Abstract
Field observations were made to find out seasonal abundance of Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae) a predatory bug of an invasive pest, the black inch worm, Hyposidra talaca Walker (Lepidoptera: Geometridae) in tea ecosystem. The trend of population study revealed that E. furcellata was abundant throughout the cropping period, with peaks during June to September. Investigations were also carried out on the biology and predatory efficiency of E. furcellata on the larvae of H. talaca. The fecundity of the predatory bug was recorded as 276.6 ± 15.8 eggs/female. The incubation period was 7.0 ± 1.0 days, total nymphal development period 21.8 ± 0.7 days and adult longevity was 30.8 ± 1.9 days. Construction of life table of E. furcellata by rearing the tea looper H. talaca indicated that, the life table parameters like, intrinsic rate of natural increase (rm) was 0.149, net reproductive rate (Ro) was 218.14 eggs/female, gross reproduction rate (∑mx) was 244.69, generation time (T) 35.49 d, doubling time (DT) 4.63 d and finite rate of increase (λ) was 1.161. The adult female consumed 10.2 ± 0.8 s instar looper in ‘no-choice’ condition, whereas in ‘free- choice’ condition the consumption was 9.8 ± 1.1 second instar and 5.4 ± 1.1 fifth instar larvae of H. talaca per day. The impact of commonly used pesticides on the predator indicated varying degree of mortality on nymphs and adults after 24 h of exposure. Hence, E. furcellata could effectively be utilized in the IPM programme envisaged for tea for effective management of H. talaca.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tea, Camelia sinensis Kuntze, ecosystem offers food, shelter and serving as a breeding ground to numerous arthropods, resulting increasing the number to about 167 pests (Das 1965) and diverse natural enemies (Das et al. 2010; Roy et al. 2014). Among all the tea pests, the polyphagous lepidopteran pests migrated to tea ecosystem from the nearby forests and make their habitat in tea plantation as well as on shade trees Albizzia spp. (A. odoratissima, A. chinensis) (Das and Mukhopadhyay 2014). The Hyposidra talaca (Walker) (Lepidoptera: Geometridae), commonly known as the black inch looper, is the dominant lepidopteran arthropod pest which causes substantial damages (11–55%) to the crop in Dooars, Terai (plains in the foothills of the eastern Himalayas in the states of West Bengal and Assam of north east India (Sinu et al. 2011; Antony et al. 2012; Prasad and Mukhopadhyay 2013; Roy et al. 2017). Regular application of synthetic chemical pesticides, especially organophosphates and pyrethroids, in the tea gardens for controlling the looper pests including H. talaca, leads to hazardous effect to the environment and human health (Mobed et al. 1992). However, in order to reduce the pesticides application and to circumvent the negative impact on environment and residues in made tea, biological control measures are being adopted worldwide (Gillespie et al. 2000). Beneficial insects like parasitoids and predators and pathogens play a vital role in the natural regulation of many tea pests (Nguyen et al. 2018; Sarkar et al. 2019). Muraleedharan and Selvasundaram (1995) stated that, the interaction between the insect natural enemies and pests in tea fields in India maintained the pest population. The stink bug, Eocanthecona furcellata (Wolff) belonging to the family Pentatomidae has received much attention in biological control of lepidopteran, coleopteran and heteropteran pests of several agricultural crops (Chakravarty et al. 2017; Lenin and Rajan 2016; Suyal et al. 2018). E. furcellata voraciously predates and completes its life cycle on larvae of Spilaricta oblique (Kumar et al. 2001), and feeds on eggs and grubs of Henosepilachna vigintioctopunctata (Kalaiyarasi et al. 2017). Das et al. (2010) reported the presence of this stink bug in tea ecosystem. Chakravarty et al. (2017) observed that higher temperatures and cloudy conditions favored the increasing of the population of this stink bug.
In the present study, an attempt was made to observe the seasonal abundance of E. furcellata and H. talaca in tea ecosystem. Biology, predatory potential and construction of life table of E. furcellata were also made by rearing on the tea looper H. talaca. Besides, the impact of commonly used pesticides on the predator under laboratory conditions was also assessed.
Materials and methods
Seasonal abundance of E. furcellata
Two experimental areas were selected at Banarhat T.E (26.78814°N, 89.0408°E) and Tea Research Association experimental plot (26°54′0″N, 88°58′0″E) in the district of Jalpaiguri of West Bengal, India. A three hectare area plot was selected in both locations and from that plot, five subplots were randomly chosen (100 tea bushes) and considered as five replications. The trends of the seasonal abundance of this predator was recorded by taking the count of average number of predators in randomly selected 30 bushes per replication at fortnightly interval from February 2017 to January 2019, following the method adopted from Puja and Sabiha (2016) and Suyal et al. (2018). Data on abiotic factors viz., humidity, maximum and minimum temperature and rainfall were collected from the meteorological observatories of Banarhat T. E and Tea Research Association experimental station during the study period. The influence of biotic and abiotic parameters on seasonal abundance of E. furcellata was ascertained using multiple regression analysis.
