasciata octodecimpustulata-C. sanguinea, C. quadrifasciata octodecimpustulata-E. connexa interactions.
IGP by adults and larvae on eggs
One adult reproductive female coccinellid of 2–3 weeks old of each species was individually isolated in the experimental units with a heterospecific egg cluster (20–30 eggs each). Similarly, one second instar larva (L2) or one fourth instar larva (L4) of each coccinellid species were confined with a heterospecific egg cluster. After 24 h, the number of eggs consumed was recorded. The number of predated eggs was analyzed as a binary response variable (consumption/non-consumption) using a Logistic Generalized Linear Model (GLM) with a binomial distribution error, with the species being the predictor factor. Odds ratios (OR) were estimated to quantify the probability of one event relative to another, such as eβ being β the model estimator (Agresti, 2015). The odd is defined as the probability that an event occurs divided by the probability it does not. The OR of the variable "species'', for example, for the combination of C. quadrifasciata octodecimpustulata and C. sanguinea, is the ratio of the probabilities of consumption (or non-consumption) of C. quadrifasciata octodecimpustulata divided by the probabilities of consumption (or non-consumption) of C. sanguinea. The analyses were carried out with the statistical package lmer of the R software (R Core Team, 2018 version 3.5.1).
IGP by adults on larvae
Synchronous cohorts of neonate larvae of each species were reared until they reached the required instars (L2 and L4). Adult females in the reproductive age of 2–3 weeks old of each coccinellid species were then isolated in the experimental unit with either one L2 heterospecific larva or one L4 heterospecific larva for 24 h, whereupon the frequency of larval mortality was tallied. The frequency of replicates in which IGP occurred was compared between treatments, using Chi-square contingency tests.
IGP between larvae
One L4 of each coccinellid species was isolated in the experimental unit with either one L2 or one L4 heterospecific larva for 24 h. After 24 h, the predation events were recorded and used to estimate the intensity and symmetry of IGP. The symmetry index was calculated as the number of replicates in which a given predator was eaten divided by the total number of replicates in which IGP occurred. This value was compared with an expected value of 50% (symmetric interaction) with the Chi-square goodness-of-fit test for each combination of species.
Results
Field sampling
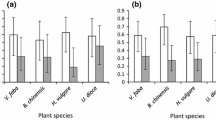
Harmonia axyridis was present throughout the entire crop cycle but it was the most abundant species in the FF stage of the crop. E. connexa and C. sanguinea also were present throughout the entire crop cycle, and they had the highest relative abundance at the beginning and end of the crop cycle, respectively. C. quadrifasciata octodecimpustulata appeared only in vegetative and flowering stages (Fig. 1a), its relative abundance was low and coincided with the highest relative abundance of E. connexa. In general, taking into account all data, the most abundant species was H. axyridis (40.6%), followed by C. sanguinea (37.7%), E. connexa (20.6%), and C. quadrifasciata octodecimpustulata (1.1%).
Relative abundance of coccinellids (bars), mean number of aphids per leaf (± SE) (line), and sample size (n), by phenological stages a) and by stratum b), in the eggplant crop during two production cycles from 2015 to 2017. Vegetative (V), flowering (F), flowering and fruiting (FF), fruiting (FR), postharvest (P), lower (L), middle (M), and upper (U)
In relation to the distribution of coccinellids in the plant strata, E. connexa was the most abundant in the lower stratum, H. axyridis and C. sanguinea were present mainly in the upper stratum, and C. quadrifasciata octodecimpustulata was only observed in the lower stratum coinciding also with the highest abundance of E. connexa (Fig. 1b). The number of aphids was higher in the vegetative (V), fruiting (FR) and postharvest (P) phenological stages (H(4, n=4323) = 83.82; P < 0.001; Fig. 1a) and in the lower stratum (H(2, n=4323) = 9.54; P = 0.008; Fig. 1b). Data of all farms and years are given in Tables S1 and S2.
