Abstract
The defoliating caterpillar Sarsina violascens causes economic losses in Brazilian Eucalyptus crops. Information on the susceptibility of Eucalyptus species and hybrids to insect pests is scarce. As such, this study aimed to assess the development of S. violascens on Eucalyptus species and hybrids under laboratory conditions. Neonate caterpillars were reared on leaves of Eucalyptus grandis, E. urophylla, E. camaldulensis and E. globulus; and hybrids of E. urophylla x E.urophylla, E. urophylla × E. grandis, E. grandis × E. camaldulensis and E. urophylla × E. camaldulensis. Biological parameters such as instar and larval phase duration, pupal weight, adult longevity and fecundity, and eclosion rate were evaluated. Eucalyptus globulus is not an S. violascens host, leading to 100% larval mortality in the first instar. Eucalyptus grandis extended the larval phase of the insect and decreased larval survivorship and female pupal weight, resulting in high levels of deformity in adults and low egg eclosion, in addition to exhibiting significant antibiosis to S. violascens. Eucalyptus camaldulensis and VM-1 extended the larval period and reduced pupal weight. Eucalyptus urophylla was better suited to the development and reproduction of S. violascens than other Eucalyptus species and hybrids. E. grandis is a promising source of resistance to S. violascens. The use of resistant species and hybrids contributes to pest management, reducing the need for chemical control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eucalyptus (Myrtaceae) is one of the most planted genera in the global forest sector, mainly because of its species’ rapid growth and adaptability to different environmental conditions (Campinhos, 1999; Wingfield et al., 2008). Commercial forest crops in Brazil occupy 7.83 million ha, providing pulp, timber, charcoal, rubber, resin, and livestock shading. Eucalyptus species and hybrids are the country’s most cultivated trees, accounting for 75% of Brazilian forest crops and producing about 36.0 m3/ha/year in 2018 (IBGE, 2017; IBA, 2019).
Caterpillars such as Sarsina violascens (Herrich-Schaeffer, 1856) (Lymantriidae), Thyrinteina arnobia (Stoll, 1782) (Geometridae), Eupseudosoma aberrans (Schaus, 1905) (Arctiidae), Eupseudosoma involute (Sepp, 1852) (Arctiidae) and Sabulodes caberata (Guenée, 1857) (Geometridae) (among other species of the families Geometridae, Arctiidae and Lymantriidae) are important pests in Eucalyptus plantations due to their severe defoliating capacity (Kowalczuck et al., 2012; Zanuncio et al., 1992b, 2006, 2016). Commonly known as the purple moth, Sarsina violascens is a native species from Argentina, Brazil, and Mexico (FAO, 2009). It feeds on several Myrtaceae species present in Brazilian vegetation and, with the introduction of Eucalyptus, expanded from these native species to Eucalyptus (Paine et al., 2011; Zanuncio et al., 2013). Leaf feeding by insects reduces wood production (Batista-Pereira et al., 2006).
Sarsina violascens is commonly associated with Eucalyptus (Zanuncio et al., 2006, 2018; Bernardi et al., 2011; Kowalczuck et al., 2012, Garlet et al., 2016, Ribeiro et al., 2016). The caterpillar attacks several species of Eucalyptus (Eucalyptus grandis, E. saligna, E. camaldulensis, E. urophylla, E. cloeziana and E. nesophylla) and Corymbia citriodora (Berti Filho, 1983; Zanuncio, 1993; Zanuncio et al., 1992a). Depending on the species of the Eucalyptus host, a S. violascens larvae can consume a leaf area of approximately 249.74 cm2 (Zanuncio et al., 1992a) during its complete development. Management of defoliating caterpillars in Eucalyptus crops is achieved mainly with insecticides (Zanuncio et al., 1993; Guedes et al., 2000) and the use of beneficial insects such as parasitoids (Oliveira et al., 2008; Pereira et al., 2008) and predators (Soares et al., 2009). Resistant Eucalyptus species and hybrids are another alternative to control phytophagous insects, including borers (Hanks et al., 1991), the bronze bug (Soliman et al., 2012), red gum lerp psyllid (Pereira et al., 2013), and caterpillars such as T. arnobia (Jesus et al., 2015).