Rearing of E. furcellata on larvae of H. talaca
Male and female moths of H. talaca were collected from the study area and placed inside a glass chimney (30x5cm) for mating and oviposition. Honey solution (10%) using cotton was provided for feeding. Dried shade tree barks were provided inside the rearing chamber for oviposition. The eggs were separated and placed in another glass chimney until hatching. After hatching, the larvae were reared by providing young tea shoots (containing three leaves and a bud) of the tea cultivar TV1 (Tocklai Vegetative 1) and the stock culture was maintained under laboratory conditions (27 ± 2 °C, 70–80% RH and 16: 10 LD photoperiod).
The field collected adult stink bugs were also kept under the same laboratory conditions inside glass chimney covered with muslin cloth for mating and laying eggs. Fresh larvae of H. talaca were provided for feeding. Shade tree barks were kept inside the glass chimney for egg laying and the egg clutches were collected periodically. A piece of wet cotton was put into the chamber for maintaining the moisture. Newly hatched first and second instar nymphal stages of the predator were reared in group and provided with 2nd and 3rd instar larvae of H. talaca. Third, fourth and fifth instars were reared individually in Tarson container (diameter 4.3 cm X 5.4 cm). The newly hatched adults were kept in separate glass chimney for recording the biological parameters of E. furcellata (Lenin and Rajan 2016; Tiwari et al. 2017).
Biology and life table studies of E. furcellata
From the stock culture, 100 eggs were separately put into glass chimney and observations were made on the hatchability and other life cycle parameters like incubation period, duration of each nymphal stages, mortality and survival rate of each life stages by providing larvae of H. talaca. Adults were sexed based on the morphological characters and sex ratio was also recorded. Newly molted healthy males and females were selected (ten pairs) randomly and kept in separate glass chimney, provided with larvae of H. talaca. Number of egg clutch and eggs were counted and recorded as and when laid. The biological parameters like pre-oviposition period, oviposition period, fecundity and longevity of adults were also recorded.
The life table parameters like age specific fecundity, age specific survival rate, gross reproductive rate (∑mx), intrinsic rate of natural increase (rm), net reproductive rate (Ro), mean generation time (Tc) and finite rate of increase (λ) under the same laboratory conditions were calculated according to Brich (1948), Deevy (1947) and Southwood (1978). Doubling time (Dt) was determined according to Mackauer (1983).
Predatory efficiency of E. furcellata on H. talaca
No-choice feeding
Predatory efficiency of E. furcellata under no-choice condition was assessed by providing specified life stages (II, III, IV or IV instar larva) of H. talaca separately under same laboratory conditions. Tea leaves were provided for the feeding of host larvae. To determine the predatory efficiency of E. furcellata, 20 host larvae were released in a glass chimney (22 cm height, 13 cm diameter of middle portion and 6 cm diameter of each opening) containing individual life stage (I, II, III, IV and V instar nymphs and adult) of E. furcellata and closed the opening with muslin cloth. Daily food consumption by each specific life stages of E. furcellata on specific larval instar of H. talaca was recorded at 24 h interval. To maintain the original prey number, new set of larvae was provided daily basis. Each set of experiment was replicated five times.
Free-choice feeding
In free-choice situation, different larval instars of H. talaca like II, III, IV and IV respectively were released together in glass chimney of same size and provided with tea shoots as food of larvae under same laboratory conditions. Individual life stage of the predator (I, II, III, IV and V instar nymphs and adults) was released into experimental set up to record the predatory potential. Observations were made at 24 h interval by counting the number of preys consumed in order to record the predatory efficiency of each life stages of the predator. Method was adopted from Barua et al. (2016).
Effect of pesticides on E. furcellata
In order to ascertain the impact of commonly used synthetic pesticides (chemical and bio-formulations) on different life stages of the predator (nymphs and adults), an experiment was conducted under laboratory conditions. From the laboratory culture, ten individuals of each life stages were placed in each glass chimney for each treatment and all the treatments were replicated five times. The selected pesticides (Table 1) were diluted in distilled water with the recommended dose and exposed. Spraying of the treatment was performed with glass atomizer, maintaining the distance of 30–40 cm for covering with fine droplets of water. Mortality and post treatment symptoms were observed at 24 h intervals and the percentage of mortality was recorded following the method of Vasanthkumar et al. (2011).
Statistical analysis
Multiple regression and coefficients were worked out between biotic and abiotic factors with the population dynamics of E. furcellata by using GraphPadInStat 3.1 software. Data on the predatory potential and effect of pesticides on E. furcellata were determined by analysis of variance (ANOVA) and means were separated by Tukey’s multiple comparison test.
