Abstract
Small walnut aphid (Chromaphis juglandicola (Kaltenbach) (Hemiptera: Aphididae) is important pest in walnut orchards in Lake Van Basin, Turkey. In this study, the life-table parameters reflecting population growth rate and predation rate of Chrysoperla carnea (Stephen) (Neuroptera: Chrysopidae), which is one of the important predators of the pest, were determined. Life table parameters were estimated according to age-stage, two-sex life table method. Variances and standart errors of population parameters were obtained according to Bootstrap method. The values obtained for the intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R0) and mean generation time (T) were 0.072 d−1, 1.070 d−1, 62.48 offspring and 57.16 d, respectively of C. carnea fed on Chromaphis juglandicola. The net predation rate (C0) was determined as 212.9 aphids. Pradation projection was performed by using timing program to prevent the predator’s decrease in predation capacity and to determine the most appropriate release quantities and the appropriate interval between releases (to fill predation gaps) for biological control. The possible effects of the findings on the bio-controls of Chromaphis juglandicola by C. carnea were discussed. Results obtained can be used in pest management program that will be prepared for this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the most commonly observed natural predators in Turkey, as in the world, Chrysoperla spp. (Neuroptera: Chrysopidae), are important predators in walnut cultivation (Karut and Kazak 1999; Karut and Şekeroğlu 1999). Due to good flying ability, long life, high fertility, and reproductive potential, they are important predators used in biological management programs (Aziza et al. 2007). Additionally, these predators are important due to their common observation in natural ecosystems, ease of mass production, high consumption and search capabilities, and early appearances in regions after the use of chemical pesticides (Obrycki et al. 1989; Bozsik 1995).
Turkey is the fourth producer in walnut production after China, the United States (US), and Iran (FAOSTAT 2018). With ecological conditions suitable for walnut cultivation, the Lake Van Basin (TUIK 2018) is third producing area in Turkey, and in this basin, species causing damage during the early vegetation period such as Panaphis juglandis (Goeze) (Hemiptera: Aphididae) and Chromaphis juglandicola (Kalt.) (Hemiptera: Aphididae), known as walnut aphids (Atlıhan et al. 2007; Toros et al. 1996) have been found. These harmful species are found almost everywhere in the world in which walnut cultivation is undertaken (Barnes et al. 1982). Chromaphis juglandicola are found scattered on the lower section of walnut leaves and feed on plant sap causing excessive sooty mold on walnut leaves. The management threshold for this pest is 15 individuals per leaf (Barnes and Moffitt, 1979). If aphid populations rise above these levels, negative effects on yield and quality are observed, and this increase has been shown to cause reductions in fruit size and wrinkling of the internal fruit (Jaskiewicz and Cichocka 2004; Jaskiewicz and Kmiec 2007; Anonymous, 2017).
Many natural predators for aphids are found in walnut fields in the Lake Van Basin, and green lacewing is one of the most commonly encountered predators having high population levels (Erol and Yaşar 1994; Erol and Atlıhan 1995; Atlıhan et al., 2007).
Life table allows us to obtain accurate information on the development and reproduction of insect species. (Yu et al. 2013a) stated that in order to increase the effectiveness of biological control, survival rate, stage differentiation, fecundity and predation rate of predator should be collected in the same frame. Studies comparing life tables of natural enemy species and predators provide information about their biological potential under controlled conditions (Chi and Su 2006). This study targeted the determination of population growth and predation potential of C. carnea feeding on C. juglandicola. The data obtained is important to provide the necessary information infrastructure in mass production, to determine the correct release time and the number of individuals required for release of C. carnea in order to design a successful biological control program for pests and projection of the ecological relationship between prey and predator.
Materials and methods
Insect rearing
Chromaphis juglandicola used as prey were collected daily from nature. C. carnea was collected from walnut plantations. Mass production of the predator was completed in a climate-controlled room with 25 ± 1 °C, 60 ± 10% relative humidity (RH) and a photoperiod 16:8 (L:D).
