Abstract
The pine cone moth, Dioryctria mendacella, is one of the most important pests affecting cones of Mediterranean pines in southern Europe. The larvae cause damages to cone tissues and seeds, but despite its pest status, the biology of D. mendacella is largely unknown, specifically the seasonal flight activity of the adults. To overcome this, we studied the annual flight activity of D. mendacella in southern Portugal with traps baited with the sex pheromone. Fifteen white delta sticky traps were placed on the lower branches of grafted Pinus pinea trees, and pyralid moths were collected weekly for one year. We captured a total of 3.555 individuals, corresponding to a flight period from February to early December. Initial captures were relatively low but subsequently increased during the summer and early autumn months, and peaking in September. However, no captures occurred from early December to late February. Multiple regression analysis detected a positive and significant correlation between moth captures and temperatures at dusk, suggesting a dominant twilight/nocturnal flight activity for D. mendacella. Overall, we conclude that Delta traps baited with the sexual pheromone are effective in capturing adult moths of D. mendacella throughout the year, and our results suggest the occurrence of two (or more) annual generations for this pest, supporting similar inferences from other studies. The use of traps baited with sex-pheromones is a new tool for the development of integrated pest management strategies against D. mendacella, offering also the possibility of studying in detail the biology and population dynamics of this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pine cone moth, Dioryctria mendacella Staudinger (Lepidotera: Pyralidae) is one of the most important pests affecting pine cones in Europe and Northern Africa, with larvae feeding on cones of all ages and causing significant damage (Romanyk and Cadahia, 1992; El Alaoui El Fels and Roques, 2006; Mutke et al., 2013; Calama et al., 2017). Attacks are particularly important in Stone pine (Pinus pinea L.) production, affecting up to 20–30% of the nut production and being more severe in years of low harvest, when the damages may impact up to 70% of the pine-cones (Calama et al., 2017). Stone pine cones are harvested for edible seeds (pine nuts) and are one of the most important non-wood forest products in Mediterranean forests, particularly in Spain and Portugal (Calama et al., 2020; Mutke et al., 2012).
The biology of D. mendacella has proven difficult to study, with all life stages present in spring and autumn and probable multiple generations which may overlap throughout the year. In the Iberian Peninsula, two annual generations can occur (Garre et al., 2022), although more generations are possible under favourable conditions (Romanyk & Cadahia, 2003). The lack of detailed knowledge on the life cycle of D. mendacella is surprising considering its importance as a forest pest, and additional research on its biology is needed to develop efficient control strategies (Calama et al., 2017; Romanyk & Cadahia, 2003).
The identification of the sex pheromone of D. mendacella offers new possibilities for using baited traps to study the seasonal flight patterns of the pest (Hall et al., 2017). Studies show that pine cone moth females produce (Z,E)-9,11-tetradecadienyl acetate (ZE9,11–14:Ac) and (Z,Z,Z,Z,Z)-3,6,9,12,15-pentacosapentaene (ZZZZZ3,6,9,12,15–25:H), with the former eliciting a strong EAG response from males while no response could be recorded for the latter. In field trapping tests, both compounds were individually unattractive to males, but blends of the two were highly attractive (Hall et al., 2017).
Considering these recent developments and appraising the importance of D. mendacella as a pest of stone pines (Naves et al., 2022; Sousa et al., 2017), in this paper we describe the flight activity of the pine cone moth in a location in southern Portugal for one year, studied with pheromone-baited traps in one of the most important cone-production regions of the country, and providing new and important data for the characterisation of the life cycle of this poorly-studied forest pest.
Materials and Methods
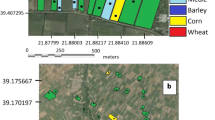
White Delta sticky traps (Kenogard), totalling fifteen, were placed on the mid-lower branches (3 to 4 m above ground) of grafted P. pinea trees (20 years old) near Canha, southern Portugal (38°45´00´´N; 8°31´55´´W; altitude 72 m). The region has a climate classification of ‘Csa’ according to the Köppen-Geiger system, corresponding to a typical Mediterranean climate with hot and dry-summers and mild and wet winters (Peel et al., 2007).