Spatially, at the plant scale, all the coccinellid combinations showed negative association (P(lt) < 0.001) except those that included C. quadrifasciata octodecimpustulata which was not associated with any other species (Table S3). The probability of temporal cooccurrence (at the phenological stage scale) of the different pairs of species was, in general, low (< 25%). Eriopis connexa and C. sanguinea were segregated (P(lt) < 0.001), except in FR and P (P(lt) > 0.05), and H. axyridis showed both negative associations with E. connexa (FF: P(lt) < 0.001 and FR: P(lt) < 0.001) and C. sanguinea (FF: P(lt) < 0.001; P: P(lt) < 0.001), as well as random associations with these both species (E connexa: F: P(lt) = 0.19; C. sanguinea: F: P(lt) = 0.42; FR: P(lt) = 0.88). The probability of cooccurrence of C. quadrifasciata octodecimpustulata with E. connexa was random during the first phenological stages (V: P(lt) = 0.67; F: P(lt) = 0.58), while no associations were registered with the other coccinellid species (Table S3).
Laboratory assays
IGP by adults and larvae on eggs
In all combinations tested, adults and larvae predated heterospecific eggs. In general, the intensity of IGP was significantly different between species (Fig. 2; Table S4). Adults and larvae of C. quadrifasciata octodecimpustulata were stronger intraguild predators on eggs of C. sanguinea and E. connexa than vice versa. Similarly, the larvae of C. quadrifasciata octodecimpustulata were stronger intraguild predators on H. axyridis eggs than vice versa, although the predation of adults on eggs was symmetrical between both species (Fig. 2).
Intensity (frequency of replicates in which IGP occurred) of IGP by adults (Ad), second (L2) and fourth (L4) larval instar of Coleomegilla quadrifasciata octodecimpustulata (Cq), Cycloneda sanguinea (Cs), Eriopis connexa (Ec) and Harmonia axyridis (Ha) on heterospecific eggs. *P < 0.05, **P < 0.01, ***P < 0.001, and ns: non-significant difference
The proportion of eggs predated by adults was generally high and significantly different between species. C. quadrifasciata octodecimpustulata preyed a similar proportion of eggs of C. sanguinea and E. connexa and, in turn, these values were higher than vice versa; however, in combination with H. axyridis this trend was reversed (Table 1). Similarly, both L2 and L4 of C. quadrifasciata octodecimpustulata consumed a similar proportion of C. sanguinea and E. connexa eggs, and these were higher than vice versa (Table 2).
IGP by adults on larvae
Adults of all coccinellid species preyed on heterospecific larvae. The intensity of the predation of C. quadrifasciata octodecimpustulata was greater on L2 and L4 of C. sanguinea and E. connexa, than vice versa; whereas H. axyridis was the strongest intraguild predator on C. quadrifasciata octodecimpustulata (Fig. 3; Table S5).
Intensity of IGP (frequency of replicates in which IGP occurred) by adults of Coleomegilla quadrifasciata octodecimpustulata (Cq), Cycloneda sanguinea (Cs), Eriopis connexa (Ec) and Harmonia axyridis (Ha) on second (L2) and fourth instar (L4) heterospecific larvae. *P < 0.05, **P < 0.01, ***P < 0.001
IGP between larvae
The IGP between L4 of C. quadrifasciata octodecimpustulata and L4 of C. sanguinea and E. connexa, was bidirectional and asymmetric being C. quadrifasciata octodecimpustulata the strongest intraguild predator; however, the interaction was unidirectional between L4 of C. quadrifasciata octodecimpustulata and L4 of H. axyridis being this latter the intraguild predator. The IGP between different larval instar was unidirectional, with the largest larvae always preying on the smaller ones (Fig. 4; Table S6).
Symmetry index of IGP between heterospecific larvae of the same and different instar of Coleomegilla quadrifasciata octodecimpustulata (Cq), Cycloneda sanguinea (Cs), Eriopis connexa (Ec), Harmonia axyridis (Ha). L2: second instar larvae, L4: fourth instar larvae. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
At temporal scale, a tendency toward segregation of coccinellid species in eggplant was observed. E. connexa appeared in greater abundance in early stages of the crop, H. axyridis in the middle and C. sanguinea at the end of the crop cycle. The temporal abundance of E. connexa and C. sanguinea was similar to those found by Rocca et al. (2021) in sweet pepper. The low frequency and abundance of C. quadrifasciata octodecimpustulata in relation to the other coccinellid species, recorded in this study, agree with the results obtained by other authors in different crops and wild vegetation (de Souza et al., 2021; Engel et al., 2020; Lixa et al., 2010; Pimenta et al., 2020; Saini, 2004). As observed in other crops in Argentina (Saini, 2004) and other countries (Grez et al., 2016; Martins et al., 2009; Roy et al., 2016), H. axyridis was one of the most abundant species of the coccinellid assemblage, along with C. sanguinea.