Adult C. carnea were left in 35 × 15 × 15 cm production jars with ventilation holes in the lid and sides covered with cheesecloth. Because adults do not feed on aphids, the inner walls of the jars were spread daily with a beer yeast, honey, and water mixture as recommended by Kişmir and Şengonca (1981), and additionally, water-soaked cotton was placed in the base of the jars with a petri dish containing drying paper. In order to allow adult individuals to lay eggs, cheesecloth pieces cut in strips were left in the jars. Larvae hatching from eggs were fed with walnut aphids until they became pupae. The adults emerging from the pupae were transferred to the same production jars for mating and the mentioned mixture was given as food as well. Mass production continuity was ensured to obtain the desired number of predators during the trials. Trials began after at least one generation was produced in order to eliminate any previous prey-related effects.
Life table study of Chrysoperla carnea
A total of 40 egg stems were cut and placed singly in Petri dishes (9 × 1.5 cm). Larvae hatching from the eggs were fed C. juglandicola ad-libitum in the same Petri dish in during the third period until they became pupae. With daily observations, the development duration of C. carnea from larval period to pupal period and the mortality rates in these periods were identified. In this study, the first mating appeared within 24 h after adults emerged and was repeated several times throughout the adult life span. After adults emerging from pupae were brought together for mating. Adults were fed with beer yeast, honey, and water mixtures. Daily observations provided information used to determine adult preoviposition, oviposition and total preoviposition periods of C. carnea, daily and total egg amounts left by females during the oviposition period, and the life expectancy of male and female individuals.
After daily observations during the trial period, jars were changed. Using data obtained about the development and reproduction of C. carnea, life table parameters were obtained. These parameters linked to age and period were calculated with the age stage two sex lifetables method (Chi 1988; Chi and Yuan 2005).
Predation rate of Chrysoperla carnea
The numbers of prey given to predators in each period under the conditions of being more than could be consumed was determined in preliminary trials. Thus, 15, 30, 45 aphids were given each day, respectively, to the first, second and third larval period of the predator. The third instar nymphs of P. juglandicola were given to the predator. Trials to determine the daily predation rate based on the age stage two sex life table, and C. carnea’s life table trials were conducted together. Daily observations about the number of aphids consumed by C. carnea were recorded. Observations continued until C. carnea reached the adult period.
Life table analysis
Based on our data related to development and reproduction of predators during trials, lifetable parameters were calculated. In order to obtain these parameters, two sex life tables linked to age and stage were used (Chi and Liu 1985; Chi 1988; Chi and Yuan 2005). Additionally, data analysis was performed with the TWOSEX–MSChart developed by Chi (2020). In order to calculate standard errors and variances of the biological parameters and population parameters, the bootstrap technique was used (Huang and Chi 2012). We used 100,000 bootstraps (Polat-Akköprü et al., 2015). The net reproduction rate (R0) is the total amount of all lxmx (in which lx is the age-specific survival rate, mx is age-specific fecundity) and was calculated as
The intrinsic rate of increase (r) was estimated according to the Euler – Lotka formula:
with age indexed from 0 by using the iterative bisection method (Goodman 1982, Chi 1988).
The finite rate of increase (λ) was calculated as
The mean generation time (T) is the time required for a population to increase to R0 times of its size as the stable age distribution is reached. It was calculated as
The life expectancy is the expected period of life duration for an individual of x age and in j stage, and it was calculated as
Where is the probability that individuals of age x and stage j will survive to age i and stage y and, is calculated by assuming s′ix = 1.
The age-stage-specific reproductive value (vxj) were calculated according to Tuan et al. (2014) as
Where is the fecundity (fxj) the number of eggs laid by female adult at age x.