Antibiosis involves both chemical and morphological plant defenses that adversely affect insect biology (Smith, 2005), influencing the survival, development, and reproduction of insects and their progeny. Effects on insects include high mortality of early instars, decreased size and/or weight, longer immature phase, and reduced adult longevity and fecundity (Padmaja, 2016; Smith, 2005; Smith & Clement, 2012). The use of plant genotypes that exhibit this resistance mechanism contributes to limiting the development and size of subsequent insect populations (Baldin et al., 2019). Despite the large number of studies in the literature that investigate Eucalyptus resistance or tolerance of different phytophagous insects, the use of species or hybrids potentially resistant to S. violascens has been poorly studied.
Planting resistant Eucalyptus species and/or hybrids may be an effective strategy to control insect pests, thereby lowering wood production costs. The present study aimed to evaluate several biological parameters of S. violascens on four species and five hybrids of Eucalyptus and identify the possible expression of antibiosis as a resistance mechanism.
Materials and methods
The Sarsina violascens caterpillars used in this study originated from a colony reared on the leaves of Eucalyptus hybrid 519 (E. urophylla × E. grandis). To establish the insect colony, S. violascens caterpillars were collected from clonal Eucalyptus crops in Alagoinhas, Bahia state, Brazil, and kept in covered plastic containers (4.5 cm in diameter and 6 cm high, with a volume of 80 mL) until the pupal phase. After emergence, the adults were placed in cylindrical PVC cages, 10 cm in diameter and 20 cm high, sealed at the top and bottom with Petri dishes and lined internally with silk paper. The eggs were removed and maintained in plastic containers until the caterpillars hatched.
A non-choice test was performed using neonate S. violascens caterpillars subjected to nine treatments: four Eucalyptus species (E. grandis, E. urophylla, E. camaldulensis and E. globulus) and five hybrids (E. urophylla x E.urophylla (117–20), E. urophylla × E. grandis (H-13 and 1404), E. grandis × E. camaldulensis (1277) and E. urophylla × E. camaldulensis (VM-1)). Each treatment was applied to thirty caterpillars. Each experimental unit consisted of a single caterpillar in a plastic container. Adult leaves were washed under running water, dried and then offered to the insects. The leaves were replaced with fresh leaves every two days. The plastic containers containing the leaves and caterpillars were stored in acclimatized rooms (12:12 L:D, 26 ± 2 °C; 60 ± 10% RH). A completely randomized design was used and each caterpillar considered one repetition (thirty repetitions per treatment, totalling 270 caterpillars).
Insect development was monitored daily. In the larval stage, larval survivorship (ratio of live/total individuals per treatment), instar duration (number of days the caterpillars remained in each instar), and number of instars (presence of exuviae in the containers) were assessed. At the end of this phase, the pupae were weighed (24 h after pupation), sexed and individually placed in plastic containers with the bottom lined with filter paper to maintain moisture.
Finally, recently emerged adults from each treatment were split in pairs (1 male and 1 female), to obtain eggs. Each pair was placed in one carton cage (10 cm in diameter and 20 cm high) sealed at the top and bottom with Petri dishes and lined with silk paper. A container with cotton soaked in a 10% aqueous honey solution was placed inside cages as food supply and replenished daily to prevent contamination or fermentation by microorganisms. Variables assessed in the adult stage were longevity, percentage of individuals with deformities (live individuals with wing curling or wings that did not fully expand), number of eggs per female and their eclosion rate.
The independent variables were tested for residual normality and homogeneity of variance using Kolmogorov–Smirnov and Levene’s tests, respectively. Data on pupal weight, adult longevity and fecundity, and eclosion rate met these assumptions. Next, one-way ANOVA was carried out, followed by Tukey’s test for comparison of the means. Data that did not meet the assumptions of residual normality and homogeneity of variance, such as larval survivorship, instar and larval phase duration, and adult deformity, were compared using the Generalized Linear Model (GLM) with binomial distribution (proportion) or Poisson distribution (count). GLM tests were followed by pairwise comparisons. All statistical analyses were performed using R software (R Development Core Team, 2019).