At spatial scale, a very low probability of cooccurrence of the coccinellid species in eggplant crop was observed. The low abundance of aphids found could affect the coccinellids cooccurrence patterns (Prescott & Andow, 2018) because they are usually aggregated on large aphid colonies (Wright & Laing, 1980; Evans & Youssef, 1992; Elliott & Kieckhefer, 2000). The low prey density could, on the one hand, promote intraguild predation due to lack of extra-guild prey, but on the other hand, by not aggregating in patches of high prey density, the encounter rate would be reduced. The spatial segregation could also be a consequence of coccinellids recognizing heterospecific chemical tracks and avoiding laying eggs in sites with such tracks (Agarwala et al., 2003; Michaud & Jyoti, 2007; Růžička & Zemek, 2008), to reduce the probability of participating in IGP (Hindayana et al., 2001; Lucas et al., 1998; Yasuda et al., 2004). The highest number of random associations among coccinellid species found in the flowering stage, would be related to the availability of food resources, such as pollen and prey, in this phenological stage. For example, Musser and Shelton (2003) found that H. axyridis and C. maculata tended to temporarily segregate in corn crops, and the highest cooccurrence was observed in periods in which pollen availability was higher.
In the laboratory, H. axyridis was the strongest intraguild predator in interaction with C. quadrifasciata octodecimpustulata. The same was found in other IGP studies involving H. axyridis-C. sanguinea (Michaud, 2002), and H. axyridis-E. connexa (Ribeiro Pereira dos Santos et al., 2009; Mirande et al., 2015). Moreover, C. quadrifasciata octodecimpustulata in interaction with C. sanguinea and E. connexa was the strongest intraguild predator. It is known that both the eggs and the larvae of some coccinellid species contain alkaloids, toxic substances for other species, avoiding their predation (Daloze et al., 1995; Hautier et al., 2011; Hemptinne et al., 2000). The toxicity of alkaloids to predators and predator ability to tolerate the toxic effect vary greatly between species and individuals of the same species (Agarwala & Dixon, 1992; Cottrell, 2004). For example, larvae and adults of Adalia bipunctata L. were reluctant to eat eggs of Coccinella septempunctata L. (Agarwala & Dixon, 1992). Accordingly, the lower consumption of C. quadrifasciata octodecimpustulata and H. axyridis eggs, by C. sanguinea and E. connexa, could be due to the presence of some toxic compounds (Kajita et al. 2010; Rocca et al. unpublished data). In relation to larvae of the same instar, C. quadrifasciata octodecimpustulata was stronger intraguild predator against C. sanguinea and E. connexa than vice versa, probably due to the presence of deterrent or toxic substances and physical defenses—dorsal spines—of C. quadrifasciata octodecimpustulata (obs. pers.); however, in interaction with H. axyridis, the latter was the strongest intraguild predator. The larvae of H. axyridis are, in general, relatively larger, with physical and chemical defenses, and more aggressive than those of other coccinellids (Michaud, 2002; Yasuda et al., 2004; Kajita, 2010). For example, Anatis ocellata (L.) larvae were little attacked by H. axyridis due to their larger size and the presence of dorsal spines (Ware et al., 2008). Otherwise, in this study it was observed that larger instars preyed on the smallest, regardless of the species, as was recorded for C. septempunctata preying on Hippodamia variegata Goeze larvae (Agarwala & Dixon, 1992; Lucas, 2012).