Results
Seasonal abundance of E. furcellata
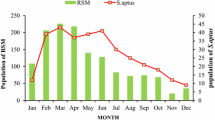
The population of E. furcellata began to show gradual increasing trend from March onwards in both the study locations (Tables 2 and 3). The population dynamics of E. furcellata coincided with cyclic oscillation of H. talaca population during the study periods (2017–2018 and 2018–2019) (Fig. 1). The highest population of this predator was recorded in the months of June, July, August, September and October in both the study locations. The peak of presence of E. furcellata at Banarhat was observed in September with an average of 2.4 ± 0.72 and in August 2.5 ± 0.95 of E. furcellata per bush in the season of 2017–2018 and 2018–2019, respectively. At TRA Experimental plot the peak of population was recorded in September with 2.2 ± 0.93 E. furcellata per bush in the season 2017–2018 and 2.7 ± 0.72 per bush in October in 2018–2019. The correlation and regression analysis fitted with the abiotic and biotic factors indicated that, the population abundance of E. furcellata showed a positive correlation with the population trends of H. talaca at both the study locations (Table 4). All the abiotic factors like maximum temperature, minimum temperature, rainfall and relative humidity showed positive correlation with the population trends of E. furcellata throughout the study period (Table 4).
The multiple regression equation fitted with certain biotic and abiotic factors to predict the incidence and abundance of E. furcellata was (Y); Y = − 2.940– 0.03841 (H. talaca population) + 0.0125 Temp. (Max.) + 0.05998 Temp. (Min) + 0.04173 Relative humidity – 0.0003758 Rain fall [R2 = 63.77%; P value is <0.0001; Multiple R = 0.7986; F = 14.436].
Biology of E. furcellata on H. talaca
The average number of eggs laid by a single female was 276.6 ± 15.8 when E. furcellata was reared by providing the larvae of H. talaca (Table 5). Newly laid eggs were whitish golden in colour, which turned into creamy to light brownish later. The mean incubation period was recorded as 7.0 ± 1.0 days. The total nymphal development was completed in a period of 21.8 ± 0.7 days. Longevity of adult female was 30.8 ± 1.9 days and male was 27.6 ± 1.1 days fed on looper. The pre- oviposition period and oviposition period were lasted for 6.2 ± 1.3 and 18.4 ± 2.3 days. Sex ratio of the predator was recorded as 1:0.9 (F: M).
Life table of E. furcellata reared on H. talaca
From a single egg clutch an average of 95.35% nymphs hatched out. Among these, 95.15% were become adult (Table 5). The age-specific survival rate (lx), age-specific fecundity (mx) and the age-specific maternity (lxmx) of the predatory bug are described in the Fig. 2. The intrinsic rate of natural increase (rm) of E. furcellata was recorded as 0.149 ± 0.0 day−1 and the net reproductive rate (Ro) was 218.14 ± 2.1 female offspring per adult female, produced during their life span expected as 276.6 ± 15.8 eggs/female. The gross reproductive rate (∑mx) was also recorded as 244.69 ± 2.9 eggs/female while the finite rate of increase (λ)was 1.161 ± 0.01 females per female per day (Table 6). The mean generation time (Tc) was 35.49 ± 2.0 day−1 and Doubling time (Dt) was 4.63 ± 0.6 days.
Predatory efficiency of E. furcellata on different stages of H. talaca
In the experiment under no-choice condition of feeding, first instar nymph of E. furcellata predated 1.8 ± 0.4 s instar larvae of H. talaca per day. Other instars like II, III, IV and V consumed 3.2 ± 0.4, 4.6 ± 1.1, 5.8 ± 1.3 and 6.0 ± 1.4 (F = 43.47, df = 6, p = 0.001) second instar larvae of H. talaca (Table 7). The adult female preyed a mean of 10.2 ± 0.8 larvae per day, which was recorded as the highest number of predation among all the life stages. First and second instar nymph preferred second instar larva of H. talaca more than the other developing stages of the prey. Instead, the adult females were observed to preferably fed on fourth and fifth instar larvae of H. talaca compared to the early instars.
The results of free-choice condition of feeding revealed that, the number of predation per day was comparatively lesser than that of no-choice (Table 8). A single V instar nymph of E. furcellata was observed to feed 4.8 ± 0.8 third instar larvae of H. talaca followed by IV instar (4.0 ± 1.0), III instar (3.0 ± 1.0), II instar (2.0 ± 0.7) and I instar (1.2 ± 0.4) nymph (F = 38.038, df = 6, p = 0.001) (Table 8). The prey consumption was inversely proportional to the stage of the prey. An adult female predated more number of second instar larvae (9.8 ± 1.1) and when compared to fifth instar larvae (5.4 ± 1.1) per day.