The pine forest where the study was carried out was subjected to grazing by cows and vegetation included herbaceous plants in the understory, Ulex shrubs and occasional cork oak (Quercus suber L.) trees. Traps were placed in pines distant ≈20 m apart, and baited with a red rubber septum impregnated with the pheromone attractant “Dioryctria mendacella” product number 50398 (Pherobank), containing (Z,E)-9,11-tetradecadienyl acetate and (Z,Z,Z,Z,Z)-3,6,9,12,15-pentacosapentaene at 0.55 mg/lure (0.79 g/kg). Pheromones were changed every four weeks, before the five-week replacement recommended by the manufacturer. Traps were placed from 18 March 2021 to 24 March 2022, collecting weekly (every 6–7 days) the insects captured and replacing the white sticky cards.
The cardboards with insects were taken to the INIAV laboratories at Oeiras for identification and counting. Adult moths were identified by the senior author (PN) to species level based on morphological characters described by Knölke (2007), considering the wing colouration and venation, unipectinate antennae, and body/wing dimensions.
When morphological identification was uncertain, the damaged or dubious specimens which could not be clearly assigned as D. mendacella were subjected to DNA sequence-based identification. Genomic DNA was extracted from individual insects using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, US) according to the manufacturer’s instructions. A fragment of the mitochondrial gene cytochrome c oxidase I gene (COI) was amplified using the universal primer pair LCO1490 and HCO2198 (Folmer et al., 1994). Polymerase chain reaction (PCR) was conducted in a 25 μL final reaction volume, containing 12.5 μL of Supreme NZYTaq II DNA polymerase Master Mix (NZYTech, Lisbon, Portugal), 1 μL of DNA template, 1 μL of each forward and reverse primer (10 μM) and 8.5 μL of molecular-grade water (Sigma-Aldrich, St. Louis, MI, USA). Amplification reactions were performed in the thermocycler Biometra TAdvanced (Analytik Jena, Germany) and the temperature program for amplification consisted of an initial denaturation of 94 °C for 2 min followed by 40 cycles of 94 °C for 1 min, 45 °C for 2 min, and 72 °C for 3 min and a final extension of 72 °C for 5 min (Folmer et al., 1994). Amplified products were visualized under UV light on a 1.5% agarose gel to confirm successful amplification. All the PCR products of the expected size were purified using ExoSAP-IT™ PCR Product Cleanup Reagent (ThermoFisher Scientific, Pittsburg, PA, USA) following the manufacturer’s instructions and then bidirectionally sequenced using the Sequencing facility at INIAV (Oeiras, Portugal). Nucleotide sequences were edited and analyzed using BioEdit v7.2.0 (Hall, 2007). The resulting consensus sequences were multi-aligned using ClustalW and the alignment revealed no differences. Molecular identification was made by comparing our sequences with those of D. mendacella and other relevant sequences of Dioryctria spp. available in the GenBank database of the National Center for Biotechnology Information (NCBI) using the Basic Local Alignment Search Tool (BLAST) homology search.
Statistical analyses
A parametric analysis of variance test (ANOVA) was used to compare mean annual captures between traps and between meteorological seasons, which in the Northern Hemisphere comprises March, April, and May for spring, June, July, and August for summer, September, October, and November for autumn, and December, January, and February for winter. The Fisher Least Significant Difference test (LSD) was used to compare means within each significant factor in the ANOVA.
To evaluate whether environmental factors were associated with weekly capture rates, we used multiple regression to model weekly captures of D. mendacella using local climatic variables provided by a conventional weather station installed 150 m from the field plot. The following variables were used in model construction: mean, maximum, and minimum daily temperatures, daily temperatures at sunrise and sunset, daily temperatures at the beginning (end of astronomical twilight) and after (beginning of astronomical twilight) “dark” night, number of days with a maximum temperature equal or above 20ºC, 25ºC, 30ºC or 35ºC, and number of days with a minimum temperature equal or below 15ºC, 10ºC or 5ºC, daily mean values (or counts of days) over the previous 7-days were backward calculated. Each week × trap combination was treated as a unit of replication for model construction. Tests were made on the STATISTICA software, version 12 (StatSoft), and incorporate at Type I error rate of α = 0.05 for assigning statistical significance.