In general, the intensity of the intraguild predation could be overestimated in experiments in small arenas by confinement in these conditions. Experiments in small arenas could show a high intensity of intraguild predation; however, in field conditions habitat structure would create refugia for intraguild prey, leading to a reduction of the encounters rate and therefore of the intraguild predation, allowing the coexistence of predators (Janssen et al., 2007). Nevertheless, this type of experiment in enclosure conditions provides basic knowledge about the intrinsic characteristics of each species in interaction with other species. Experimental results observed in small enclosures and over small-time frames may, in some cases, still scale up to predict field-wide patterns (Lucas & Rosenheim, 2011). The results obtained in our laboratory study suggest that C. quadrifasciata octodecimpustulata could exclude C. sanguinea and E. connexa from this system. Otherwise, H. axyridis is a strong intraguild predator on C. quadrifasciata octodecimpustulata, as well as on C. sanguinea (Michaud, 2002) and E. connexa (Mirande et al., 2015). In this context, native coccinellid species would be vulnerable to the exotic and invasive H. axyridis; however, the lack of cooccurrence of this species with the native ones, under conditions of low density of extra-guild prey (Agarwala & Dixon, 1992; Lucas et al., 2009; Rocca et al., 2017), would indicate that spatial segregation could be the mechanism by which these species coexist in the eggplant crop.
Data availability
We added supplementary material.
Abbreviations
- V:
-
Vegetative
- F:
-
Flowering
- FF:
-
Flowering and fruiting
- FR:
-
Fruiting
- P:
-
Postharvest
- L2:
-
Second instar
- L4:
-
Fourth instar
- OR:
-
Odds ratio
- IGP:
-
Intraguild predation
- Cq:
-
Coleomegilla quadrifasciata octodecimpustulata
- Cs:
-
Cycloneda sanguinea
- Ec:
-
Eriopis connexa
- Ha:
-
Harmonia axyridis
References
Agarwala, B. K., & Dixon, A. F. G. (1992). Laboratory study of cannibalism and interspecific predation in ladybirds. Ecological Entomology, 17, 303–309. https://doi.org/10.1111/j.1365-2311.1992.tb01062.x
Agarwala, B. K., Yasuda, H., & Kajita, Y. (2003). Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: Role of fecal cues in predator avoidance. Journal of Chemical Ecology, 29, 357–376. https://doi.org/10.1023/a:1022681928142
Agresti, A. (2015). Foundations of Linear and Generalized Linear Models. Wiley & Sons.
Albajes, R., Gullino, L. M., Van Lenteren, J. C., & Elad, Y. (2002). Integrated Pest and Disease Management in Greenhouse Crops. Kluwer Academic Publishers.
Andorno, A. V., Botto, E. N., La Rossa, F. R., Möhle, R. (2015). Control biológico de áfidos por métodos conservativos en cultivos hortícolas y aromáticas. Ediciones. INTA.
Burgio, G., Santi, F., & Maini, S. (2002). On intra-guild predation and cannibalism in Harmonia axyridis (Pallas) and Adalia bipunctata L. (Coleoptera: Coccinellidae). Biological Control, 24, 110–116. https://doi.org/10.1016/S1049-9644(02)00023-3
Brown, P. M., & Roy, H. E. (2018). Native ladybird decline caused by the invasive harlequin ladybird Harmonia axyridis: Evidence from a long-term field study. Insect Conservation and Diversity, 11, 230–239. https://doi.org/10.1111/icad.12266
Camacho-Cervantes, M., Ortega-Iturriaga, A., del-Val, E. (2017). From effective biocontrol agent to successful invader: the harlequin ladybird (Harmonia axyridis) as an example of good ideas that could go wrong. PeerJ, 5, e3296. https://doi.org/10.7717/peerj.3296.
Castresana, J., Paz, R. (2019). Manejo agroecológico del pulgón en cultivo de pimiento. EEA Concordia, Ediciones INTA. inta_concordia_manejo_agroecologico_del_pulgon_en_cultivo_de_pimiento.pdf. Accessed 15 Sept 2022.
CHBA. (2005). Censo Hortiflorícola de Buenos Aires. Buenos Aires: Dirección Provincial de Economía Rural del Ministerio de Asuntos Agrarios, Dirección Provincial de Estadísticas del Ministerio de Economía, Secretaría de Agricultura, Ganadería, Pesca y Alimentos de la Nación, Consejo Federal de Inversiones.