Effect of pesticides on immature and mature stages of E. furcellata
The results of the present investigation showed that, among all the tested pesticides, quinalphos and thiamethoxam were more toxic on all the life stages of E. furcellata. Emamectin benzoate, deltamethrin, bifenthrin, flubendiamide, fenpyroxymate, Beauveria bassiana formulation and azadirachtin were found to be less toxic (Table 9). Maximum mortality (100%) of nymphs and adults was observed within 24 h of the spraying of quinalphos and thiamethoxam. After 72 h of the exposure to deltamethrin and bifenthrin a mortality of 53.3 ± 3.3% and 50.0 ± 5.8% was observed on nymphal stages. Fenpyroximate caused only 33.3 ± 3.3% mortality on nymphal stages and 30.0 ± 5.8% on the adult. The treatment with B. bassiana formulation showed 10.0 ± 0.0% nymphal and 6.7 ± 3.3% adult mortality after 72 h of the exposure, before that no toxic symptom was noticed. No mortality was found in the treatment with Azadirachtin 5% EC exposure till 72 h of observation.
Discussion
In both the study areas, throughout the two seasons (2017–2018 and 2018–2019), E. furcellata appeared in March during the initial flush of the tea plant after the winter dormancy prevailing in this region. At this time scanty average population of 0.6 ± 0.01 and 0.7 ± 0.02 bugs/bush were recorded at Banarhat TE in 2017–2018 and 2018–2019, respectively. The same trend was noticed in the other location (TRA experimental plot). The dynamic of E. furcellata with the pest population (H. talaca) was positively correlated. Chakravarty et al. (2017) also observed a similar trend of population with 1.2 bugs/sq. m in pigeon-pea Cajanus cajan (L.) field. A gradual increase in the population was noticed with a peak population in September and August both the season at Banarhat TE. A similar trend was recorded at TRA plot also during the month of September and October in all years. Similar results were reported by Suyal et al. (2018), recorded 3.8 bugs/soybean plant in September. For better understand of the influence of temperature, rainfall and relative humidity on the population trends of E. furcellata, the collected metrological data were correlated with the population dynamics of the bug. The multiple regression showed that, population build-up of E. furcellata in tea ecosystem of both the locations showed significant positive correlation with all the weather parameters. Chakravarty et al. (2017) reported that, the population dynamic showed a significantly positive correlation with maximum temperature and rainfall, whereas with other meteorological parameters the correlation was found to be non-significance. Pillai and Agnihotri (2011) observed that the predatory stink bug population abundance was positively correlated with maximum and minimum temperature and evening relative humidity in green gram cultivated plot.
In general, biological parameters of E. furcellata were almost similar to the study of Lenin and Rajan (2016) who reared the predator on Corcyra cephalonica, Yasuda and Wakamura (1992) reared on the larvae of Spodoptera litura, Kumar et al. (2001) on Spilaricta oblique and Siddaiah and Devi (2015) on vapourer tussk moth and tasar silkworm. Developmental period of E. furcellata from egg to fifth instar nymph was 21 to 28.8 days, which was identical as reported by Ganguli et al. (2000); Yasuda and Wakamura (1992); Ray and Khan (2011); Bhatnagar et al. (2019). The first instar nymphs emerged from the dorsal surface of eggs by removing the upper lid (Ray and Khan 2011; Siddaiah and Devi 2015); they were reddish with black in colour which later turned slowly into bluish and red. The longevity of male and female of this stink bug was 27.6 ± 1.1 and 30.8 ± 1.9 days, in the line with the findings of Siddaiah and Devi (2015). This study clearly indicates that it could be possible to maintain the rearing of E. furcellata on larvae of H. talaca for further study.
The present investigation showed that, the egg hatchability rate was 95.35%, when fed on larvae of H. talaca. Lenin and Rajan (2016) showed 90% egg hatchability rate of E. furcellata fed on larvae of C. cephalonica. Instead, Nielsen et al. (2008) got only an average of 61% survival rate of closely related pentatomid bug Halyomorpha halys, reared at 250 C. The highest nymphal mortality was observed in II instar nymph compared to other life stages.