Predation rate analysis based on age-stage, two-sex life table
To determine the predation rate, The raw data for proportion of aphid numbers consumed by predators in each stage were analyzed with the computer program CONSUME-MSChart (Chi 2020a) The age-specific predation rate (kx) is defined as the average number of prey consumed by the predator at age x and was calculated as;
where Cxj is the age-stage-specific predation rate of individuals C. carnea at age x and stage j. The age-specific net predation rate (qx) is the number of prey consumed by C. carnea at age x, and was calculated as;
The net predation rate‘C0’ is the average number of prey consumed by an individual during their life span including individuals that died in the preadult stages and adults of both sexes, also defined as the predatory capacity of the predatory population
Qp, is the number of prey required by a predatory to grow a generation, and the ratio (Qp) was calculated as:
Finite predation rate (ω) is used to compare the predation capacity of a predator and was calculated as:
where λ is the finite rate of the predator population; ψ is the stable predation rate; axj is the rate of individuals at to age x and stage j; Cxj is the age-stage specific predation rate.
We used the same bootstrap samples in order to provide linking between the life table and pradation rate. The Bootstrap method is available in both TWOSEX and CONSUME programs.
Population and predation projection of C. carnea
The life table and predation rate data from the earlier studies were used to Population and Predation projection of C. carnea and their uncertainties using the program TIMING (Chi, 2020b). The population projection and predation projection at time t was calculated by according to Chi (1990) and Huang (2018). Population projection; The newly laid ten eggs as the initial population were used to population development for 60 days.
Predation projection; different simulations were made to close the gap in predation capacity during the period when the predator did not consume prey (egg, pupa and adult). In the initial release simulation, 10 eggs (2 d) of C. carnea were used as the starting population. In the second release simulation, a second release was made 10 days after the first release with the same population structure. In the last simulation, the population structure was changed to15 eggs (2 d) and a second release was made 10 days after the first release.
The variability of population growth and predation was projected according to Huang et al. (2018) by using the life tables representing the 0.025th and 0.975th percentiles of the bootstrap results of the finite rate of increase.
Results
Development time and mortality of Chrysoperla carnea
Developmental time for egg stage was four days and for the f1st, 2nd and 3rd larval instar 3.4, 3.56 and 3.75 days respectivelly, and the longest development period being 9.58 days for the pupal period. The total preadult stage development time for the predator was determined as 24.46 days. The mortality rates total preadult observed as 27% (Table 1).
The age-stage survival rate (sxj) shows the probability that a newly hatched individual will survive to age x and stage j (Fig. 1a). Because the variable developmental rates found among individuals were included in the age-stage, two-sex life table, the overlaps between different stages could be observed in Fig. 1a. The survival probability of a newly laid C. carnea egg to adulthood was 0.24 and 0.48 for females and males, respectively. As the survival curve for female predators fed only with C. juglandicola remained horizontal, the period without deaths was identified between the 29th and 86th days (Fig. 1a).
Reproduction and population parameters of Chrysoperla carnea
Based on the analyses, the fecundity rate was calculated as 257.75 eggs/female days. The adult preoviposition period (APOP) was eight days, and the total preoviposition period (TPOP) was 33.0 days for C. carnea fed with C. juglandicola. From the population parameters, r was 0.072 days−1, R0 was 62.484 eggs/generation, λ was 1.075 days−1, and T was 57.16 days (Table 2).
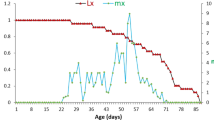
The age specific survival rate (lx), age-specific fecundity (mx), and age-specific maternity (lxmx) of C. carnea are shown in Fig. 1b. Age-specific fecundity (mx) and age specific fertility for total population for C. carnea fed with C. juglandicola was determined to be 3.25 offspring (Fig. 1b).
The mean life expectancy for a newly put one-day C. carnea egg until death was 66.72 days with the life expectancy for a one-day old larva determined to be 62.72 days (Fig. 2a). The life expectancy of females (70.29 days) was identified to be longer than that of males (63.24 days).