Results
Over a period of one year, we captured a total of 3.555 male D. mendacella, with a flight period extending for more than eight months.
In 2021 insects were captured in the first week of sampling, from 25 March (Julian day 84) onwards, with initial captures relatively low during the spring months but increasing throughout the summer/autumn months. There was a peak in September, with the highest catches on September 23rd (Julian day 266) with 332 males in one week, and captures continued until December 9th (Julian day 343), this being the last capture of the civil calendar year.
The absence of captures occurred during 11 weeks of the year, from early December to late February, with the first moths appearing on February 24th (Julian day 55) 2022 (Figs. 1 and 2).
Overall, each trap captured a mean of 237 ± 78 (mean ± SD) moths during the year, with significant differences between traps (F = 3.1435; df = 14; p < 0.001). The highest weekly captures by a single trap were 34 moths, occurring during the week ending on September 23rd.
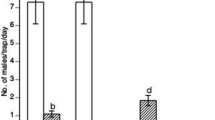
There were significant differences in captures between meteorological seasons (F = 14.5747; df = 3; p < 0.001), with a contrast of lower captures in winter and spring and higher numbers in summer and autumn (Fig. 3).
The multiple regression model was a relatively strong fit for predicting weekly trap captures (R2 = 0,798; F = 10,767; df = 3; p < 0.001), and only detected a significant (and positive) correlation between captures and temperatures at the beginning of the “dark” night (end of astronomical twilight) (Table 1), while for the other variable’s the correlations were not statistically significant.
Most of the insects were alive and in good conditions when collected and could be clearly identified as D. mendaccella by morphological characters. In order to assert the identity of 21 dubious specimens, a molecular identification was performed by sequencing a fragment of the COI gene, resulting in a PCR product of about 675 bp in agreement with the expected size established for this region. BLAST hits showed that the partial sequences of COI gene were 100% identical (e-value 0.0) to D. mendacella isolates PP17 and PP16 from Portugal (accession numbers OK559625 and OK559626) (Naves et al., 2022), and to voucher BC ZSM Lep 51,355 (accession number KX040713) from Spain. Novel sequences from this study were deposited in GenBank, accession numbers OP269725 to OP269745.
Very few other insect species were caught in the traps, and by-catch captures consisted mainly of Muscidae flies (Diptera).
Discussion
The Delta trap baited with the “Dioryctria mendacella” 50,398 pheromone was effective in capturing adult male moths of D. mendacella throughout the year and was very specific with very few by-catch captures of other insects.
We report evidence for a prolonged period of flight activity extending from February to early December, with a peak in September. The seasonal flight activity of D. mendacella had not been studied in detail until now: isolated captures were reported from central Portugal from May to June and from August to November (Pires & Corley, 2007), while in the Algarve (southern Portugal) specimens are sporadically caught from February to November each year (Banza, 2022), resulting in an overall pattern of seasonal activity compatible with the one we report.
In Spain, the seasonal flight activity is reported on April-June and October–November, suggesting a bivoltine life cycle of two annual generations (Gómez de Aizpirúa, 1991), although more generations are possible under favourable conditions (Romanyk & Cadahia, 2003). Our continuous captures throughout the year do not allow us to discriminate different generations; nevertheless, the increasing captures from June onwards suggest a first annual generation completed in early summer, with a subsequent second, or even third generation completed in early autumn and/or with overlapping/sister generations also possible. The overlap of life stages among generations is common to several Dioryctria species worldwide (Merkel & Fatzinger, 1971; Whitehouse et al., 2011).
Other species of the Genus Dioryctria also exhibit extended seasonal flight activity (Grant et al., 1993; Merkel & Fatzinger, 1971; Roe et al., 2006; Whitehouse et al., 2011), although not as prolonged as we report for D. mendacella. Flight peaks of other Dioryctria species usually occur in June-July (Menassieu et al., 1989; Whitehouse et al., 2011) or August (Roe et al., 2006), and not in September as reported here for D. mendacella.
