Abstract
An intensive survey was conducted throughout India in order to study the intensity of damage caused by the exotic spiralling whitefly, Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae) as well as the incidence on different host plants and its natural enemies. Interestingly, the incidence was found only in 12 geographical regions of India. Extreme damage intensity of A. dispersus was observed in Tamil Nadu, India (99.17%); Kerala, India (97.72%); and Karnataka, India (95.31%). The spiralling whitefly is reported for the first time from Andaman and Nicobar and Himachal Pradesh, which were both on guava. Nitidulid predator, Cybocephalus sp. was the predominant predator of A. dispersus predator on cassava. The most abundant parasitoids in cassava were Encarsia guadeloupae Viggiani and Encarsia meritoria Gahan. Of the recorded 147 host plants (from 53 families), 56 hosts were new host records for A. dispersus. Cotton, mulberry, papaya and cassava showed the highest incidence (100%), while the least incidence was observed on Nephelium (2.40%). Teak (168.3 ± 14.2 leaf−1) and cabbage (0.07 ± 0.07 leaf−1) had the highest and least spiralling whitefly population, respectively. Fifty eight host species are identified as preferred hosts based on host frequencies, incidence (>75%) and the level of population (10 individuals/leaf), which may require additional management. Given the widespread and severe incidence of exotic spiralling whitefly on a variety of host plants in India, this species is likely to pose a threat to the cultivation of economically important crops in India in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aleurodicus dispersus Russell, the spiralling whitefly, is native to Caribbean region (Russell 1965). It has been reported to occur in several Pacific Islands and Cape Verte Islands in Central America, North America, the Caribbean Islands, Africa, South America, and Asia. Spiralling whitefly is of tropical and subtropical origin, like most whiteflies (Mound and Halsey 1978). The insect was first introduced during 2000 on the West African coast; by then, the species has done huge damage losses to food crops, and several indigenous plants (Monteiro 2004). A. dispersus has a wide distribution with steady spread to nearly all countries from its native islands and is gaining economic importance. Because of its economic importance, it now has official quarantine status.
In India, it was first recorded on cassava (Palaniswami et al. 1995) at Thiruvananthapuram (Kerala, India) in 1993 and later at many locations in peninsular India (Mani et al. 2001; Boopathi et al. 2013, 2015a, b). Since then, the pest got distributed over the Southern, and North-Eastern India and has now become a major insect pest of horticultural, agricultural, and forest crops since the 2000s. Production of honeydew and premature leaf fall were caused by the dense A. dispersus population and honeydew act as a substratum for sooty mould growth during feeding (Akinlosotu et al. 1993). The sooty mould causes blackening of leaves, reduces the activity of photosynthesis and the plant vigour which sometimes disfigures the host.
The level of spiralling whitefly damage varies depending on the host species and the condition of the plant. A loss in fruit yield, amounting to >80% was observed on guava in Taiwan (Wen et al. 1995). Heavy spiralling whitefly incidences in cassava resulted in yield reductions of up to 50–80% (Geetha 2000; Boopathi et al. 2016). A. dispersus is currently one of the main insect pests of several field, vegetable, fruit and ornamental crops (Lambkin 1999; Boopathi et al. 2014a, b). Banana, cassava, coconut, eggplant, guava, hibiscus, Indian almond, papaya, rose and tomato are the important host plants of A. dispersus (Geetha 2000; Srinivasa 2000; Mani et al. 2001; Boopathi 2013, Boopathi et al. 2019).
Following the introduction of spiralling whitefly from neighboring countries such as Sri Lanka (Ranjith et al. 1996), Maldives (Muniappan 1996), and Myanmar (Burma) (Boopathi 2008; Boopathi et al. 2014c), it invaded and got established in agricultural, natural, and urban areas. Several native plants, forest trees, ornamentals and food crops have been damaged by this pest since then (Palaniswami et al. 1995; Geetha 2000; Mani et al. 2001; Boopathi 2013; Boopathi et al. 2017a). However, the geographic source and colonization process of the original population are still unknown. Despite its economic significance and serious threat to agricultural production, except for a few survey reports in India (David and Regu 1995; Ranjith et al. 1996; Mani et al. 2000; Charati et al. 2003; Boopathi 2008), little is documented about the occurrence, level and patterns of distribution of spiralling whitefly populations. Therefore, a study was formulated based on two objectives, (i) to determine the distribution and damage patterns of A. dispersus on an economically important agricultural crops and other alternate host plants in India, which will help to prevent the spread of this species from infested regions to other regions by complying with strict domestic quarantine regulations and also aid in enforcing better control measures to prevent further spread and (ii) to identify the potential natural enemies that can be further evaluated for their efficiency, which can then be included in the IPM program for effective control of A. dispersus.
Material and Methods
Assessment of potential distributive areas and intensity of damage
An intensive survey was conducted in various geographical locations comprising all the states of India between August 2012 and December 2018 to study the potential distributive areas and intensity of damage caused by the spiralling whitefly. Sample units were randomly chosen at five places in each location and surveys were conducted on the most preferred plants by spiralling whitefly as hosts (cassava, guava, and rose). A standard assessment system was developed based on the damage intensity (percent) caused by the A. dispersus (Boopathi et al. 2014c). Based on the intensity (%), the damage was categorized into seven grades (Table 1).
Assessment of host plants and sampling method
Host plant surveys were conducted in agriculturally important areas and also in areas with significant biological diversity. The incidence and presence of adults, nymphs, and spiral eggs of the pest were examined on the plants harbouring the spiralling whitefly. Host plants, which could not be identified in the field during the survey, were collected and brought back to the laboratory for identification by referring to plant botany and weed science manuals and also by consulting with experts in the Botanical Survey of India, Coimbatore, Tamil Nadu (India). A digital camera (Nikon model no. D5200) was used to take colored photographs of host plants, whitefly nymphs and adults and spiral eggs.