Cottrell, T. E. (2004). Suitability of exotic and native lady beetle eggs (Coleoptera: Coccinellidae) for development of lady beetle larvae. Biological Control, 31, 362–371. https://doi.org/10.1016/j.biocontrol.2004.06.004
Daloze, D., Braekman, J. C., Pasteels, J. M. (1995). Ladybird defence alkaloids: structural, chemotaxonomic and biosynthetic aspects (Col.: Coccinellidae) Chemoecology, 5, 173–183. https://doi.org/10.1007/BF01240602.
De La Fuente, E. B., Lenardis, A. E., Suárez, S. A., Gil, A., & Ghersa, C. M. (2006). Insect communities related to wheat and coriander cropping histories and essential oils in the Rolling Pampa, Argentina. European Journal of Agronomy, 24, 385–395.
De Souza, T. S., Aguiar-Menezes, E. D. L., Guerra, J. G. M., Fernandes, V. J., Pimenta, A. G., Dos Santos, C. A. A. (2021). Faunistic analysis and seasonal fluctuation of ladybeetles in an agro-ecological system installed for organic vegetable production. Bioscience Journal, 37, e37016. https://doi.org/10.14393/BJ-v37n0a2021-53540.
Del Pino, M., Massi, M., & Carpintero, D. (2012). Principales plagas y enemigos naturales del cultivo de berenjena bajo producción orgánica en invernadero en La Plata. Horticultura Argentina, 31, 24.
Dode, M., & Romero Sueldo, M. (2013). Coccinélidos (Coleoptera: Coccinellidae) asociados a Brassica rapa (Brassicaceae), en invierno y primavera en Tucumán, Argentina. Acta Zoológica Lilloana, 57, 217–220.
Dos Santos, D. S., Trindade, R. C. P., Torres, J. B., De lima, M. S., Dos Santos, L., Batista, F. C. (2020). Predation of Brevicoryne brassicae and Aphis craccivora by Eriopis connexa depending on availability. Acta Biológica Colombiana, 26, 99–104. https://doi.org/10.15446/abc.v26n1.83303.
Elliott, N., & Kieckhefer, R. (2000). Response by coccinellids to spatial variation in cereal aphid density. Population Ecology, 42, 81–90. https://doi.org/10.1007/s101440050012
Engel, E., Batistella Pasini, M. P., Hörz, D. C., Pivotto Bortolotto, R., & Zamberlan, J. F. (2020). Predatory arthropods on alternative host plants in area surrounding by soybean-corn succession system. Biologia, 75, 1591–1599. https://doi.org/10.2478/s11756-019-00410-z
Evans, E. W., & Youssef, N. N. (1992). Numerical responses of aphid predators to varying prey density among Utah alfalfa fields. Journal of the Kansas Entomological Society, 65, 30–38. https://doi.org/10.2307/25085324
Fonseca, M. M., Lima, E., Lemos, F., Venzon, M., & Janssen, A. (2017). Non-crop plant to attract and conserve an aphid predator (Coleoptera: Coccinellidae) in tomato. Biological Control, 115, 129–134. https://doi.org/10.1016/j.biocontrol.2017.10.005
García, M. F., Becerra, V. C., & Reising, C. E. (1999). Harmonia axyridis Pallas (Coleoptera, Coccinelidae) Estudio biológico. Revista de la Facultad de Ciencias Agrarias: Universidad Nacional de Cuyo, 31, 85–91.
González, G. (2014). Los Coccinellidae de Argentina [online]. Available in World Wide Web: http://www.coccinellidae.cl/paginasWebArg. Accessed 15 Sept 2022.
Grez, A. A., Zaviezo, T., Roy, H. E., Brown, P. M. J., & Bizama, G. (2016). Rapid spread of Harmonia axyridis in Chile and its effects on local coccinellid biodiversity. Diversity and Distributions, 22, 982–994. https://doi.org/10.1111/ddi.12455
Griffith, D. M., Veech, J. A., March, C. J. (2016). cooccur: Probabilistic Species Co-Occurrence Analysis in R. Journal of Statistical Software, Code Snippets, 69, 1–17. https://doi.org/10.18637/jss.v069.c02.