The life table study of E. furcellata indicated that the mean generation time was (Tc) = 35.49 days, corrected generation time (T) =35.9 4 days while the λ = 1.61and the net reproductive rate (Ro) 218.14 with the rm. value 0.149. These findings are in the line with the research of Tuan et al. (2015), while rearing the stink bug on Plutella xylostella larvae, got the rm. = 0.138, λ = 1.149, Ro = 292.4 and Tc = 40.9. All the values were found to be comparatively lower than reared on larvae of S. litura. Nielsen et al. (2008) studied the life table of polyphagous stink bug H. halys and recorded the rm. = 0.07, Ro = 60.02 and Tc = 56.59 reared on green bean (Phaseolus vulgaris), Spanish peanut (Arachis hypogaea) and corn (Zea mays). The intrinsic rate of natural increase (rm) is a key demographic parameter among all the life table parameters, useful for predicting the potential of population growth of an animal under given environmental conditions (Andrewartha and Birch 1954; Southwood and Henderson 2000).When a predator possesses population growth rate either equal to or greater than its prey, may have the efficiency to regulate the prey population (Sabelis 1992). For the development of a biological control agent, the ‘rm’ are progressively used on the basis of biological control agent’s reproductive potential and to predict the outcome of pest-natural enemy interactions (Dent and Walton 1997; Jervis and Copland 1996). However, other factors like predation potential, early detection ability and longevity may also equally contribute to the efficiency to regulate its prey (Roy et al. 2003). Therefore, E. furcellata with its predatory efficiency has the ability to regulate the population of H. talaca in tea field
There is no published evidence on the predatory efficiency of E. furcellata on larvae of H. talaca in tea. The predatory efficiency of E. furcellata has gradually increased with the development of the nymphal instars (Nyunt 2008; Tuan et al. 2015). First instar nymphs were found to predate in and feed on second and third instar larvae of H. talaca and not prefer big sized larvae, similar observation reported by Lenin and Rajan (2016). Yasuda (2000) reported that E. furcellata could detect the prey by chemical cues emitted by the prey while feeding. Nyunt (2008) observed the prey detection of E. furcellata on American Bollworm feeding on four different host plants (cabbage, cotton, chickpea and tomato plants). In the present study it was observed the maximum consumption by fifth instar among all the nymphal instars in both the feeding conditions. During this study, when the larvae were provided into the rearing glass chimney in front of E. furcellata nymphs, the feeding started by injecting the rostrum and sucking the haemolymph of larvae. Similar result was highlighted by Kalaiyarasi et al. (2017) when E. furcellata feeding on the eggs and grubs of H. vigintioctopunctata. In the present experiment the number of prey consumption depended on the stages and size of prey. This observation is in the line with Kumar et al. (2001) who examined the prey consumption of E. furcellata on larvae of Spilaricta oblique (Walk.) in mulberry plantation. In free-choice condition the prey consumption per day by I, II, III, IV and V instar nymphs of E. furcellata on second instar larvae of H. talaca were 1.8 ± 0.4, 2.6 ± 0.5, 4.0 ± 1.0, 5.0 ± 0.7 and 5.6 ± 1.1, respectively. More consumption efficiency was reported by Nyunt (2008); the daily consumption of II, III, IV and V instar nymphs of E. furcellata was 4.1, 4.3, 6.0, and 8.5 on diamondback moth larvae. The results on no-choice condition of feeding of adult female revealed that, it can consume 10.2 ± 0.8, 9.0 ± 1.0, 7.4 ± 1.8 and 7.0 ± 1.6 number of II, III, IV and V instar larvae of H. talaca per day. Similar trends of feeding were observed by Kumar et al. (2001) on larvae of S. oblique. This may be attributed to the predator’s adaptation to balance its required nutrition for achieving the physiological needs. The efficiency of predation by the developing stages and adult stages could be an added advantage of the predator helpful to regulate the population of H. talaca in tea field. Hence this predator could consume more number of early instars (II and III) larvae of H. talaca, it may be found useful to prevent the pest proliferation and yield loss.
The awareness on impact of commonly used pesticides on beneficial arthropods is necessary for the successful integration of biological control programme in the respective agro-ecosystems (Croft 1990). In the current investigation, among the tested pesticides, quinalphos and thiamethoxam at the field recommended concentration caused 100% mortality of both immature and adult stages in E. fuecellata after 24 h of the application. Cloyd et al. (2009); Delbeke et al. (1997); Veire et al. (2002) reported that, the neonicotinoid-based insecticide thiamethoxam was directly harmful to adults of Athetacoriaria and many bio-control agents, in particular, to Orius laevigatus. In the present study, deltamethrin caused 53.3 ± 3.3% nymphal and 43.3 ± 3.3% adult mortality after 72 h with the recommended concentrations followed by bifenthrin, flubendiamide, emamectin benzoate, fenpyroximate, B. bassiana formulation and azadirachtin. We found that flubendiamide, emamectin benzoate and fenpyroximate were comparatively less toxic to E. furcellata which is in agreement with the work of Amor et al. (2012). The microbial formulation of B. bassiana showed less pathogenicity on E. furcellata. A similar trend was reported by Thungrabeab and Tongma (2007) who found non-pathogenic nature of B. bassiana to natural enemies and beneficial soil insects. Application of azadirachtin formulation on immature and adult stages of E. furcellata, showed no mortality till 72 h of exposure, however, some abnormal behaviour in feeding, movement and moulting of nymphal instar were noticed which may be due to the repellency and growth regulating nature. Zanuncio et al. (2016) reported the toxic symptoms of neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus.