Twenty plants were selected for the population survey for annual crops or shrubs or small plants. For tree crops, 10 trees were chosen for survey of A. dispersus population. In each tree, four terminal branches were randomly chosen from the whole canopy. Therefore, a total of 40 shoots were sampled growing in all directions (Boopathi et al. 2015b). At each location/host plant, the ‘leaf turn’ technique was applied to determine the densities of spiral eggs, nymphs and adults by recording individuals on the upper-and under- sides of three leaves from the middle, bottom, and top using a 10x folding pocket magnifier (Boopathi et al. 2015a).
Assessment of natural enemies
An intensive survey of natural enemies of A. dispersus was carried out in southern India (Coimbatore, Bengaluru, Erode, Namakkal, Salem, Tiruchirappalli, and Tiruppur) between 2012 and 2015 to investigate the abundance of natural enemies.
Sampling method for parasitoids
In each location/host plant, the densities of immature aphelinids, Encarsia meritoria Gahan and Encarsia guadeloupae Viggiani attacking A. dispersus were determined by collecting 30 leaves from ten plants (3 leaves/plant). The leaves chosen for the investigation were colected from the terminal’s 7th mainstem node. E. meritoria and E. guadeloupae that emerged from pupae and 4th instar nymphs of A. dispersus were recorded in the laboratory. The presence of the parasitoid larvae was decided on the basis of the host’s mycetomes being displaced, but it was not possible to distinguish the parasitoid species in these cases. I determined a parasitism index as per the ratio of parasitized 4th-stadium nymphs combined by both parasitoid species. A sub-leaf sample (n = 20) from each location was observed to assess the composition of emerging adult species (Boopathi et al. 2017b). Parasitized insects were kept in a ventilated box at 28 ± 1 °C and 14 L:10D photoperiod for 2 weeks.
Sampling method for arthropod predators
Arthropod predators were sampled at each location/host plant from 20 plants for annual crops, whereas in tree species four terminal branches were randomly chosen from the whole canopy in 10 plants. Density of arthropod predators; both adult and larval, were determined by visually recording individuals on the top and bottom sides of the whole plant canopy (Boopathi et al. 2017b). Predators consuming on different life stages of spiralling whitefly were observed.
Statistical analysis
Analyses were carried out using version 9.3 of SAS Software (SAS 2011). The survey data of the incidence of A. dispersus and its natural enemies (NEs) in various geographic locations of India and on several host plants was subjected to statistical analysis and the means were separated with a standard error (SE) at P ≤ 0.01. Distribution of arthropod predators were subjected one way analysis of variance and data interaction was estimated using the post hoc Tukey’s Honestly Significant Difference test, and the average values were separated at P ≤ 0.001.
Results
Potential distributive areas and intensity of damage of Aleurodicus dispersus
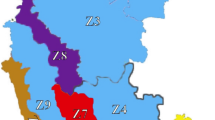
Results on the distribution pattern and damage intensity of spiralling whitefly in various geographic locations of India showed extreme damage intensity in Tamil Nadu (99.17%), Kerala (97.72%) and Karnataka (95.31%) (Fig. 1). The intensity was very high in Mizoram and Andaman and Nicobar (76.57% and 72.67%), but the damage intensity was found to be low in Meghalaya (13.96%) and Telengana (24.07%). Differences in intensity of damage by A. dispersus (F = 54.069; df = 11,44; P < 0.001) and populations (F = 75.049; df = 11,44; P < 0.001) were statistically significant in 12 geographic regions of India. Tamil Nadu (247.1 leaf−1) and Karnataka (219.6 leaf−1) had the highest population density of A. dispersus. Telengana (11.8 leaf−1) and Meghalaya (17.8 leaf−1) had the least dense population of A. dispersus.
Host plants of Aleurodicus dispersus
Survey conducted in India showed that A. dispersus occurred on 147 host plants (Table 2 and Fig. 2). Of the recorded 147 host plants, 56 were new hosts of A. dispersus. The level of incidence of spiralling whitefly on various host plants ranged from 2.4 to 100.0%. Gossypium hirsutum L., Morus alba L., Carica papaya L., and Manihot esculenta Crantz had the highest incidence (100%). the least percent incidence was recorded on Nephelium lappaceum L. (2.4 ± 0.98). The characteristic pattern of spiral egg laying was observed throughout the reported host plants. The adult female preferred young apical leaves for oviposition. However, it was observed that the adult female occasionally oviposits on upper surface of leaves. Eggs were also noticed on fruit-parts of plants like eggplant, papaya and tomato. The most and least egg spiral/leaf were found on Tectona grandis L.f. (17.13 ± 2.13) and Citrullus lanatus (Thunb.) Matsum. and Nakai (0.13 ± 0.08), respectively. The adults were observed to be engaging in migratory flight especially for reproduction during the early morning hours (5.30 to 7.30 h). The spiralling whitefly preferred the lower and middle leaves for feeding. Teak had the highest spiralling whitefly nymph population (91.73 ± 11.14 leaf−1) and adult population (76.60 ± 6.56 leaf−1). Host plants such as Vigna unguiculata (L.) Walp., Cucumis sativus L., C. lanatus, Plectranthus amboinicus (Lour.) Spreng., Butea monosperma (Lam.) Taub., and Ardisia elliptica Thunb. had no nymphal stages. Similarly, in the host plants such as Grevillea robusta A.Cunn. ex R.Br., and Cordia sebestena L., the adult stage was not present. The highest density of population of spiralling whitefly was observed on teak (168.3 ± 14.2 leaf−1), whereas the least density was found on Brassica oleracea L. (Capitata Group) (0.07 ± 0.07 leaf−1).