Results
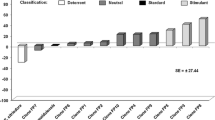
Feeding on different Eucalyptus treatments affected S. violacens development. In all treatments, larval mortality remained under 25%, irrespectively of the eucalyptus species, or of the hybrids used, except for E. globulus, which caused 100% mortality of first instar S. violascens caterpillars (χ2 = 132; df = 8, P < 0.01) (Fig. 1). The duration of the first to third instar was shorter on E. urophylla and generally longest on E. grandis (except for the third instar, where the duration was slightly longer in two of the other treatments). These differences were significant when compared to some, but not all, treatments (First instar: χ2 = 44.79; df = 7; P < 0.01, Second instar: χ2 = 19.42; df = 7; P < 0.01, Third instar: χ2 = 18.00; df = 7; P < 0.01) (Table 1). The fourth, fifth and sixth instars did not differ between the Eucalyptus material (fourth: χ2 = 8.06; df = 7; P = 0.33, fifth: χ2 = 5.01; df = 7; P = 0.66, six: χ2 = 12.30; df = 7; P = 0.09) (Table 1). Only those reared on E. grandis, E. camaldulensis, and VM-1 leaves reached the seventh instar, with no significant difference in its duration between treatments (χ2 = 2.60; df = 2; P = 0.27). The number of instars varied between five and eight, with only one caterpillar fed on E. grandis leaves reaching this instar. Insects that consumed E. urophylla leaves had the shortest mean larval period duration, followed by 1404, H-13, 117–20, and 1277. Eucalyptus grandis, E. camaldulensis, and hybrid VM-1 prolonged the larval phase of S. violascens (χ2 = 169.23; df = 7; P < 0.01) (Table 1).
Larval mortality of Sarsina violascens when reared on Eucalyptus species and hybrids (12:12 L:D; 26 ± 2 °C; 60 ± 10% RH). Parental hybrids: 117–20 (E. urophylla), 1277 (E. grandis × E. camaldulensis), VM-1 (E. urophylla × E. camaldulensis), H-13, and 1404 (E. urophylla × E. grandis). Bars followed by the same lowercase letter do not differ significantly (GLM with Binomial distribution (P ≥ 0.05), followed by pairwise comparisons)
Regarding weight, male pupae weighed less when fed on VM-1, E. camaldulensis, and E. grandis and more when fed on hybrids 1404, 117–20, and E. urophylla leaves (F = 20.51; df = 7, 101; P < 0.01). A similar trend was observed for female pupae, which were lighter in the VM-1, E. camaldulensis, and E. grandis treatments and heavier for hybrid 1404 (F = 30.64; df = 7, 96; P < 0.01) (Table 2).
Adult deformity was significantly higher for insects fed on E. grandis, differing from all treatments, except to H-13; and followed by 1404, 117–20, and 1277 (χ2 = 35.25; df = 7; P < 0.01) (Fig. 2). Deformed adults were not observed for E. urophylla, E. camaldulensis, and VM-1 treatments. The highest longevity was observed for E. urophylla, differing significantly from E. camaldulensis, E. grandis, 117–20, H-13 and VM-1 (F = 3.59; df = 7; P < 0.01) (Table 2).
Adult deformity of Sarsina violascens fed on Eucalyptus species and hybrids (12:12 L:D; 26 ± 2 °C; 60 ± 10% RH). Parental hybrids: 117–20 (E. urophylla), 1277 (E. grandis × E. camaldulensis), VM-1 (E. urophylla × E. camaldulensis), H-13, and 1404 (E. urophylla × E. grandis). Bars followed by the same lowercase letter do not differ significantly (GLM with Binomial distribution (P ≥ 0.05), followed by pairwise comparisons)
Oviposition of S. violascens was significantly higher in treatments 1404, E. urophylla, and 1277 (F = 2.35; df = 7, 77; P < 0.05) than on treatments E. grandis and H-13 (Table 3). Successful egg eclosion was higher among females reared on VM-1 significantly different only from those fed on E. grandis leaves (F = 2.25; df = 7, 77; P < 0.05) (Table 3).