Conclusion
Studies on seasonal abundance of E. furcellata provided that its occurrence is coincide with the cyclic oscillations of the looper (H. talaca) population. Statistically significant correlation and regression values with all the abiotic factors indicated that, the populations of E. furcellata can easily build-up in tea ecosystem. The survival fitness of this stink bug and fecundity rate confirmed the possibility of maintaining and mass rearing of this predator under laboratory conditions. The predatory efficiency of E. furcellata on the different larval stages of H. talaca has reconfirmed that, later life stages and adults of E. furcellata preferred later stages of prey for fulfilling the nutritional requirements. More studies are needed on the possibility of augmenting and conservation of such beneficial insect predators for incorporating the same in IPM programme through field release. Hence, E. furcellata could be utilized in the bio-control based looper pest management programme in tea fields.
References
Amor, F., Medina, P., Bengochea, P., Cánovas, M., Vega, P., Correia, R., García, F., Gómez, M., Budia, F., Viñuela, E., & López, J. A. (2012). Effect of Emamectin benzoate under semi-field and field conditions on key predatory biological control agents used in vegetable greenhouses. Biocontrol Science and Technology, 22(2), 219–232.
Andrewartha, H. G., & Birch, L. C. (1954). The distribution and abundance of animals. Chicago: University of Chicago Press.
Antony, B., Sinu, P. A., & Rehman, A. (2012). Looper caterpillar invasion in north east Indian tea agro-ecosystem, change of weather and habitat loss may be possible causes? Journal of Tea Science Research, 2, 1–5.
Barua, A., Babu, A., & Rajkhowa, R. C. (2016). Seasonal abundance and predatory potential of Stethorus aptus Kapur (Coleoptera, Coccinellidae), a biocontrol agent of tea red spider mite Oligonychus coffeae Nietner (Acarina, Tetranychidae). Preceding of the Zoological Society, 71(3), 224–228.
Bhatnagar, V. R., Maurya, R. P., Brijwal, L., Dobhal, P., Suyal, P., & Singh, N. K. (2019). Study on variability among predatory stink bug, Eocanthecona furcellata (Wolff) (Hemiptera, Pentatomidae) population thriving in different agro-ecosystems of North-Western Himalayas, India. Journal of Entomology and Zoology Studies, 7(3), 726–736.
Brich, L. C. (1948). The intrinsic rate of natural increase of an insect population. Journal of Animal Ecology, 17, 15–26.
Chakravarty, S., Agnihotri, M., & Jagdish, J. (2017). Seasonal abundance of predatory bugs, Eocanthecona furcellata (Wolff.) and Rhynocoris fuscipes (F.) and its olfactory responses towards plant and pest mediated semiochemical cues in Pigeonpea ecosystem. Legume Research, 40(2), 351–357.
Cloyd, R. A., Timmons, N. R., Goebel, J. M., & Kemp, K. E. (2009). Effect of pesticides on adult rove beetle Atheta coriaria (Coleoptera, Staphylinidae) survival in growing medium. Journal of Economic Entomology, 102(5), 1750–1758.
Croft, B. A. (1990). Arthropod biological control agents and pesticides. New York: Jhon Wiley and Sons.
Das, G. M. (1965). Pests of tea in north-East India and their control. Tocklai Experimental Station, Tea Research Association, Jorhat, Assam, India.
Das, S., & Mukhopadhyay, A. (2014). Host-based life cycle traits and detoxification enzymes of major looper pests (Lepidoptera, Geometridae) of tea from Darjeeling Terai, India. Phytoparasitica, 42, 275–283.
Das, S., Roy, S., & Mukhopadhyay, A. (2010). Diversity of arthropod natural enemies in the tea plantations of North Bengal with emphasis on their association with tea pests. Current Science, 99(10), 1457–1463.
Deevy, E. S. (1947). Life table for natural populations of animals. The Quarterly Review of Biology, 22, 283–314.
Delbeke, F., Vercruysse, P., Tirry, L., Clercq, D. P., & Degheele, D. (1997). Toxicity of Diflubenzuron, Pyriproxifen, Imidacloprid and Diafenthiuron to the predatory bug Oriuslae vigatus (Het., Anthocoridae). Entomophaga, 42(3), 349–358.
Dent, D., & Walton, M. P. (1997). Methods in ecological and agricultural entomology. In Wallingford, Oxon, UK. New York: CAB International.
Ganguli, J., Chandrakar, S., & Puri, C. (2000). Canthecona furcellata Wel., a predatory bug on caterpillars of Clostera sp. Insect Environment, 6(2), 79–80.