The reproductive value (vxj) indicates the additive features of a C. carnea of x age and j stage to the next population. Completing the preadult stage development stage, occurrence of females and beginning of reproduction significantly increased the reproductive value. The highest value of the reproductive value curve shows the highest contribution a female can make to a population. Females of C. carnea fed with C. juglandicola first reach adult on the 21st day. Contrary to this, the reproductive value reached highest levels on the 54th day (v54 = 84.34). Due to the reduction in reproduction as females begin to age, a reduction occurs in the reproductive value curve. The survival curve for female C. carnea ends at the 101st day with the fertility curve reaching zero on the 91st day. In parallel with this result, the reproductive value curve for females appears to have ended on the 91st day (Fig. 2b).
Population projection of Chrysoperla carnea
Population projection was used to predict stage structure and growth trends in the future of C. carnea populations. The population projection demonstrated that the growth of the C. carnea was 265 individuals after 60 days, and there were 77 eggs, 48 larva 1, 40 larva 2, 39 larva 3, 38 Pupa, 7 females and 15 males (Fig. 3a). In the original cohort, it was seen that the total population would reach about 264 individuals after 60 days (Fig. 3b). Our results showed that the variability could range from 116 to 408.
a. Population projection with change of age-stage structure of C. carnea during population growth., b. Population projection by using the life tables of the original cohort, the cohorts constructed based on the 2.5 and 97.5% percentiles of finite rate (λ). Total population size for the time period 0–60 d
Predation rate for Chrysoperla carnea
The mean numbers consumed by C. carnea feeding on only C. juglandicola in the first, second, and third larval periods were determined as 32.97, 75.19, and 140.75, respectively. The net predation rate (C0) indicated that the average number of C. juglandicola eaten by per individual of C. carnea during its lifespan was 212.9. The transformation rate (Qp) was 3.41. The value of stable predation rate (ψ) was 8.21. The finite predation rate (ω) of C. carnea was 8.83 (Table 3).
The predation rate (Cxj) of C. carnea feeding on C. juglandicola is shown in Fig. 4a. The non-predatory stages created two gaps in predation capacity, such as eggs, pupae, and adults.
Age-specific predation rate (kx) and age-specific net predation rate (qx) are shown for C. carnea feeding on C. juglandicola (Fig. 4b). kx gives the average number of aphids eaten by C. carnea of age x. qx gives the weighted number of aphids eaten by C. carnea of age x. As C. carnea feed with aphids only in the larval period, the increase in kx and qx were linked to lx until adult period with a stepwise reduction beginning from the larval period in the transition to the adult period. There were three gaps in the curves for kx and qx. The reason for these gaps was lack of consumption in the egg, pupal, and adult stages.
Predation projection of Chrysoperla carnea
The population growth and predation capacity of C. carnea was projected in a way similar to that used in Chi (1990), Huang and Chi (2012) and Yu et al. (2013a, b). The predation capacity linked to population growth of larva and adult obtained from 10 eggs at 2 days old of C. carnea was compared and is shown in Fig. 5.
The appearance of larvae causes an increase in predation capacity linked to the amount of aphids consumed on the second day. Due to some larvae transitioning to the pupal period, and all changing period by the sixteenth day, predation capacity begins to decline and causes formation of gaps at the locations marked. When adults begin to emerge from pupae (adults do not consume aphids), these adults contribute to creation of new larvae, and their consumption leads to another increase in predation capacity (Fig. 5a).
As C. carnea do not feed on aphids in the pupal and adult periods, extra releases of the predator are needed to prevent reductions occurring in consumption capacity in this time interval. If the second release is changed to the 10th day, the larvae newly emerging from eggs increase the predation capacity; however, they were determined not to fill the gap between the 26th and 34th days. It was observed that adults had emerged, but new larvae had not formed. Hence, release on the 10th day was determined not to prevent predation capacity reductions (Fig. 5b).
In order to fill the predation gap, a third release after 10 days of 15 individuals from 2-day eggs was performed. The reduction in consumption capacity was identified to be less with sufficient numbers of larvae in the environment at the correct time closing the predation gap between pupal and adult periods and no time interval was observed to have formed (Fig. 5c).