Discussion
Although S. violascens is commonly associated with Eucalyptus (Bernardi et al., 2011; Kowalczuck et al., 2012; Ribeiro et al., 2016), few studies have investigated how different species and hybrids can affect its development and survival. Eucalyptus grandis prolonged the larval phase, reduced the weight of female pupae, and produced more deformed adults and lower eclosion rate. Eucalyptus camaldulensis and VM-1 prolonged the larval cycle and reduced the pupal weight of males and females. These treatments were unfavorable to the development of S. violascens. By contrast, E. urophylla favor its development based on a shorter larval period, lower number of larval instars, high adult longevity and fecundity.
In general, the choice and establishment of phytophagous insects in eucalyptus is related to the presence of secondary compounds (Branco et al., 2019). Possibly such substances may be related to the unfavorable development of S. violacens in the species E. grandis and E. camaldulensis. Similarly, E. camaldulensis showed resistance to defoliation by coleopteran Anoplognathus spp. (Leach) (Edwards et al., 1993), but E. grandis was classified as highly susceptible to T. arnobia, another important Eucalyptus defoliator caterpillar (Jesus et al., 2015). Eucalyptus urophylla was best suited to S. violascens development, as observed for Hylesia paulex, a lepidopteran from the family Saturniidae (Pereira et al., 2009).
Our findings show that E. globulus is an unsuitable host for S. violascens larvae, while species such as E. urophylla favor its development mostly due to a shorter larval period and higher adult longevity. We observed 100% of S. violascens mortality for larvae fed on E. globulus leaves. For autumn gum moth larvae Mnesampela privata (Guenée, 1857), the high tannin levels of E. globulus leaves have been associated with reduced survival (Rapley et al., 2008). These authors suggested that tannins act as toxins and/or antifeedants for the larval phase of M. privata. Additionally, wax compounds are indicated as a possible source of E. globulus resistance to M. privata (Jones et al., 2002).
Caterpillars reared on E. grandis, E. camaldulensis, and VM-1 had more instars than immature individuals fed on other materials. The variation in the number of S. violascens instars demonstrates that some Eucalyptus species and hybrids do not favor the insect’s development. Moreover, lengthening of the developmental phases of the insect or its cycle may indicate that the plant has an unfavorable effect on its biology, characterizing antibiosis-type resistance. However, this may also be related to the non-feeding preference, due to low levels of food intake by the insect (Baldin et al., 2019).
Caterpillars fed with E. grandis and E. camaldulensis leaves produced the lightest female pupae, and females reared on E. grandis and H-13 produced fewer eggs. In general, the weight and/or size of females is positively correlated with egg production (Haukioja & Neuvonen, 1985; Marshall, 1990; Tammaru, 1998). However, only for 1404 we observed higher weight of female pupae and higher fertility.
Eucalyptus urophylla and hybrids 1277 and 1404 produced females with the highest fecundity. These values are higher than that reported previously for S. violascens, namely approximately 150 eggs per female (Nascimento et al., 2000; Zanuncio et al., 1992b). Unexpectedly, eggs on E. urophylla exhibited a lower eclosion rate than that observed by Zanuncio et al. (1992b) (72.45%) on the same species. A lower eclosion rate was recorded for eggs laid by females reared on E. grandis leaves, less than half the 49.5% reported by Nascimento et al. (2000) on the same species.
Interestingly, hybrid 1277 (between E. grandis and E. camaldulensis) favored larval development, while its parental species had a negative effect on the larval stage of S. violascens. Hybrids seem to be more susceptible to pests than pure species. Therefore, intense selection to obtain elite hybrids is important (Potts & Dungey, 2004). Species E. grandis and E. urophylla and their hybrids (E. grandis x E. urophylla) comprise most Eucalyptus forest crops in Brazil (Assis et al., 2015). Eucalyptus hybrids are important in forest crops, particularly in subtropical and tropical regions, since clonal propagation is frequently used.