Regression analysis detected a positive and significant correlation only between moth captures and temperatures at the beginning of the “dark” night (end of astronomical twilight), which in the longest summer days of June/July initiates at 22:03 (10:03 PM), local time. Our results suggest that D. mendacella flight activity may take place mainly during the astronomical twilight/early night hours, which agrees with reports for other species of the genus that are active soon after dark (Fatzinger & Asher, 1971; Trudel et al., 1995; Menassieu et al., 1989). For Dioryctria disclusa Heinrich the flight of males in response to sex pheromone occurs between 3 to 5 h after sunset (DeBarr & Berisford, 1981), while for D. abietivorella it begins 2 h after sunset, peaks 4 h after sunset, and ends 1 h after dawn (Whitehouse et al., 2011). Furthermore, Triggiani (1986) mentions that Dioryctria pineae has crepuscular habits in Italy, while Menassieu et al. (1989) report nocturnal reproductive flight activity for Dioryctria sylvestrella in south-west France.
Although the traps were placed relatively close to each other (≈20 m apart), there were significant differences in the number of captured moths. The discrepancies could result from differences in population density between trees with traps, considering that D. mendacella has a low dispersal propensity at the local scale (Calama et al., 2017), although additional studies are needed to clarify this.
The pine cone moth D. mendacella is one of the most important pests affecting cone production in the Mediterranean region, and, furthermore, has recently caused damage to P. pinea grafted shoots in southern Portugal (Naves et al., 2022). Considering the experience of other species of the Dioryctria genus, the use of traps baited with sex-pheromones allows for to study biology and population dynamics of D. mendacella and offers a convenient, inexpensive, and specific survey technique to monitor the population levels between years and locations (DeBarr & Berisford, 1981; DeBarr et al., 2000; Grant et al., 1993; Hanula et al., 1984a, 1984b; Löfstedt et al., 2012; Roe et al., 2006; Strong et al., 2008; Whitehouse et al., 2011). According to Whitehouse et al. (2011), there is great potential for the use of synthetic sex pheromones to control pestiferous Dioryctria species, with trapping used on mating-disruption approaches within integrated pest management strategies incorporating silvicultural, mechanical, and biological techniques (Hall et al., 2017). Pheromone-based control tactics require information derived from reproductive-behaviour studies, which for D. mendacella should focus on characterising the phenology and seasonal flight activity in regions with contrasting climatic conditions, on assessing the most efficient location of traps on the tree canopy and trap density within the stands, and on quantifying the production/economic benefits in using traps as monitoring and control tools against this pest.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Banza, P. (2022). Monitorização de Lepidópteros - Relatórios sobre borboletas noturnas. Publicações A ROCHA. Retrieved May 04, 2022, from https://arocha.pt/pt/conservacao-e-ciencia/publicacoes/.
Calama, R., Fortin, M., Pardos, M., & Manso, R. (2017). Modelling spatiotemporal dynamics of Pinus pinea cone infestation by Dioryctria mendacella. Forest Ecology and Management, 389, 136–148. https://doi.org/10.1016/j.foreco.2016.12.015
Calama, R., Gordo, J., Mutke, S., Conde, M., Madrigal, G., Garriga, E., Arias, M. J., Piqué, M., Gandía, R., Montero, G., & Pardos, M. (2020). Decline in commercial pine nut and kernel yield in Mediterranean stone pine (Pinus pinea L.) in Spain. iForest, 13, 251–260. https://doi.org/10.3832/ifor3180-013
DeBarr, G., & Berisford, C. (1981). Attraction of webbing coneworm males to female sex pheromone. Environmental Entomology, 10, 119–121.
DeBarr, G. L., Hanula, J. L., Niwa, C. G., & Nord, J. C. (2000). Synthetic pheromones disrupt male Dioryctria spp. moths in a loblolly pine seed orchard. The Canadian Entomologist, 132, 345–351.
El Alaoui El Fels, M. A., & Roques, A. (2006). Guide d’identification des ravageurs des cônes et des graines des résineux autochtones du Haut Atlas occidental. El Watanya.
Fatzinger, C. W., & Asher, W. C. (1971). Observations on the pupation and emergence behavior of Dioryctria abietella (Lepidoptera: Pyralidae (Phycitinae)). Annals of the Entomological Society of America, 64, 413–418.