The variability is distinctive of population growth. It was reflected by using life tables from the 2.5th and 97.5th percentiles of the finite rate (Fig. 6). The total consumption and its variability are illustrated in Fig.6 Eggs, pupae, and adults were in non-prey stages, and total consumption is reduced when the egg (thin arrow), pupa stage (thick arrow), and the adult stage (striped arrow) take longer. In the original cohort, it was seen that the total consumption would reach about 25,000 individuals after 60 days (Fig. 6b).
Population predation projected by using the life tables and predation rate of the original cohort, the cohorts constructed based on the 2.5 and 97.5% percentiles of finite rate (λ). Total consumption for the time period 0–60 d. (a) Total consumption for the time period 0–30 d. (b) Total consumption for the time period 30–60 d. Thin arrow shows the cohort contains mainly eggs, thick arrow shows the cohort contains mainly.pupae and striped arrow shows the cohort contains mainly adults
Discussion
Chrysoperla carnea fed on walnut aphids for 24.46 days in the preadult development period and presented a total mortality rate of 27%. The total preadult development time for C. carnea feeding on Schizaphis graminum (Hemiptera: Aphididae) was determined as 24.60 days by Khan et al. (2013). Another study by Kasap et al. (2003) identified the preadult development time for C. carnea feeding on Aphis pomi (Hemiptera: Aphididae) as 25.68 days. The development duration obtained in the study appears to correspond to data in the literature even though the predator hunted different aphids. A variety of researchers have stated that the preadult development time for C. carnea changes is linked to the prey type and larval nutrition quality (Liu and Chen 2001; Sattar et al. 2011; Khuhro et al. 2012; Saljoqi et al. 2015; Takalloozadeh 2015; Farrokhi et al. 2017).
The total number of eggs (fecundity) for the predator fed with C. juglandicola was 257.75 (Table 2). Polat-Akköprü and Atlıhan (2014) identified total egg numbers as 170.43 when fed with P. juglandis. In the other study with the same prey and pradator, Yarımbatman and Atlıhan (2008) identified the fecundity as 117.50 at 23 °C and 244.92 at 28 °C for C. carnea feeding on P. juglandis. The prey we use in our study and the prey used in the literature are the aphids that share the same walnut leaf. The results of fecundity stated is lower than that of our. Kasap et al. (2003) determined the fecundity put was 641.28 by C. carnea feeding on Aphis pomi while The fecundity laid by the polyphagous predator C. carnea appears to vary and may be linked to food and temperature differences.
Age-specific fecundity (mx), is a term equivalent to fertility (productivity) and yields the number of females produced by females of x age in unit time. In other words, the expected generation numbers are produced by every female during every age interval of life (Sharov 2012). Yu et al. (2013a, b) stated the maximum fertility value (mx) was 1.28 eggs for Chrysopa pallens (Neuroptera: Chrysopidae) fed with Aphis craccivora (Hemiptera: Aphididae) The total number of eggs left by C. pallens fed with A. craccivora (660.7) was much higher than the total number of eggs determined in our study (170.43) (Table 2), which is considered to be due to the differences in the selection of predator and prey species. Jafari and Bazgir (2015) stated that prey size is an important factor in predator selection.
The finite and intrinsic rates of increase are the most convenient instruments for characterizing the growth potential of predators. Polat-Akköprü and Atlıhan (2014) worked on predation rate and two-sex life table of C. carnea fed on Panaphis juglandis, and they determined that the intrinsic rate of increase (r), the finite rate of increase (λ), the net reproductive rate (R0), and the mean generation time (T) of were 0.103 d−1, 1.108 d−1, 59.91 offsprings and 39.72 days respectively. The same researchers in another study (Polat-Akköprü and Atlıhan, 2016) identified r, R0, λ, and T as 0.114 d−1, 122.5 days, 1.119 d−1 and 40.48 days for Chrysoperla carnea fed with Myzus persicae (Hemiptera: Aphididae); Kasap et al. (2003) determined r as 0.138, R0 as 155.7, and T as 36.7 days for C. carnea fed with Aphis pomi. In terms of R0, r, and T, there appear to be differences between the literature and our study results. These differences are considered to be due to differences in the type of prey fed to the predators. There are many studies indicating that differences in population growth parameters stem from food quality (Bouras and Papadoulis 2005; Momen and Abdel-Khalek 2008).