Plant resistance to insects is an alternative strategy to decrease pest populations and can be adopted in conjunction with other control techniques, contributing to reducing insect infestation in the field. The current trend is sustainable pest management, seeking to limit the use of chemical insecticides in forestry. The results obtained here may be useful in breeding programs focused on developing Eucalyptus resistant to S. violascens.
References
Assis, T. F., Abad, J. I. M., & Aguiar, A. M. (2015). Melhoramento genético do eucalipto. Informe Agropecuário Belo Horizonte (Brazil), 18, 32–51.
Baldin, E. L. L., Vendramim, J. D., & Lourenção, A. L. (2019). Resistência de plantas a insetos: fundamentos e aplicações. Fealq, 493 p.
Batista-Pereira, L. G., Fernandes, J. B., Corrêa, A. G., Silva, F., & Vieira, P. C. (2006). Electrophysiological responses of eucalyptus brown looper Thyrinteina arnobia to essential oils of seven Eucalyptus species. Journal Brazilian Chemical Society, 17(3), 555–561.
Branco, S., Mateus, E. P., Silva, M. D. R. G., Mendes, D., Pereira, M. M. A., Schutz, S., & Paiva, M. R. (2019). Identification of pheromone candidates for the eucalyptus weevil, Gonipterus platensis (Coleoptera, Curculionidae). Journal Applied Entomology, 144, 41–53.
Bernardi, O., Garcia, M. S., Silva, E. J. E., Zazycki, L. C. F., Bernardi, D., & Finkenauer, É. (2011). Levantamento populacional e análise faunística de Lepidoptera em Eucalyptus spp. no município de Pinheiro Machado, RS. Ciência Florestal, 21(4), 735–744.
Berti Filho, E. (1983). Insetos da Ordem Lepidoptera associados com Eucalyptus spp. no Brasil. Silvicultura, 8(32), 623–624.
Campinhos, E. (1999). Sustainable plantations of high-yield shape Eucalyptus trees for production of fiber: the Aracruz case. New Forests, 17, 129–143.
Edwards, P. B., Wanjura, W. J., & Brown, W. V. (1993). Selective herbivory by Christmas beetles in response to intraspecific variation in Eucalyptus terpenoids. Oecologia, 95(4), 551–557.
FAO. (2009). Global review of forest pests and diseases. FAO Forestry Paper – Food and Agriculture Organization of the United Nations. [on line] Available from: http://www.fao.org/docrep/011/i0640e/i0640e00.htm. Accessed 3 Oct 2020.
Garlet, J., Costa, E. C., & Boscardin, J. (2016). Levantamento da entomofauna em plantios de Eucalyptus spp. por meio de armadilha luminosa em São Francisco de Assis-RS. Ciência Florestal, 26(2), 365–374.
Guedes, R. N. C., Zanuncio, T. V., Zanuncio, J. C., & Medeiros, A. G. B. (2000). Species richness and fluctuation of defoliator Lepidoptera populations in Brazilian plantations of Eucalyptus grandis as affected by plant age and weather factors. Forest Ecology Management, 137, 179–184.
Hanks, L. M., Paine, T. D., & Milla, J. G. (1991). Mechanisms of resistance in Eucalyptus against larvae of the Eucalyptus longhorned borer (Coleoptera: Cerambycidae). Environmental Entomology, 20, 1583–1588.
Haukioja, E., & Neuvonen, S. (1985). The relationship between size and reproductive potential in male and female Epirrita autumnata (Lepidoptera, Geometridae). Ecological Entomology, 10(3), 267–270.
IBA - The Brazilian Tree Industry. (2019). Relatório 2019. Available from: https://iba.org/eng/datafiles/publicacoes/relatorios/relatorioiba2019-final.pdf. Accessed 29 Sept 2020.
IBGE – INSTITUTO BRASILEIRO DE GEOGRAFIA E ESTATÍSTICA. (2017). Diretoria de Pesquisas. Coordenação de Agropecuária. Produção da extração vegetal e silvicultura. Rio de Janeiro. Available from: https://agenciadenoticias.ibge.gov.br/media/com_mediaibge/arquivos/15f538e9095614fc3204f828b22fa714.pdf. Accessed 29 Sept 2020.