Of the different host plants determined, 25% plant species (37) had >10 individuals per leaf, including A. tricolor L., Acalypha hispida Burm. f., Achyranthes aspera L., Aleurites fordii Hemsl., Alternanthera triandra Lam., Amaranthus viridis L., C. capsularis L., Calotropis gigantean (L.) W.T.Aiton, Capsicum annuum L., Cassia sp., Cleome viscosa L., Commelina benghalensis L., Convolvulus arvensis L., Corchorus olitorius L., Datura metel L., E. geniculata Ortega, E. heterophylla L., E. hirta L., E. ingens E.Mey. ex Boiss., Euphorbia pulcherrima Willd. ex Klotzsch, M. esculenta, Jacaranda mimosifolia D. Don, Millettia pinnata (L.) Panigrahi, Moringa oleifera Lam., Musa paradisiaca L., Parthenium hysterophorus L., Ruellia tuberosa L., S. elaeagnifolium Cav., S. lycopersicum L., S. xanthocarpum Schrad. and Wendl., Sida acuta Burm. f., Simarouba glauca DC., Solanum melongena L., T. grandis, Tecoma stans (L.) Juss. ex Kunth., Terminalia catappa L., and Vernonia cinerea (L.). A. fordii, A. hispida, Abelmoschus esculentus (L.) Moench, Acalypha indica L., Annona squamosa L., Arachis hypogaea L., C. annuum, C. olitorius, C. papaya, Cajanus cajan (L.) Millsp., Cocos nucifera L., D. metel, Datura discolor Bernh., E. hirta, E. pulcherrima, Gossypium hirsutum L., J. mimosifolia, Lablab purpureus (L.) Sweet, M. alba, M. esculenta, M. paradisiaca, M. pinnata, Ocimum gratissimum (L.), P. hysterophorus, P. guajava, Punica granatum L., Ricinus communis L., S. glauca, S. lycopersicum, S. melongena, S. nigrum L., S. torvum Sw., S. trilobatum L., T. catappa, T. grandis, T. stans, Tridax procumbens L., and V. unguiculata are highly preferred host plants by spiralling whitefly in India based on percentage incidence (> 75%).
Spiralling whitefly infested fifty-three (53) plant families (Table 3). Thirty-three plant families were found to be a susceptible host plants for spiralling whitefly. The highest number of host plants infested by the spiralling whitefly in the plant families viz., Euphorbiaceae (14), Fabaceae (14), Solanaceae (12), Asteraceae (9), Amaranthaceae (8), Cucurbitaceae (8), Malvaceae (7) and Lamiaceae (6) which contributed >50% of host species. The percentage distribution of plant families due to the spiralling whitefly incidence ranged from 0.68 to 9.46%. The highest percentage distribution of plant families due to the spiralling whitefly incidence was recorded from Euphorbiaceae and Fabaceae (9.46).
Natural enemies of Aleurodicus dispersus
Survey was carried out to investigate the abundance of natural enemies (parasitoids and predators) of spiralling whitefly (Fig. 3).
Arthropod predators
Surveys conducted to study the distribution and occurrence of arthropod predators feeding on spiralling whitefly in seven geographic regions of India as well as on 15 host species confirmed the occurrence of 28 species of arthropod predators such as 16 coccinellid species, 5 chrysopid species, 2 drosophilid species and one species of each cybocephalid, lycaenid, mantodea, reduviid and oxyopid species.
Among the seven geographical regions of India, the total population of predators was found to be highest in Coimbatore, Tamil Nadu (134.8 ± 14.76/10 plants) compared to other geographical regions. Tiruchirappalli, Tamil Nadu (23.0 ± 1.64/10 plants) and Tiruppur, Tamil Nadu (24.75 ± 1.78/10 plants) recorded the lowest population of predators. Among the 15 predators, Cybocephalus sp. (111.0 ± 21.57/10 plants) and Mallada astur (Banks) (100.5 ± 23.80/10 plants) were the most abundant predators reported. Less abundant was found to be Acletoxenus indicus Malloch (5.0 ± 2.86/10 plants). There were statistically significant differences in population density of Cryptolaemus montrouzieri Muls., Axinoscymnus puttarudriahi Kapur & Munshi, Anegleis cardoni (Weise), Micraspis sp., Jauravia sp., Cybocephalus sp., M. astur, Mallada desjardinsi (Navas), Chrysoperla zastrowi sillemi (Esben-Petersen), A. indicus, praying mantis (Mantodea: Mantidae) and spiders (Arachnida: Araneae) from seven geographical locations of India. However, there were no significant differences in the population of Cheilomenes sexmaculata (F.), Scymnus coccivora Ayyar and Chilocorus nigrita (F.) in seven geographic regions of India. Interestingly, M. astur, Cybocephalus sp., and A. puttarudriahi were fund to be more prevalent throughout the study period in Bengaluru, Coimbatore, Erode, Namakkal, Salem, Tiruppur, and Trichy (Fig. 4). In Namakkal (Tamil Nadu), Cybocephalus sp., A. puttarudriahi, C. montrouzieri and S. coccivora were more abundant. M. astur, C. sexmaculata, Micraspis sp., S. coccivora, praying mantis and spiders were found in more numbers at Coimbatore. A. indicus and M. desjardinsi were recorded only in Bengaluru, Karnataka.