Hautier, L., San Martin, G., Callier, P., De Biseau, J. C., & Grégoire, J. C. (2011). Alkaloids provide evidence of intraguild predation on native coccinellids by Harmonia axyridis in the field. Biological Invasions, 13, 1805–1814. https://doi.org/10.1007/s10530-010-9935-0
Hemptinne, J. L., Dixon, A. F. G., Gauthier, C. (2000). Nutritive cost of intraguild predation on eggs of Coccinella septempunctata and Adalia bipunctata (Coleoptera: Coccinellidae). European Journal of Entomology, 97, 559–562. https://doi.org/10.14411/eje.2000.087.
Hindayana, D., Meyhöfer, R., Scholz, D., & Poehling, H. M. (2001). Intraguild Predation among the hoverfly Episyrphus balteatus de Geer (Diptera: Syrphidae) and other aphidophagous predators. Biological Control, 20, 236–246. https://doi.org/10.1006/bcon.2000.0895
Hodek, I., & Evans, E. W. (2012). Food relationships. In I. Hodek, H. F. van Emden, & A. Honěk (Eds.), Ecology and Behaviour of Ladybird Beetles (Coccinellidae) (pp. 141–274). Blackwell Publishing Ltd.
Janssen, A., Sabelis, M. W., Magalhaes, S., Montserrat, M., & Van der Hammen, T. (2007). Habitat structure affects intraguild predation. Ecology, 88, 2713–2719. https://doi.org/10.1890/06-1408.1
Lixa, A. T., Campos, J. M., Resende, A. L. S., Silva, J. C., Almeida, M. M. T. B., & Aguiar-Menezes, E. L. (2010). Diversidade de Coccinellidae (Coleoptera) em plantas aromáticas (Apiaceae) como sítios de sobrevivência e reprodução em sistema agroecológico. Neotropical Entomology, 39, 354–359. https://doi.org/10.1590/S1519-566X2010000300007
Lucas, É., Coderre, D., & Brodeur, J. (1998). Intraguild predation among aphid predators: Characterization and influence of extraguild prey density. Ecology, 79, 1084–1092. https://doi.org/10.1890/0012-9658(1998)079[1084:IPAAPC]2.0.CO;2
Lucas, E., Fréchette, B., & Alomar, O. (2009). Resource quality, resource availability, and intraguild predation among omnivorous mirids. Biocontrol Science and Technology, 19, 555–572. https://doi.org/10.1080/09583150902883460
Lucas, É. (2012). Intraguild Interactions. In I. Hodek, H. F. van Emden, & A. Honěk (Eds.), Ecology and Behaviour of the Ladybird Beetles (Coccinellidae) (pp. 343–374). Blackwell Publishing Ltd.
Lucas, E., & Rosenheim, J. A. (2011). Influence of extraguild prey density on intraguild predation by heteropteran predators: A review of the evidence and a case study. Biological Control, 59, 61–67. https://doi.org/10.1016/j.biocontrol.2011.05.010
Martins, C. B. C., Almeida, L. M., Zonta-de-Carvalho, R. C., Castro, C. F., & Pereira, R. A. (2009). Harmonia axyridis: A threat to Brazilian Coccinellidae? Revista Brasileira de Entomologia, 53, 663–671. https://doi.org/10.1590/S0085-56262009000400018
Michaud, J. P., & Grant, A. K. (2003). Intraguild predation among ladybeetles and a green lacewing: Do the larval spines of Curinus coeruleus (Coleoptera: Coccinellidae) serve a defensive function? Bulletin of Entomological Research, 93, 499–505. https://doi.org/10.1079/BER2003269
Michaud, J. P., Jyoti, J. L. (2007). Repellency of conspecific and heterospecific larval residues to Hippodamia convergens (Coleoptera: Coccinellidae) ovipositing on sorghum plants. European Journal of Entomology, 104, 399–405. https://doi.org/10.14411/eje.2007.059.