Gillespie, D. R., Opit, G., & Roitberg, B. (2000). Effects of temperature and relative humidity on development, reproduction and predation in Feltiella acarisuga (Vallot) (Diptera, Cecidomyiidae). Biological Control, 17, 132–138.
Jervis, M. A., & Copland, M. J. W. (1996). The life cycle. In M. Jervis & B. Kidd (Eds.), Insect natural enemies; practical approaches to their study and evaluation (pp. 6–161). London: Chapman and Hall.
Kalaiyarasi, L., Livingstone, A. R., & Miracline, S. F. (2017). A report on the predatory behaviour of stink bug Eocanthecona furcellata Wolff (Hemiptera, Pentatomidae) on the eggs and grubs of Henosepilachna vigintioctopunctata. International Journal of Recent Science Research, 8(8), 19550–19554.
Kumar, V., Morrison, M. N., Rajadurai, S., Babu, A. M., Thiangarajan, V., & Datta, R. K. (2001). Studies on the biology and predatory behaviour of Eocanthecona furcellata (Wolff.) predating on Spilaricta oblique (walk.) in mulberry plantation. International Journal of Industrial Entomology, 2(2), 173–180.
Lenin, E. A., & Rajan, S. J. (2016). Biology of predatory bug Eocanthecona furcellata Wolff (Hemiptera, Pentatomidae) on Corcyra cephalonica Stainton. Journal of Entomology and Zoology Studies, 4(3), 338–340.
Mackauer, M. (1983). Quantitative assessment of Aphidius smithi (Hymenoptera, Aphidiidae), fecundity, intrinsic rate of increase and functional response. Canadian Entomologist, 115, 399–415.
Mobed, K., Gold, E. B., & Schenker, M. B. (1992). Occupational health problems among migrant and seasonal farm workers. The Western Journal of Medicine, 157, 367–373.
Muraleedharan, N., & Selvasundaram, R. (1995). Natural enemies of tea pests. In, Ecofriendly Tea Farming- Planters’ Alternative Approach, pp22–28). UPASI – KVK, Coonoor, Nilgiris.
Nguyen, T. T., Suryamohan, K., Kuriakose, B., Janakiraman, V., Reichelt, M., Chaudhuri, S., Guillory, J., Divakaran, N., Rabins, P. E., Goel, R., Deka, B., Sarkar, S., Ekka, P., Tsai, Y. C., Vargas, D., Santhosh, S., Mohan, S., Chin, C. S., Korlach, J., Thomas, G., Babu, A., & Seshagiri, S. (2018). Comprehensive analysis of single molecule sequencing-derived complete genome and whole transcriptome of Hyposidra talaca nuclear polyhedrosis virus. Scientific Reports, 8, 8924.
Nielsen, A. L., Hamilton, G. C., & Matadha, D. (2008). Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera, Pentatomidae). Environmental Entomology, 37(2), 348–355.
Nyunt, K. T. (2008). Potential of the predatory pentatomid Eocanthecona furcellata (Wolff) as a bio-control agent on American bollworm in cotton in Myanmar. Ph.D. Thesis, Georg-August University Göttingen, Germany.
Pillai, K. A., & Agnihotri, M. (2011). Population dynamics of the predatory bug Eocanthecona furcellata (Wolff.). Indian Journal of Applied Entomology, 25, 162–163.
Prasad, A. K., & Mukhopadhyay, A. (2013). A technique to measure the loss in tea crop by the defoliating pest (Hyposidra talaca Walker) on the basis of dry mass and leaf area parameters. International journal of Bio-resource and Stress Management, 4(2s), 358–361.
Puja, D., & Sabiha, K. (2016). A field study of population dynamics of major insect pests and their natural enemies on cauliflower of Ajmer district. International Journal of Agriculture Sciences, 8(53), 2642–2645.
Ray, S. N., & Khan, M. A. (2011). Biology of predatory bug, Canthecona furcellata Wolff. (Hemiptera, Pentatomidae) on poplar defoliator, Clostera fulgurita Walker (Lepidoptera, Notodontidae). Journal of Biopesticides, 4(2), 109–111.
Roy, M., Brodeur, J., & Cloutier, C. (2003). Effect of temperature on intrinsic rates of natural increase (rm) of a coccinellid and its spider mite prey. Bio-control, 48, 57–72.
Roy, S., Das, S., Handique, G., Mukhopadhyay, A., & Muraleedharan, N. (2017). Ecology and management of the black inch worm, Hyposidra talaca Walker (Geometridae, Lepidoptera) infesting Camellia sinensis (Theaceae), a review. Journal of Integrative Agriculture, 16(10), 2115–2127.
Roy, S., Muraleedharan, N., & Pujari, D. (2014). Acatalogue of arthropod pests and their natural enemies in the tea ecosystem of India. Two and a Bud, 61(1&2), 11–39.