Of the 15 host species, guava, cassava, mulberry and teak had the highest total predator population than other host species. The total population of predators was lowest in banana and custard apple. M. astur (483.7 ± 16.75/10 plants) and Cybocephalus sp. (462.7 ± 2.21/10 plants) were the most abundant of the 15 predators. Acletoxenus indicus was found to be the less abundant one (13.0 ± 3.06/10 plants). There were significant variations in the population density of A. cardoni, A. indicus, A. puttarudriahi, C. montrouzeiri, C. nigrata, C. zastrowi sillemi, Cybocephalus sp., Jauravia sp., M. astur, M. desjardinsi, M. sexmaculata, Micraspis sp., praying mantis, S. coccivora, and spiders from 15 host species (Table 4). Axinoscymnus puttarudriahi, C. sexmaculata, Cybocephalus sp. and Micraspis sp. were found to have the highest density in cassava when compared to other host plants. In comparison with other host plants, highly dense populations of A. cardoni, C. montrouzeiri, Jauravia sp., M. desjardinsi and praying mantis were found in guava. Populations of C. nigrata and M. astur were highest in teak when compared to other plants in the host. The densest population of A. indicus, C. zastrowi sillemi, S. coccivora, and spider were found on acalypha, cotton, mulberry and chilli, respectively.
Parasitoids
In order to identify the parasitoids attacking A. dispersus, seven geographic regions and 15 host plants were surveyed. Two species of parasitoids, E. meritoria and E. guadeloupae, were confirmed to occur.
Coimbatore, Tamil Nadu (58.8 ± 5.76/5 plants) recorded the highest population of parasitoids among the seven geographical regions of India. At Tiruchirappalli, Tamil Nadu (8.8 ± 1.32/5 plants), the total population of parasitoids was found to be least. Among the two parasitoids, E. guadeloupae (110.8 ± 14.23/5 plants) was the most abundant parasitoid in all geographical regions than E. meritoria (79.55 ± 2.72/5 plants). There were significant variations in the parasitism (%), parasitoid emergence (%) and populations of E. meritoria and E. guadeloupae from seven geographic locations of India (Fig. 5). Bengaluru (54.2%) and Tiruchirappalli (12.8%) recorded the highest and lowest parasitism. At Coimbatore (89.3) and at Tiruppur (35.2) the percentage of parasitoid emergence was highest. The most abundant parasitoids in Coimbatore were E. guadeloupae (27.0/5 plants) and E. meritoria (28.3/5 plants) when compared to other geographic regions.
Of the 15 host plants, cassava (56.3 ± 0.88/5 plants) recorded the highest total population of parasitoids when compared to other host plants. In pigeon pea (3.7 ± 1.76/5 plants) the total population of parasitoids was found to be least. Among the two parasitoids, E. guadeloupae (187.3 ± 17.57/5 plants) was the most abundant parasitoid on all host plants than E. meritoria (145.7 ± 15.07/5 plants). There were significant variations in the parasitism (%), parasitoid emergence (%) and populations of E. meritoria and E. guadeloupae from 15 host plants (Fig. 6). The highest and lowest level of parasitism were documented from acalypha (58.4%) and banana (6.4%), respectively. The percentage of parasitoid emergence was highest on acalypha (89.7) and almond (89.0). The most abundant parasitoids in cassava were E. guadeloupae (26.7/5 plants) and E. meritoria (29.7/5 plants).
Discussion
A severe incidence of spiralling whitefly was observed in most of the surveyed states in southern India (Kerala, Karnataka, Tamil Nadu, and Maharashtra except Andhra Pradesh) and in northeastern states (Mizoram and Meghalaya). In India, this pest was first reported during 1993 in Thiruvananthapuram (Kerala, India) (Palaniswami et al. 1995). It was then reported from multiple places of Tamil Nadu (David and Regu 1995), Kerala (David and Regu 1995; Ranjith et al. 1996), Karnataka (Mani et al. 2000), Andhra Pradesh (Charati et al. 2003), Maharashtra (Charati et al. 2003), Mizoram (Boopathi 2008), and Meghalaya (Boopathi et al. 2014c) were reported later. This pest might have got introduced to India from the neighboring countries such as Sri Lanka (Ranjith et al. 1996), Maldives (Muniappan 1996), and Myanmar (Burma) (Boopathi 2008; Boopathi et al. 2014c). The spiralling whitefly is reported for the first time from Andaman and Nicobar and Himachal Pradesh, which were both on guava. Currently, the spiralling whitefly is emerging as a major and economically important insect pest of many food crops in India. Host availability and climate certainly play a significant role in assessing the spiralling whitefly incidence, but accidental spread is a key factor in the latest outbreaks of this pest.
In the present investigation, as many as 147 crop species belonging to 53 families were reported as host plants of spiralling whitefly in India. Out of these, 56 host plants are new host records in India. The host range includes vegetables, fruits, flowers, ornamentals, shade trees, perennial trees, shrubs, annuals and alternative weed hosts. Reports are being made from several countries where the occurrence of this pest has been reported on a broad range of host species. A. dispersus, as already reported, is highly polyphagous and capable of attacking around 500 plants in various countries (Srinivasa 2000). This pest was first noticed on coconut in Florida (Russell 1965). Palaniswami et al. (1995) first reported the pest on cassava in India. The number of host species infested by spiralling whitefly were recorded by several authors in the past, and the numbers ranged from 22 to 128 (David and Regu 1995; Prathapan 1996; Ranjith et al. 1996; Gajendra Babu and David 1999; Mani and Krishnamoorthy 1999a; Muralikrishna 1999; Asia Mariam et al. 2000; Mallappanavar 2000; Ramani 2000; Srinivasa 2000; Geetha and Swamiappan 2001; Aiswariaya et al. 2007). The current investigation is a major update to this data, which reported 147 crop species, as infested by spiralling whitefly. This may suggest that not all available host species in India have been overwhelmingly colonized by the spiralling whitefly. The pest affected about 80–90% of the crops examined, with losses ranging from 2.4% to 100%. A few of the host species reported in the present survey have been identified previously in other parts of the world as host plants.