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Garre, M. J., Girdley, J., Guerrero, J. J., Rubio, R. M., & Ortiz, A. S. (2022). An annotated checklist of the Pyralidae of the region of Murcia (Spain) with new records, distribution and biological data (Lepidoptera, Pyraloidea, Pyralidae). Biodiversity Data Journal, 10, e79255.
Gómez de Aizpirúa, C. (1991). Algunos lepidópteros huéspedes del pino silvestre, Pinus sylvestris Linne. C. Boletin de Sanidad Vegetal, Plagas, 17(2), 213–234.
Grant, G., Katovich, S., Hall, D., Lombardo, D., & Lessor, K. (1993). Sex pheromone identification and trapping of Dioryctria resinosella (Lepidoptera: Pyralidae). Environmental Entomology, 22, 154–161.
Hall, T. (2007). BioEdit. Biological Sequence Alignment Editor for Win95/98/NT/2K/XP. Carlsbad, CA, Ibis Biosciences. Retrieved May 4, 2022, from http://www.mbio.ncsu.edu/BioEdit/bioedit.html.
Hall, D. R., Farman, D., Domínguez, J. C., & Pajares, J. A. (2017). Female sex pheromone of the cone moth, Dioryctria mendacella: Investigation of synergism between type I and type II pheromone components. Journal of Chemical Ecology, 43, 433–442.
Hanula, J., Berisford, C., & DeBarr, G. (1984a). Pheromone cross-attraction and inhibition among four coneworms, Dioryctria spp. (Lepidoptera: Pyralidae) in a loblolly pine seed orchard. Environmental Entomology, 13, 1298–1301.
Hanula, J., DeBarr, G., Harris, W., & Berisford, C. (1984b). Factors affecting catches of male coneworms, Dioryctria spp. (Lepidoptera, Pyralidae), in pheromone traps in southern pine seed orchards. Journal of Economic Entomology, 77, 1449–1453.
Knölke, S. (2007). A revision of the European representatives of the microlepidopteran genus Dioryctria Zeller, 1846 (Insecta: Lepidoptera: Pyralidae: Phycitinae). Ludwig Maximilians-Universität München.
Löfstedt, C., Svensson, G. P., Jirle, E. V., Rosenberg, O., Roques, A., & Millar, J. G. (2012). 3Z,6Z,9Z,12Z,15Z)-Pentacosapentaene and (9Z,11E)-tetradecadienyl acetate: Sex pheromone of the spruce coneworm Dioryctria abietella (Lepidoptera: Pyralidae. Journal of Applied Entomology, 136, 70–78.
Menassieu, P., Stockel, J., & Levieux, J. (1989). Données actuelles sur la biologie de Dioryctria sylvestrella (Ratz.) (Lep., Pyralidae) ravageur du Pin maritime (Pinus pinaster Ait) dans le Sud Ouest de la France. Journal of Applied Entomology, 107, 238–247.
Merkel, E. P., & Fatzinger, C. W. (1971). Periodic abundance of pine cone-infesting Lepidoptera in black light traps and sleeve cages in North Florida. Florida Entomologist, 54, 53–61.
Mutke, S., Calama, R., Gonzalez-Martínez, S. C., Montero, G., Gordo, F. J., Bono, M., & Gil, L. (2012). Mediterranean Stone Pine: Botany and Horticulture. In J. Janick (Ed.), Horticultural Reviews Volume 39 (pp. 153–201). Hoboken: Wiley-Blackwell.
Mutke, S., Piqué, M., & Calama, R. (2013). AGROPINE 2011 Meeting conclusions. In Mutke, S., Piqué, M., & Calama, R. (Eds.), Mediterranean stone pine for agroforestry. Options Méditerranéennes Série A. Séminaires Méditerranéens, 105, 111–112.
Naves, P., Silva, C., Nóbrega, F., & Sousa, E. (2022). Not just the cones: Dioryctria mendacella (Lepidotera Pyralidae) also attacks grafted pine shoots. Bulletin of Insectology, 75(1), 55–58.
Peel, M. C., Finlayson, B. L., & McMahon, T. A. (2007). Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633–1644.