The life expectancy data in our study differ from that described in the literature. It is known that nutritional variety has different effects on the life expectancy of natural predators. For example, Jokar and Zarabi (2012) determined the mean life expectancy of a newly laid one-day egg of C. carnea fed on S.graminum as 24.05 days. Yu et al. (2013a) found that the mean life expectancy for a newly laid one-day egg of C. pallens feeding on A. craccivora to be 49 days, with the life expectancy 78 days for C. pallens females. The life expectancy, exj, obtained using age stage two-sex life tables shows the differences between individuals of the same age and differences between sexes and periods. As the study was performed in climate-controlled cabins, negative effects due to natural conditions (climate factors, natural predators, competition among others) were prevented. Thus, the aging of the pest reduced the mean life expectancy in different stages (Fig. 2a).
The reproductive value is a significant factor in management programs. The reproductive value of a newborn of C. carnea feeding on C. juglandicola and finite rate of increase (λ) of the population was found to be 1.070 (Fig. 2b). Yu et al. (2013b) reported that the reproductive value of a newborn of C. pallens feeding on A. craccivora was 1.134 and the finite rate of increase value was equal to that value.
In this study, the future status of the natural enemy population was tried to be revealed with the 60-day population projection based on the age-stage, two-sex life table(Fig. 3a). The variability range of C. carnea’s population growth was simulated, with a high level of variability in natural enemy populations(Fig. 3b).
Results demonstrated that C. carnea fed on C. juglandicola nymphs had the highest predation capacity in the third larval period compared to other periods (Table 3.) Most researchers have reported that the predation rate of preadult stages of C. carnea generally increase with age and period structure (Klingen et al. 1996; Silva et al. 2002; Huang and Enkegaard 2010; Bailey et al. 2011; Khan et al. 2013; Yu et al., 2013a; Polat-Akköprü and Atlıhan, 2014;).
In the present study, the net predation rate (C0) was 212.9 aphids (Table 3.). Polat-Akköprü and Atlıhan (2014) stated that the net predation rate of the predator was 143.94 aphids of C. carnea fed with Panaphis juglandis. The results of the net predation rate mentioned is lower than that of our. Polat-Akköprü and Atlıhan (2016) also reported the net predation rate (C0) of C. carnea fed on Myzus persicae as 324.67 aphids. However, Khan et al. (2013) studied predatory potential of C. carnea fed on Schizaphis graminum and the results showed that the larvae consumed 414.6 aphids. The results stated is higher than that of our, the mismatch with literature may be attributed to the different prey used in the experiments.The transformation rate (Qp) of C. juglandicola that a C. carnea needs to produce a single live egg is 3.41 (Table 3), that is, about three C. juglandicola are needed for production of a C. carnea egg.
Biological control is a slow process, it takes time for biological agents to suppress the pest, In the period required until natural enemies control pest population, the pests may be present in intolerable populations (Kok, 1999). Reasons such as being sensitive to environmental conditions, and biological periods not compatible with pest cause fluctuations in pest populations (Reichelderfer, 1980; Van Emden and Service, 2004.).
Yu et al. (2013b) reported that predation capacity of predators were reset during their biological periods that could not be fed with prey and pest populations increased. Chi (1990) and Yu et al. (2013b) showed with population estimations based on age-stage two sex life tables variations in period structure of insects during population growth and predation capacity. We have simulated the population growth and predation capacity of the predator in order to prevent pest population increases that will occur in nature during these periods. In the projection we made, we determined that the predation gap was closed with a third release made from 15 eggs of 2 days, 10 days after the second release. In another study on this subject, Yu et al. (2013b) compared the population estimation of C. pallens for 3-day old larvae from 10 eggs and adults for population growth and predation capacities. When they changed the date of the second release to the 10th day, they reported that the time interval did not occur and there was no reduction in predation capacity.