In the current investigation, 37 plant species (25%) had >10 individuals of A. dispersus per leaf. Montiero (2004) reported earlier that 45% of host plants viz., A. wilkesiana var. musaica Müll. Arg., M. paradisiaca, M. esculenta, E. pulcherrima, Hibiscus rosasinensis L., Hymenocallis senegambica Kunth & Bouché, Malvastrum cordifolium Rojas Acosta, C. papaya, Parietaria debilis G.Forst. and S. nigrum (L.) had more than 10 individuals per cm2. These 58 species are preferred hosts based on the spiralling whitefly population (10 individuals per leaf), which may require additional management. About 38 plant species are highly preferred host plants by spiralling whitefly in India based on percentage incidence (> 75%). Palaniswarni et al. (1995), Mani and Krishnamoorthy (1999b) and Geetha et al. (1998, 1999) also reported the highest incidence of whitefly in annona, banana, cassava, cassia, citrus, chilli, coconut, eggplant, fig, guava, jasmine, Leucinia, mango, Ocimum sanctum L., O. basilicum L., okra, rose and sapota. Breeding and feeding hosts of spiralling whitefly are host species such as banana, cashew, castor, chilli, citrus, cocoa, coconut, eggplant, guava, jackfruit, okra, papaya, pigeon pea, pepper, sapota, and tomato (Ranjith 1998). In Tamil Nadu (India), this pest was also found to feed on wild cassava and rubber (David and Regu 1995).
Majority of host plants belonged to Euphorbiaceae (9.46%), Fabaceae (9.46%), Solanaceae (8.11%), Asteraceae (6.08%), Amaranthaceae (5.41%), Cucurbitaceae (5.41%), Malvaceae (4.73%) and Lamiaceae (4.05%). Earlier, Monteiro (2004) reported that the majority of host plants were in the Fabaceae (23%), Euphorbiaceae (23%), Malvaceae (13%), and Solanaceae (12%) families. Francis et al. (2016) reported that Arecaceae (22%), Burseraceae (16%), Clusiaceae (10%), Lauraceae (9%), Combretaceae (4%) and Anacardiaceae (3%) were the host families affected by the rugose spiralling whitefly (Aleurodicus rugioperculatus Martin). Plant families such as Euphorbiaceae, Fabaceae and Solanaceae were more susceptible to spiralling whitefly incidence in the present investigation. The findings are similar to those of Asia Mariam (1999) who reported plant species belonging to Euphorbiaceae, Solanaceae and Fabaceae were more susceptible to infestation with A. dispersus in earlier studies.
Spiralling whitefly nymphs and adults gathered and heavily infested the lower leaf surface of all plant varieties (Boopathi et al. 2014c). When the spiralling whitefly infestation was severe, yellow speckling, curling, and crinkling of the leaves was observed (Palaniswami et al. 1995). Damage in young plants by spiralling whitefly would result in reduced growth. Tree species may have escaped death, but would have significantly reduced vigor (Ranjith 1998). Wen et al. (1995) estimated that spiralling whitefly could result in an 80% loss of fruit yield in guava through four months of infestation. David and Regu (1995) reported that this insect’s occurrence and spread was alarming on rubber and the infestation caused unseasonal leaf collapse and consequent yield reduction as a result of reduced latex flow. Banana production decreased as a result of the spiralling whitefly attack (Ranjith 1998). Some of the host species reported are likely to be minor host plants that are unable to sustain A. dispersus populations for long-time and therefore need minimum or no control measures. However, 58 host species are preferred hosts based on host frequencies, incidence (>75%) and most dense population (10 individuals/leaf), which may require additional control. The spiralling whitefly had a drastic detrimental impact on the agricultural sectors (Alam et al. 1998). Considering the wide spread and severe incidence of spiralling whitefly on a variety of plants in India, it is likely that this species may soon pose a threat to cultivation of economically important field and plantation crops in India.
Extensive survey was conducted in the present study to find out natural enemies of this introduced pest. In Tamil Nadu and Karnataka, 28 predators belonging to the families of Cybocephalidae, Coccinellidae, Chrysopidae, Drosophilidae, Lycaenidae, Anthocoridae, praying mantis and spiders and two hymenopteran parasitoids species were observed as natural enemies of A. dispersus. These natural enemies have a vital role to play in A. dispersus population regulation. Several workers in many countries have previously documented the natural enemy complex of A. dispersus (Nechols 1982; Kumashiro et al. 1983; Waterhouse and Norris 1989; Blanco-Metzler and Laprade 1998; Geetha 2000). In India (Karnataka, Kerala, Maharashtra, Tamil Nadu, Lakshadweep Islands, and Andhra Pradesh), 45 predators, 3 parasitoids, and 2 pathogens of A. dispersus were reported (Mani 2010). Seventeen Coleopteran, five Neuropteran, two Dipteran and one in each of the lycaenid, anthocorid, mantid and spider groups were found in Tamil Nadu and Karnataka (India). A total 45 predators were reported in India, mostly generalists and a few species are host specific (Mani 2010). Known to attack A. dispersus were predators numbering 22 from Karnataka (Mani et al. 2004), 15 from Tamil Nadu (Geetha 2000) and 40 from Karnataka and Lakshadweep (Ramani 2000). The current study showed that the predominant predator of A. dispersus on cassava was Cybocephalus sp. Previously, Cybocephalus sp. was first recorded from Minicoy (Ramani 2000) and was later discovered to occur frequently in Bangalore (Karnataka, India), notably at highest densities (PDBC 2000; Mani and Krishnamoorthy 1999a). Geetha (2000) have recorded higher numbers of Cybocephalus sp. on cassava.
Axinoscymnus puttarudriahi, C. montrouzieri, C sexmaculata, C. nigrita and S. coccivora were the commonly found coccinellids on cassava in colonies of A. dispersus. A. puttarudriahi is found to be specific to A. dispersus and occurs all year round. A. puttarudriahi presence was previously recorded as a potential predator on A. dispersus in Sri Lanka (Wijesekera and Kudagamage 1990) and in Karnataka, India (Mani 2010). C. montrouzieri was found to be praying on A. dispersus and found to decrease in large numbers the population of A. dispersus to some extent. Mani and Krishnamoorthy (1997), Asia Mariam (1999) and Geetha (2000) found that in many areas of India, C. montrouzieri preyed on whitefly almost all year round. The abundance of C. nigrita on guava was reported earlier by Mani and Krishnamoorthy (1999b) and Geetha (2000).