Michaud, J. P. (2002). Invasion of the Florida citrus ecosystem by Harmonia axyridis (Coleoptera: Coccinellidae) and asymmetric competition with a native species, Cycloneda sanguinea. Environmental Entomology, 31, 827–835. https://doi.org/10.1603/0046-225X-31.5.827
Mirande, L., Desneux, N., Haramboure, M., & Schneider, M. I. (2015). Intraguild predation between an exotic and a native coccinellid in Argentina: The role of prey density. Journal of Pest Science, 88, 155–162. https://doi.org/10.1007/s10340-014-0597-z
Musser, F., & Shelton, A. M. (2003). Factors altering the temporal and within-plant distribution of coccinellids in corn and their impact on potential intra-guild predation. Environmental Entomology, 32, 575–583. https://doi.org/10.1603/0046-225X-32.3.575
Pimenta, A. G., De Souza, T. S., Fernandes, V. J., Aguiar-Menezes, E. D. L., & Guerra, J. G. M. (2020). Análise faunística de joaninhas (Coleoptera: Coccinellidae) em um sistema agroecológico de produção orgânica de hortaliças. Revista Multidisciplinar De Educação e Meio Ambiente, 1, 1.
Prescott, K. K., & Andow, D. A. (2018). Co-occurrence among intraguild Predators: Avoidance or aggregation? Environmental Entomology, 47, 559–566. https://doi.org/10.1093/ee/nvy016
R Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. URL https://www.R-project.org/. Accessed 8 Dec 2022.
Ribeiro Pereira Dos Santos N, Monteiro Dos Santos Cividanes T, Cividanes FJ, Ribeiro Dos Anjos AC, Vaz Leite De Oliveira L (2009) Aspectos biológicos de Harmonia axyridis alimentada com duas espécies de presas e predação intraguilda com Eriopis connexa. Pesquisa Agropecuária Brasileira, 44, 554–560. https://doi.org/10.1590/S0100-204X2009000600002.
Rizzo, E. (2020). Interacciones dentro del ensamble de parasitoides y depredadores de áfidos y su efecto sobre el control biológico en el cultivo de berenjena. PhD Thesis Universidad Nacional de La Plata, Argentina.
Rocca, M., Díaz Lucas, M. F., & Greco, N. (2021). Effect of spatiotemporal association and trophic interactions between aphidophagous coccinellids towards aphid control. Environmental Entomology, 51, 44–51. https://doi.org/10.1093/ee/nvab127
Rocca, M., Rizzo, E., Greco, N. M. (2019) Larval interactions between two aphidophagous coccinellids in sweet pepper. Anais da Academia Brasileira de Ciências, 92(1). https://doi.org/10.1590/0001-3765202020181163.
Rocca, M., Rizzo, E., Greco, N., & Sánchez, N. (2017). Intra- and interspecific interactions between aphidophagous ladybirds: The role of prey in predator coexistence. Entomologia Experimentalis Et Applicata, 162, 284–292. https://doi.org/10.1111/eea.12527
Rondoni, G., Borges, I., Collatz, J., Conti, E., Costamagna, A. C., Dumont, F., Evans, E. W., Grez, A. A., Howe, A. G., Lucas, E., Maisonhaute, J. E., Soares, A. O., Zaviezo, T., & Cock, M. J. W. (2021). Exotic ladybirds for biological control of herbivorous insects – a review. Entomologia Experimentalis Et Applicata, 169, 6–27. https://doi.org/10.1111/eea.12963
Roy, H. E., Brown, P. M. J., Adriaens, T., Berkvens, N., Borges, I., Clusella-Trullas, S., & Estoup, A. (2016). The harlequin ladybird, Harmonia axyridis: Global perspectives on invasion history and ecology. Biological Invasions, 18, 997–1044. https://doi.org/10.1007/s10530-016-1077-6
Růžička, Z., & Zemek, R. (2008). Deterrent effects of larval tracks on conspecific larvae in Cycloneda limbifer Z. BioControl, 53, 763–771. https://doi.org/10.1007/s10526-007-9109-x
Saini, E. D. (2004). Presencia de Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) en la provincia de Buenos Aires. Aspectos biológicos y morfológicos. RIA, 33, 151–160.