Sabelis, M. W. (1992). Predatory arthropods. In M. J. Crawley (Ed.), Natural enemies. The population biology of predators, parasites and disease (pp. 22–264). Oxford: Blackwell.
Sarkar, S., Babu, A., Chakraborty, K., & Deka, B. (2019). Study on the biology, feeding behaviour and predatory potential of Sycanus collaris (Fabricius) (Heteroptera, Reduviidae), a new predator of Hyposidra talaca (walk.) (Lepidoptera, Geometridae), a major tea pest and mass rearing on Corcyra cephalonica (Stainton) in laboratory. International Journal of Current Advanced Research, 08(06), 19258–19262.
Siddaiah, A. A., & Devi, A. R. (2015). Biology of a predatory bug Eocanthecona furcellata Wolff (Hemiptera, Pentatomidae) on Vapourer tussock moth larvae, a major pest of tasar silkworm food plants. International Journal of Industrial Entomology, 30(1), 26–30.
Sinu, P. A., Antony, B., & Mallick, S. (2011). The occurrence of nucleopolyhedrovirus infecting Hyposidra talaca (Geometridae, Lepidoptera), a tea defoliator from north- East India. Biocontrol Science and Technology, 21, 999–1003.
Southwood, T. R. E. (1978). The construction, description and analysis of age- specific life-tables. In T. R. E. Southwood (Ed.), Ecological methods with particular reference to the study of insect population (pp. 356–387). London: Champ and Hall.
Southwood, T. R. E., & Henderson, P. A. (2000). Ecological methods 3rd edition. Backwell.
Suyal, P., Gaur, N., Rukesh, P. K. N., & Devrani, A. (2018). Seasonal incidence of insect pests and their natural enemies on soybean crop. Search Results Journal of Entomology and Zoology Studies, 6(4), 1237–1240.
Thungrabeab, M., & Tongma, S. (2007). Effect of entomopathogenic fungi, Beauveria bassiana (balsam) and Metarhizium anisopliae (Metsch) on non-target insects. Science and Technology Journal, 7(1), 8–12.
Tiwari, S., Mayuri, R. P., & Pandey, A. K. (2017). Effect of different insect hosts on biology and predation efficiency of Eocanthecona furcellata Wolff (Hemiptera, Pentatomidae). The bioscan, 12(1), 193–197.
Tuan, S. J., Yeh, C. C., Atlihan, R., & Chi, H. (2015). Linking life table and predation rate for biological control, a comparative study of Eocanthecona furcellata (Hemiptera, Pentatomidae) fed on Spodoptera litura (Lepidoptera, Noctuidae) and Plutella xylostella (Lepidoptera, Plutellidae). Journal of Economic Entomology, 1–13, 1–12.
Vasanthkumar, D., Roobakkumar, A., Rahaman, V. K. J., & Babu, A. (2011). Impact of temperature and pesticide applications on the prey consumption of Mallada desjardins (Navas) (Neuroptera, Chrysopidae), a predator of red spider mite infesting tea. Two and a Bud, 59, 43–48.
Veire, V. D. M., Sterk, G., Staaij, V. D. M., Ramakers, P. M. J., & Tirry, L. (2002). Sequential testing scheme for the assessment of the side-effects of plant protection products on the predatory bug Oriuslae vigatus. BioControl, 47, 101–113.
Yasuda, T. (2000). Role of semiochemicals in prey- locating behavior of a generalist predatory stink bug Eocanthecona furcellata (Heteroptera, Pentatomidae). Japan Agricultural Research Quarterly, 34, 15–20.
Yasuda, T., & Wakamura, S. (1992). Rearing of the predatory stink bug, Eocanthecona furcellata (Wolff) (Heteroptera: Pentatomidae), on frozen larvae of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Applied Entomology and Zoology, 27(2), 303–305.
Zanuncio, J. C., Mourão, S. A., Martínez, L. C., Carlos Frederico Wilcken, C. F., Ramalho, F. S., Rueda, A. P., Soares, M. A., & Serrão, J. E. (2016). Toxic effects of the neem oil (Azadirachta indica) formulation on the stink bug predator, Podisus nigrispinus (Heteroptera, Pentatomidae). Scientific Reports, 6, 30261.
Acknowledgments
Authors are thankful to the authority and the management team of Banarhat T.E. of Jalpaiguri district, West Bengal, India for supporting in field survey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar, S., Babu, A., Chakraborty, K. et al. Eocanthecona furcellata (Wolff) (Hemiptera: Pentatomidae), a potential biocontrol agent of the black inch worm, Hyposidra talaca Walker (Lepidoptera: Geometridae) infesting tea. Phytoparasitica 49, 363–376 (2021). https://doi.org/10.1007/s12600-021-00888-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-021-00888-x