Among the two parasitoids recorded, the most abundant parasitoids in Coimbatore were E. guadeloupae and E. meritoria. These two parasitoids might have been accidentally entered in India with the host insect (A. dispersus). They were found in Tamil Nadu and Karnataka parasitising A. dispersus nymphs on several host species. Earlier in Kerala (Beevi et al. 1999) and Karnataka (Srinivasa et al. 1999), similar parasitoid activity was reported. These two parasitoids are exotic and have never been reported in India on other whiteflies. The study of relative abundance of parasitoids in various locations of Tamil Nadu and Karnataka revealed that Coimbatore recorded more numbers of parasitoids and the least was documented from Tiruchirappalli. This variation in parasitoid abundance at a location may be due to local climate conditions and availability of host food. Host plants also affected parasitoid abundance. Parasitoids harbored more in cassava (56.7), cotton (45.7) and acalypha (43.0), while pigeon pea had less parasitoids (3.7). Survey in Thrissur (Kerala, India) found high levels of parasitism on banana, balsam, chilli, eggplant, guava, rubber, and tapioca (PDBC 2002). In this study, parasitism level was up to 58.8% and parasitoid emergence on acalypha was up to 89.7%. Earlier, Mani and Krishnamoorthy (2006) recorded E. guadeloupae parasitism in rose to 96%, Hibiscus to 86.45%, Poinsettia to 90.4% and Acalypha to 39.86%.
Given the widespread and severe incidence of exotic spiralling whitefly on a variety of host plants in India, this species is likely to pose a threat to the cultivation of economically important crops in India in the near future. Arthropod predators, Cybocephalus sp. and M. astur were the predominant predators of A. dispersus. E. guadeloupae and E. meritoria are expected to spread to even more places, resulting in a major decline in the A. dispersus population in India. In order to prevent further spread of A. dispersus, these four natural enemies, Cybocephalus sp., M. astur, E. guadeloupae and E. meritoria can be included in the IPM program for regulating the population of A. dispersus.
References
Aiswariaya, K. K., Manjunatha, M., & Naik, M. (2007). Biology and host range of spiralling whitefly. Karnataka J Agricul Sci, 20, 149–152.
Akinlosotu, T. A., Jackai, L. E. N., Nitonifor, N. N., Hassan, A. T., Agyakwa, G. W., Odfbiyi, J. A., Akingbohungbe, A. E., & Rossel, H. W. (1993). Spiralling whitefly, Aleurodicus dispersus in Nigeria. FAO Plant Prot Bull, 41, 127–129.
Alam, S., Islam, M. N., Alam, M. Z., & Islam, M. S. (1998). Effectiveness of three insecticides for the control of the spiralling whitefly (Aleurodicus dispersus Russell) of guava. Bangladesh J Entomol, 8, 53–58.
Asia Mariam (1999) Biology and management of spiralling whitefly Aleurodicus dispersus (Russell) (Homoptera: Aleyrodidae) on mulberry. M.Sc. (Ag.) Thesis, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India.
Asia Mariam, M., Douressamy, S., & Chandramohan, N. (2000). New hosts of spiralling whitefly (SWF) Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). Insect Environ, 6, 70.
Beevi, S. P., Lyla, K. R., & Vidya, P. (1999). Report of Encarsia (Hymenoptera: Aphelinidae) on spiralling whitefly Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). Insect Environ, 5, 44.
Blanco-Metzler, H., & Laprade, S. (1998). Natural enemies of the spiralling whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae): Parasitoids and Predators. Agron Mesoamer, 9, 41–44.
Boopathi T (2008) Monitoring and management of pest complex of fruits, vegetables and spices in Mizoram. In: Annual report of ICAR Research Complex for NEH Region 2008–2009. ICAR Research Complex for NEH Region, Umiam, Meghalaya. p289.
Boopathi T (2013) Biological control and molecular characterization of spiralling whitefly, Aleurodicus dispersus Russell on cassava and brinjal. Ph.D. Thesis, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India.
Boopathi T, Karuppuchamy P, Kalyanasundaram M, Mohankumar S, Ravi M (2013) Pathogenicity, ovicidal action, and median lethal concentrations (LC50) of entomopathogenic fungi against exotic spiralling whitefly, Aleurodicus dispersus Russell. J Path 1–7. https://doi.org/10.1155/2013/393787.
Boopathi, T., Karuppuchamy, P., Kalyanasundaram, M., Mohankumar, S., Ravi, M., & Singh, S. B. (2014a). Effects of botanicals, fish oil rosin soap and organic salt on eggs of spiralling whitefly, Aleurodicus dispersus. Indian J Plant Prot, 42, 86–88.
Boopathi, T., Karuppuchamy, P., Kalyanasundaram, M., Mohankumar, S., Ravi, M., & Singh, S. B. (2014b). Toxicity of newer insecticides against spiralling whitefly, Aleurodicus dispersus under laboratory conditions. Indian J Plant Prot, 42, 178–180.
Boopathi T, Mohankumar S, Karuppuchamy P, Kalyanasundaram M, Ravi M, Breetha P, Aravintharaj R (2014c) Genetic evidence for diversity of spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) populations in India. Florida Entomologist 97: =1115–1122. https://doi.org/https://doi.org/10.1653/024.097.0318.
Boopathi, T., Karuppuchamy, P., Kalyanasundaram, M., Mohankumar, S., Ravi, M., & Singh, S. B. (2015a). Microbial control of the exotic spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) on eggplant using entomopathogenic fungi. African Journal of Microbiology Research, 9, 39–46.