Survival rates, times of developmental stages, stage differentiation, fecundity, and population parameters of C. carnea fed on C. juglandicola were determined. Predation rates linked to life tables were detected. Furthermore, by collating all of the resulting data, the population projection based on the age-stage was created. The results obtained in this study provide useful knowledge for the use of C. carnea as an important control agent in biological management studies in the walnut orchards and other insect pest species. The use of the age-stage, two-sex life table is recommended on a large scale for studies that concern integrated pest management and biological control, and in all related research areas.
Change history
24 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12600-020-00851-2
References
Anonymous (2017). Chromaphis juglandicola. http://influentialpoints.com/Gallery/Chromaphisjuglandicola_Small_walnut_aphid.htm. (accessed 22 May 2017).
Atlıhan, R., Kaydan, B. M., & Yarımbatman, A. (2007). Callaphis juglandis ile beslenen Adalia fasciatopunctata revelierei’nin işlevsel tepkisi, Türkiye II. Bitki Koruma Kongresi Bildirileri. 27–29 Ağustos 2007, Isparta, S: 19.
Aziza, M., El-Gantiry, El-Arnaouty, S. A., Badawy, H. M., & Nevien, M. G. (2007). Biochemical variation in the life stages and populations of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). Egyptian Journal of Agricultural Research, 85(6), 2121–2138.
Bailey, R., Chang, N., & Lai, P. (2011). Two-sex life table and predation rate of Cybocephalus flavocapitis smith (Coleoptera: Cybocephalidae) reared on Aulacaspis yasumatsui Takagi (Hemiptera: Diaspididae), in Taiwan. Journal of Asia-Pacific Entomology, 14, 433–439.
Barnes, M. M., Barbett, W. W., Culver, D. J., Davis, C. S., Olson, W. H., Ridel, H., & Schreader W. R. (1982). Insect and mites. İn:Integrated pest management for walnut (M.L. Flint eds.) niversity of California statewide integrated pest management project division of agricultural science publication 3270, California, Usa.
Barnes, M. M., & Moffitt, H. R. (1979). A five-year study of the effects of the walnut aphid and the european red mite on persian walnut productivity in coastal orchards12. Journal of Economic Entomology, 71(1), 71–74.
Bouras, S. L., & Papadoulis, G.-T. H. (2005). Influence of selected fruit tree pollen on life history of Euseius stipulatus (Acari: Phytoseiidae). Experimental & Applied Acarology, 36, 1–14. https://doi.org/10.1007/s10493-005-2381-5.
Bozsik, A. (1995). Effect of some zoocides on Chrysoperla carnea adults (Planipennia, Chrysopidae) in The laboratory. Anzeiger für Schadlingskunde, Pflanzenschutz, Umweltschutz, 68, 5859. https://californiaagriculture.ucanr.org/fileaccess.cfm?article=64128&p...pdf. Accessed 06.02.2019
Chi, H., & Liu, H. (1985). Two new methods for the study of ınsect populatıon ecology. Bull. Inst. Zool. Academia Sinica, 24(2), 225–240.
Chi, H. (1988). Life-table analysis ıncorporating both sexes and variable development rates among ındividuals. Environmental Entomology, 17(1), 26–34.
Chi, H. (1990). Timing of control based on the stage structure of pest populations: Asimulation approach. Journal of Economic Entomology, 83(4), 1143–1150.
Chi, H., & Yuan, S. H. (2005). Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead)(Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer)(Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environmental Entomology, 35(1), 10–21.
Chi, H., & Su, H. (2006). Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and ıts host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proffof the relationship between famale fecundity and the net reproductive rate. Environmental Entomology, 35(1), 10–21.
Chi, H. (2020). TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. (http://140.120.197.173/Ecology/Download/TWOSEX-MSChart.zip). (accessed 09 May 2020).