Jesus, F. G., Nogueira, L., Boiça-Junior, A. L., Ribeiro, Z. A., Araújo, M. S., & Zanuncio, J. C. (2015). Resistance of Eucalyptus spp. genotypes to Eucalyptus brown looper Thyrinteina arnobia (Lepidoptera: Geometridae). Australian Journal of Crop Science, 9, 1016–1021.
Jones, T. H., Potts, B. M., Vaillancourt, R. E., & Davies, N. W. (2002). Genetic resistance of Eucalyptus globulus to autumn gum moth defoliation and the role of cuticular waxes. Canadian Journal of Forest Research., 32(11), 1961–1969.
Kowalczuck, M., Carneiro, E., Casagrande, M. M., & Mielke, O. H. H. (2012). The Lepidoptera associated with forestry crop species in Brazil: a historical approach. Neotropical Entomology, 41, 345–354.
Marshall, L. D. (1990). Regulation of meal size and growth of fifth instar nymphs of Locusta migratoria (Orthoptera, Acrididae) in different conditions of starvation and temperature. Annales Nutrition Et De La Alimentation, 31, 85–90.
Nascimento, M. L., Wilcken, C. F., Ottati, A. L. T., & Orlato, C. (2000). Biologia de Sarsina violascens Herrich-Schaeffer, 1856 (Lepidoptera: Lymantriidae) em Eucalyptus grandis. Floresta, 30(1–2), 176.
Oliveira, H. N., Zanuncio, T. V., Zanuncio, J. C., & Serrão, J. E. (2008). The eucalypt defoliator Thyrinteina arnobia (Lepidoptera: Geometridae) protects its eggs from parasitism a novel mechanism of a Eucalypt defoliator to avoid parasitism of its eggs. Biological Letters, 45, 23–28.
Padmaja, P. G. (2016). Insect pest resistance in sorghum. In I. K. Das & P. G. Padmaja (Eds.), Biotic stress resistance in millets (pp. 105–145). Academic.
Paine, T. D., Lawson, S. A., & Steinbauer, M. J. (2011). Native and exotic pests of eucalyptus: a worldwide perspective. Annual Review of Entomology, 56, 181–201.
Pereira, F. F., Zanuncio, J. C., Tavares, M. T., Pastori, P. L., Jacques, G. C., & Vilela, E. F. (2008). New record of Trichospilus diatraeae as a parasitoid of the eucalypt defoliator Thyrinteina arnobia in Brazil. Phytoparasitica, 36(3), 304–306.
Pereira, A. I. A., Zanuncio, V. V., Lorenzon, A. S., Bolognani, H., Fernandes, B. V., Mielke, O. H. H., et al. (2009). Biological and morphological characteristics of Hylesia paulex (Lepidoptera: Saturniidae) fed with Eucalyptus urophylla (Myrtaceae). Interciencia, 34(9), 645–649.
Pereira, J. M., Baldin, E. L. L., Soliman, E. P., & Wilcken, C. F. (2013). Attractiveness and oviposition preference of Glycaspis brimblecombei Moore in Eucalyptus spp. Phytoparasitica, 41, 117–124.
Potts, B. M., & Dungey, H. S. (2004). Interspecific hybridization of Eucalyptus: key issues for breeders and geneticists. New Forests, 27, 115–138.
R Development Core Team. (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.r-project.org/. Accessed 19 Nov 2019.
Rapley, L. P., Allen, G. R., Potts, B. M., & Davies, N. W. (2008). Constitutive or induced defences – how does Eucalyptus globulus defend itself form larval feeding? Chemoecology, 17, 235–243.
Ribeiro, G. T., Zanuncio, J. C., de S tavares, W., de S Ramalho, F., & Serrão, J. E. (2016). Constancy, distribution, and frequency of Lepidoptera defoliators of Eucalyptus grandis and Eucalyptus urophylla (Myrtaceae) in four Brazilian regions. Neotropical Entomology, 45(6), 629–636.
Smith, C. M. (2005). Plant resistance to arthropods: molecular and conventional approaches (p. 423). Springer.