Pires, P., & Corley, M. F. V. (2007). The Lepidoptera of Baixo Mondego (Beira Litoral, Portugal) (Insecta: Lepidoptera). SHILAP Revista De Lepidopterología, 35(138), 187–230.
Roe, A. D., Stein, J. D., Gillette, N. E., & Sperling, F. A. H. (2006). Identification of Dioryctria (Lepidoptera: Pyralidae) in a seed orchard at Chico, California. Annals of the Entomological Society of America, 99, 433–448.
Romanyk, N. & Cadahia, D. (2003). Plagas de insectos en las masas forestales. Sociedad Española de Ciencias Forestales, Madrid, España: Mundi-Prensa.
Sousa E., Pimpão M., Valdiviesso T., Naves P., & Branco, M. (2017). Cone pests of stone pine in the Mediterranean Basin. In: Carrasquinho, I., Correia, A.C., & Mutke, S., (Eds.). Mediterranean pine nuts from forests and plantations. Options Méditerranéennes Série A. Séminaires Méditerranéens, 122, 91–107.
Strong, W., Millar, J., Grant, G., Moreira, J., Chong, J., & Rudolph, C. (2008). Optimization of pheromone lure and trap design for monitoring the fir coneworm, Dioryctria abietivorella. Entomologia Experimentalis Et Applicata, 126, 67–77.
Triggiani, O. (1986). Observations on the biology and behaviour of Dioryctria pineae (Stgr.) (Lepidoptera: Phycitidae) and on its parasitoid Elachertus geniculatus (Ratz.) (Hymenoptera: Chalcidoidea). Entomologica, 21, 141–153.
Trudel, R., Bauce, R., Cabana, J., & Guertin, C. (1995). Rearing technique for Dioryctria abietivorella (Lepidoptera: Pyralidae). Journal of Economic Entomology, 88, 640–643.
Whitehouse, C., Roe, A. D., Strong, W., Evenden, M., & Sperling, F. A. H. (2011). The biology and management of North American cone-feeding Dioryctria species. The Canadian Entomologist, 143, 1–34.
Acknowledgements
We would like to thank the proprietaries of Herdade da Abegoaria (Canha), namely Mrs Mónica Soares and Mr João Soares, for allowing the studies to be conducted on the Estate and for their continuous logistic support throughout the year. We would also like to thank Pherobank BV company (The Netherlands), in the person of Mr Frans Griepink, for kindly supplying the “Dioryctria mendacella” pheromone attractants, and to Bárbara Machado (Florgénese) for providing the Delta traps. We also acknowledge Drª Alexandra Correia (INIAV) for providing the local climatic data for analysis, and Drª Ana Dias (FCUL, Lisboa) and Dr. Seth Davies (University of Colorado, USA) for critical comments on an earlier version of the manuscript. Funding was provided by the Operational Group “Gestão integrada de agentes bióticos associados à perda de produção do pinhão—+PINHÃO, PDR2020-101-031185”, and by the Fundação para a Ciência e a Tecnologia (Portugal) through the R&D Unit UIDB/04551/2020 GREEN-IT—Bioresources for Sustainability.
Funding
This study was funded by the Operational Group “Gestão integrada de agentes bióticos associados à perda de produção do pinhão—+ PINHÃO, PDR2020-101–031185”, and by the Fundação para a Ciência e a Tecnologia (Portugal) through the R&D Unit UIDB/04551/2020 GREEN-IT—Bioresources for Sustainability.
Author information
Authors and Affiliations
Contributions
Pedro Naves and Edmundo de Sousa prepared the field trials, Pedro Naves conducted the field trials and identified the insects, Filomena Nóbrega made the molecular analyses, Pedro Naves, Filomena Nóbrega and Edmundo Sousa wrote the manuscript text, Pedro Naves prepared the figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed with the content and give explicit consent to submit.
Competing interests
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naves, P., Nóbrega, F. & de Sousa, E. Annual flight activity of Dioryctria mendacella (Lepidoptera: Pyralidae) in southern Portugal. Phytoparasitica 51, 41–48 (2023). https://doi.org/10.1007/s12600-022-01036-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-01036-9