Boopathi, T., Karuppuchamy, P., Singh, S. B., Kalyanasundaram, M., Mohankumar, S., & Ravi, M. (2015b). Microbial control of the invasive spiraling whitefly on cassava with entomopathogenic fungi. Brazilian J Microbiol, 46, 1077–1085. https://doi.org/10.1590/S1517-838246420141067
Boopathi T, Singh SB, Ravi M, Manju T (2016) Distribution and biology of Mallada desjardinsi (Neuroptera: Chrysopidae) in India and its predatory potential against Aleurodicus dispersus (Hemiptera: Aleyrodidae). J Eco Entomol 109:1988–1994. https://doi.org/https://doi.org/10.1093/jee/tow154.
Boopathi, T., Mohankumar, S., Karuppuchamy, P., Singh, S. B., Ravi, M., Preetha, B., Aravindraj, R., & Manju, T. (2017a). SSR markers based identification of genetic variability of spiraling whitefly, Aleurodicus dispersus populations in Tamil Nadu, India. Indian Journal of Biotechnology, 16, 276–282 http://nopr.niscair.res.in/handle/123456789/43340
Boopathi, T., Sankari Meena, K., Ravi, M., & Thirunavukarasu, K. (2017b). Impact of insecticides on spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae) and its natural enemy complex in cassava under open field conditions. Crop Protection, 94, 137–143. https://doi.org/10.1016/j.cropro.2016.12.021
Boopathi, T., Mohankumar, S., Gayacharan, Kalyanasundaram, M., Singh, S. B., Aravintharaj, R., Preetha, B., Sankari Meena, K., & Chandrasekar, K. (2019). Host-based genetic divergence in populations of an exotic spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae). Euro J Entomol, 116, 221–228. https://doi.org/10.14411/eje.2019.024
Charati, S. N., Pokharkar, D. S., & Ghorpade, S. A. (2003). Abundance of spiralling whitefly, a newly introduced pest in Maharashtra State. J Maharashtra Agricul Univer, 28, 83–84.
David BV, Regu K. (1995) Aleurodicus dispersus Russell (Aleyrodidae: Homoptera), a whitefly pest new to India. Pestology 19:5–7.
Francis, A. W., Stocks, I. C., Smith, T. R., Boughton, A. J., Mannion, C. M., & Osborne, L. (2016). Host plants and natural enemies of rugose spiralling whitefly (Hemiptera: Aleyrodidae) in Florida. Florida Entomologist, 99, 150–153.
Gajendra Babu, B., & David, P. M. M. (1999). New host plant records and host range of the spiralling whitefly, Aleurodicus dispersus Russell. (Hemiptera: Aleyrodidae). Madras Agricul. J., 86, 305–313.
Geetha B (2000) Biology and management of spiralling whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). Ph.D. Dissertation, Department of Agricultural Entomology, Centre for Plant Protection Studies, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India.
Geetha, B., Loganathan, M., & Swamiappan, M. (1998). Record of spiralling whitefly, Aleurodicus dispersus Russell on groundnut. Insect Environ, 4, 55.
Geetha B, Swamiappan M (2001) Host range and natural enemies of spiralling whitefly, Aleurodicus dispersus in Tamil Nadu. In: National seminar on emerging trends pests and diseases management. Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India. pp. 11.
Geetha, B., Swamiappan, M., & Loganathan, M. (1999). New hosts for spiralling whitefly, Aleurodicus dispersus Russell in Tamil Nadu. Insect Environ., 5, 80.
Kumashiro, B. R., Lai, P. Y., Funasaki, G. Y., & Teramoto, K. K. (1983). Efficacy of Nephaspis amnicola and Encarsia haitiensis in controlling Aleurodicus dispersus in Hawaii. Proceedings of the Hawaiian Entomological Society, 24, 261–269.
Lambkin, T. A. (1999). A host list for Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae) in Australia. Australian J Entomol, 38, 373–376.
Mallappanavar MC (2000) Bioecology and management of spiralling whitefly Aleurodicus dispersus Russell by Verticillium lecanii (Zimm.) on guava. M.Sc. (Ag.) Thesis, University of Agricultural Sciences, Dharwad, Karnataka, India.
Mani, M. (2010). Origin, introduction, distribution and management of the invasive spiralling whitefly Aleurodicus dispersus Russell in India. Karnataka J Agricul Sci, 23, 59–75.
Mani M, Dinesh MS, Krishnamoorthy A (2000) Biological control studies on the spiralling whitefly, Aleurodicus dispersus Russell. (Aleyrodidae: Homoptera). In: Entomology Congress 2000, Trivandrum, Kerala, India.
Mani, M., & Krishnamoorthy, A. (1997). Discovery of Australian ladybird beetle (Cryptolaemus montrouzieri) on spiralling whitefly (Aleurodicus dispersus) in India. Insect Environ, 3, 5–6.
Mani, M., & Krishnamoorthy, A. (1999a). Natural enemies and host plants of spiralling whitefly Aleurodicus dispersus Russell (Homoptera: Aleyrodidae) in Bangalore, Karnataka. Entomon, 24, 5–80.
Mani M, Krishnamoorthy A (1999b) Predatory potential and development of Australian ladybird beetle, Cryptolaemus montrouzieri Muls. on the spiralling whitefly, Aleurodicus dispersus Russell. Entomon 24:166–171.
Mani M, Krishnamoorthy A (2006) Colonization of introduced parasitoid, Encarsia guadeloupae Viggiani, on the exotic spiralling whitefly, Aleurodicus dispersus Russell, infesting ornamentals, J Horti Sci 1:148–151.