Smith, C. M., & Clement, S. L. (2012). Molecular bases of plant resistance to arthropods. Annual Review of Entomology, 57, 309–328.
Soliman, E. P., Wilcken, C. F., Pereira, J. M., Dias, T. K. R., Zaché, B., Dal Pogetto, M. H. F. A., & Barbosa, L. R. (2012). Biology of Thaumastocoris peregrinus in different eucalyptus species and hybrids. Phytoparasitica, 40, 223–230.
Soares, M. A., Zanuncio, J. C., Leite, G. L. D., Wermelinger, E. D., & Serrão, J. E. (2009). Does Thyrinteina arnobia (Lepidoptera: Geometridae) use different defense behaviours against predators? Journal of Plant Diseases and Protection, 116(1), 30–33.
Tammaru, T. (1998). Determination of adult size in a folivorous moth: constraints at instar level? Ecological Entomology, 23(1), 80–89.
Wingfield, M. J., Slippers, B., Hurley, B. P., Coutinho, T. A., Wingfield, B. D., & Roux, J. (2008). Eucalypt pests and diseases: growing threats to plantation productivity. Southern Forests: a Journal of Forest Science, 70(2), 139–144.
Zanuncio, J. C. (1993). Manual de pragas em florestas. Lepidoptera desfolhadores de eucalipto: biologia, ecologia e controle. IPEF/SIF.
Zanuncio, J. C., Fagundes, M., Araújo, M. S. S., & Evaristo, F. D. C. (1992a). Monitoramento de lepidópteros, associados a plantios de eucalipto da região de Açailândia (Maranhão), no período de agosto/90 a julho/91. Acta Amazônica, 22(4), 615–622.
Zanuncio, J.C., Guedes R.N.C., Garcia J.F. and Rodrigues L.A. (1993). Impact of two formulations of deltamethrin in aerial application against Eucalyptus caterpillars and theirs predaceous bugs. Medical Faculteit Landbouwkundige, 58(2), 469–475.
Zanuncio, J. C., Santos, G. P., Saraiva, R. S., & Zanuncio, T. V. (1992b). Ciclo de vida e consumo foliar de Sarsina violascens (Herrich-Schaeffer, 1856) (Lepidoptera: Lymantriidae), em Eucalyptus urophylla. Revista Brasileira Entomologia, 36(4), 843–850.
Zanuncio, T. V., Zanuncio, J. C., de Freitas, F. A., Pratissoli, D., Sediyama, Z., Camilla, A., & Maffia, V. P. (2006). Main lepidopteran pest species from an Eucalyptus plantation in Minas Gerais, Brazil. Revista de Biologial Tropical, 54(2), 553–560.
Zanuncio, J. C., Soares, M. A., Zanuncio, T. V., Mielke, O. H. H., de S Ramalho, F., Assis Júnior, S. L., & Wilcken, C. F. (2013). Euselasia hygenius occulta (Riodininae): first report of feeding on Psidium guajava (Myrtaceae) in Minas Gerais State, Brazil. Journal of the Lepidopterists’ Society, 67, 221–224.
Zanuncio, J. C., Tavares, W. D. S., Ramalho, F. D. S., Leite, G. L. D., & Serrão, J. E. (2016). Sarsina violascens spatial and temporal distributions affected by native vegetation strips in Eucalyptus plantations. Pesquisa Agropecuária Brasileira, 51(6), 703–709.
Zanuncio, J. C., Cruz, A. P., Ramalho, F. S., Serrão, J. E., Wilcken, C. F., Silva, W. M., Santos Júnior, V. C., & Ferreira-Filho, P. (2018). Environmental determinants affecting the occurrence of defoliator caterpillars on Eucalyptus (Myrtaceae) plantations in the Brazilian Amazonian Region. Florida Entomologist, 101(1), 480–485.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no conflicts or competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pereira, J.M., dos Santos, T.T.M., Soliman, E.P. et al. Survival and performance of Sarsina violascens (Lepidoptera:Lymantriidae) larvae on Eucalyptus species and hybrids. Phytoparasitica 50, 13–20 (2022). https://doi.org/10.1007/s12600-021-00933-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-021-00933-9