Schellhorn, N. A., & Andow, D. A. (1999). Mortality of coccinellid (Coleoptera: Coccinellidae) larvae and pupae when prey become scarce. Environmental Entomology, 28, 1092–1100. https://doi.org/10.1093/ee/28.6.1092
Srinivasan, M. (2009). Insect and mite pests on eggplant. A field guide for identification and management. AVRDC—The World Vegetable Center, 10–13.
Veech, J. A. (2014). The pairwise approach to analyzing species co-occurrence. Journal of Biogeography, 41, 1029–1035. https://doi.org/10.1111/jbi.12318
Wagner, L. S., Fenoglio, M. S., & Salvo, A. (2017). Alien species numerically dominate natural enemy communities in urban habitats: A preliminary study. Journal of the Entomological Research Society, 19(2), 31–42.
Ware, R. L., & Majerus, M. E. N. (2007). Intraguild predation of immature stages of British and Japanese coccinellids by the invasive ladybird Harmonia axyridis. In H. E. Roy & E. Wajnberg (Eds.), From biological control to invasion: The ladybird Harmonia axyridis as a model species (pp. 169–188). Springer.
Ware, R. L., Ramon-Portugal, F., Magro, A., et al. (2008). Chemical protection of Calvia quatuordecimguttata eggs against intraguild predation by the invasive ladybird Harmonia axyridis. BioControl, 53, 189–200. https://doi.org/10.1007/s10526-007-9129-6
Werenkraut, V., Baudino, F., Roy, H. E. (2020). Citizen science reveals the distribution of the invasive harlequin ladybird (Harmonia axyridis Pallas) in Argentina. Biological Invasions, 22, 2915–2921. https://springerlink.bibliotecabuap.elogim.com/article/https://doi.org/10.1007/s10530-020-02312-7. Accessed 8 Dec 2022.
Wright, E., & Laing, J. (1980). Numerical response of coccinellids to aphids in corn in southern ontario. The Canadian Entomologist, 112, 977–988. https://doi.org/10.4039/Ent112977-10
Yasuda, H., Evans, E. W., Kajita, Y., Urakawa, K., & Takizawa, T. (2004). Asymmetric larval interactions between introduced and indigenous ladybirds in North America. Oecologia, 141, 722–731. https://doi.org/10.1007/s00442-004-1680-6
Zalazar, L., & Salvo, A. (2007). Entomofauna associated to horticultural crops under organic and conventional practices in Córdoba, Argentina. Neotropical Entomology, 36, 765–773. https://doi.org/10.1590/s1519-566x2007000500019
Acknowledgements
We thank Eliana Nieves and Martha Roggiero for the maintenance of the insect colonies and the eggplant plants and Graciela Minardi for the statistical analyses. Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.
Funding
This research was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica PICT 2015–1427, PICT 2019–1745 and by the Universidad Nacional de La Plata Programa de Incentivos N834.
Author information
Authors and Affiliations
Contributions
María Estefanía Rizzo carried out the laboratory assays, performed the analysis of data, interpretation and discussion of results and prepared the draft of the manuscript. Adriana Salvo collaborated in the interpretation and discussion of results, and in the writing of the final version of the manuscript. Margarita Rocca participated in the planning of the assays, collaborated in the analysis of data, interpretation, and discussion of results, and in the writing of the final version of the manuscript. She also obtained the funds to carry out the investigation. Nancy Greco participated in the planning of the assays, interpretation, and discussion of results, as well as in the writing of the final version of the manuscript. She also obtained the funds to carry out the investigation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript and the authors of the manuscript are not involved in any potential conflicts of interest, including financial interests and relationships and affiliations, and all authors gave their consent to participate in the manuscript.
Consent for publication
All authors gave their consent for the publication of the manuscript.
Competing interests
Authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rizzo, M.E., Salvo, A., Rocca, M. et al. Spatial and temporal cooccurrence among Neotropical native coccinellids and the exotic Harmonia axyridis. Phytoparasitica 51, 89–99 (2023). https://doi.org/10.1007/s12600-022-01040-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-01040-z