Mani, M., Krishnamoorthy, A., Venugopalan, R., & Pattar, G. L. (2004). Biological control of exotic spiralling whitefly Aleurodicus dispersus Russell on guava by Encarsia haitiensis Dozier and Encarsia guadeloupae Viggiani in India. Pest Manage Horticul Ecosys, 10, 29–39.
Mani, M., Ragunatha, R., Dinesh, M. S., & Krishnamoorthy, A. (2001). Spiralling whitefly Aleurodicus dispersus Russell in Hyderabad. Insect Environ, 7, 82.
Montiero AHRR (2004) Introduction of Aleurodicus dispersus (Russell, 1965) (Hemiptera: Aleyrodidae), in Cape Verde: molecular characterization, host range and phytosanitary measures. Dissertation presented the University of Brasilia during August 2004.
Mound LA, Halsey SH (1978) Whitefly of the world. A systematic catalogue of the Aleyrodidae (Homoptera) with host plant and natural enemy data. British Museum (Natural History). John Wiley & Sons, London. 340p.
Muniappan R (1996) Spiralling whitefly threat. The Hindu, dated 16 March 1996, p12.
Muralikrishna M (1999) Bioecology, host range and management of spiralling whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae). M.Sc. (Ag.) Thesis, University of Agricultural Sciences, Bengaluru, Karnataka, India.
Nechols JR (1982) Entomology: biological control. Annual Report 1982. Guam Agricultural Experimental Station, 33–49.
Palaniswami, M. S., Pillai, K. S., Nair, R. R., & Mohandas, C. (1995). A new cassava pest in India. Cassava Newslett, 19, 6–7.
PDBC (2000) Annual Report of 1999–2000. Project Directorate of Biological Control, Banglaore, Karnataka, India. 216p.
PDBC (2002) Annual Report of 2001–2002. Project Directorate of Biological Control, Banglaore, Karnataka, India. 221p.
Prathapan, K. D. (1996). Outbreak of the spiralling whitefly, Aleurodicus dispersus Russell (Aleyrodidae, Homoptera) in Kerala. Insect Environ, 2, 36–37.
Ramani, S. (2000). Fortuitous introduction of an aphelinid parasitoid of the spiralling whitefly, Aleurodicus dispersus Russell (Homoptera: Aleyrodidae), in to the Lakshadweep Islands with notes on host plants and other natural enemies. J Biol Cont, 14, 55–60.
Ranjith AM (1998) Mealy whitefly, pest with a wide host range. The Hindu, Dated 29January 1998, p4.
Ranjith, A. M., Rao, D. S., & Thomas, J. (1996). New host records of the "Mealy Whitefly", Aleurodicus dispersus Russell in Kerala. Insect Environ, 2, 35–38.
Russell, L. M. (1965). A new species of Aleurodicus Douglas and two close relatives. Florida Entomologist, 48, 47–55.
Srinivasa, M. V. (2000). Host plants of the spiralling whitefly, Aleurodicus dispersus (Hemiptera: Aleyrodidae). Pest Manage Horticul Ecosys, 6, 79–105.
Srinivasa, M. V., Viraktamath, C. A., & Reddy, C. (1999). A new parasitoid of the spiralling whitefly Aleurodicus dispersus Russell (Hemiptera: Aleyrodidae) in South India. Pest Manage Horticul Ecosys, 5, 59–61.
Statistical Analysis System (SAS®) (2011) SAS® 9.3 System options: reference, second edition. SAS Institute Inc., SAS Campus Drive, Cary, North Carolina.
Waterhouse DF, Norris KR (1989) Aleurodicus dispersus Russell. In Biological Control: Pacific Prospects - Supplement 1, ACIAR Monograph No.12, ACIAR, Canberra, Australia. pp. 13–22.
Wen HC, Hsu TC, Chen CN (1995) Yield loss and control of spiralling whitefly (Aleurodicus dispersus Russell). J. Agricul. Res., China 44:147–156.
Wijesekera, G. A. W., & Kudagamage, C. (1990). Life history and control of 'spiralling' white fly Aleurodicus dispersus (Homoptera: Aleyrodidae): fast spreading pest in Sri Lanka. Quarterly Newslett. Asia and Pacific Plant Protect. Comm., 33, 22–24.
Acknowledgements
The author is grateful to the Director, Centre for Plant Protection Studies, Tamil Nadu Agricultural University, Coimbatore, Tamil Nadu, India and the Professor and Head, Department of Agricultural Entomology for providing the facilities and support. I am very thankful to Dr. Prasad, Associate Professor, Andhra Pradesh, India; Dr. K. Rajasekar, Scientist (Soil Science), ICAR Research Complex for NEH Region, Mizoram Centre, Kolasib, Mizoram, India; Dr. A.N. Shylesha, Principal Scientist, ICAR-NBAIR, Bengaluru, Karnataka, India; Dr. U. Amala, Scientist (Agricultural Entomology), ICAR-NRC on Grapes, Pune, Maharashtra, India; Dr. Anjitha Alexander, Scientist (Agricultural Entomology), ICAR-NRC on Citrus, Nagpur, Maharashtra, India; Mr. Amitosh, RA, ICAR Research Complex for NEH Region, Mizoram Centre, Kolasib, Mizoram, India; and Dr. T. Nagaraja, Ph.D. Scholar, Bengaluru, Karnataka, India for providing samples and also for the help rendered during the survey. The support given to recording the observations by Dr. Govindaraj (Seed Science and Technology), Mr. Govindaraj (Entomology), Mr. Arulnayagam, Mr. Muthumariaapan, and Mr. Mani is recognized.
Availability of Data and Material
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boopathi, T. New host plants, natural enemy complex and newly distributed potential areas of exotic spiralling whitefly (Hemiptera: Aleyrodidae) in India. Phytoparasitica 50, 335–357 (2022). https://doi.org/10.1007/s12600-021-00969-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-021-00969-x