Chi, H. (2020a). CONSUME-MSChart: A computer program for the predation rate analysis based on age-stage, two-sex life table. (http://140.120.197.173/Ecology/Download/Consume-MSChart.zip). (accessed 09 May 2020).
Chi, H. (2020b). TIMING-MSChart: A computer program for the population projection based on age-stage, two-sex life table. (http://140.120.197.173/ecology/download/timing-MSChart.Zip). (accessed 09 May 2020).
Erol, T., & Atlıhan, R. (1995). Değişik yaprakbiti (Homoptera:Aphididae, Callaphididae) türleriyle beslenen Adalia fasciatopunctata revelierei (Mulsant) (Coleoptera: Coccinellidae) nin biyolojisi üzerinde araştırmalar. Türkiye Entomoloji Dergisi, 19(4), 277–286.
Erol, T., & Yaşar, B. (1994). Van ili elma ağaçlarında bulunan zararlı ve yararlı böcek türleri ile nemlilerinin popülasyon yoğunlukları üzerinde araştırmalar. TÜBİTAK Tarım Ormancılık Araştırma Grubu, Proje No:769.
Farrokhi, M., Gharekhani, G., Iranipour, S., & Hassanpour, M. (2017). Effect of different artificial diets on some biological traits of adult green lacewing Chrysoperla carnea (Neuroptera: Chrysopidae) under laboratory conditions. Journal of Entomology and Zoology Studies, 5(2), 1479–1484.
Goodman, D. (1982). Optimal life histories, optimal notation, and the value of reproductive value. The American Naturalist, 119, 803–823.
Huang, N., & Enkegaard, A. (2010). Predation capacity and prey preference of Chrysoperla carnea on Pieris brassicae. Biocontrol., 55(3), 379–385.
Huang, Y., & Chi, H. (2012). Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate a case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). Journal of Agriculture and Forestry, 61(1), 37–45.
Huang, H. W., Chi, H., & Smith, C. L. (2018). Linking demography and consumption of Henosepilachna vigintioctopunctata (Coleoptera:Coccinellidae) fed on Solanum photeinocarpum (solanales: solanaceae): with a new method to project the uncertainty of population growth and consumption. Journal of Economic Entomology, 111(1), 1–9.
Jafari, S., & Bazgir, F. (2015). Life history traits of predatory mite Typhlodromus (Anthoseius) bagdasarjani (Phytoseiidae) fed on Cenopalpus irani (Tenuipalpidae) under laboratory conditions. Systematic & Applied Acarology, 20(4), 366–374.
Jaskiewicz, B., & Cichocka, E. (2004). Aphids on European walnut (Juglans regia L.) in the urban conditions of Lublin. Aphids and other hemipterious ınsect. PAS Skierniewice, 10, 3–46.
Jaskiewicz, B., & Kmiec, K. (2007). The occurrence of Panaphis Juglandis (Goetze) and Chromaphis Juglandicola (Kalt.) on walnut under the urban condıtıons of Lublın Agricultural University in Lubliacta Sci. Pol. Hortorum Cultus, 6(3), 15–26.
Jokar, M., & Zarabi, M. (2012). Surveying effect kind of food on biological parameters on Chrysoperla carnea (Neuroptera: Chrysopidae) under laboratory conditions. Egyptian Academic Journal of Biological Sciences. A, Entomology, 5(1), 99–106.
Karut, K., & Kazak, C. (1999). Zakkum (Nerium oleander L.) bitkilerinden toplanan Chrysoperla carnea (Stephens) yumurtalarının doğal ölüm, açılma ve parazitlenme oranlarının belirlenmesi. Türkiye 4. Biyolojik Mücadele Kongresi Bild. 269-276.
Karut, K., & Şekeroğlu, E. (1999). Chrysoperla carnea (Stephens) yumurtalarının laboratuvar koşullarında depolanma olanakları. Türkiye 4. Biyolojik Mücadele Kongresi Bild. 463-472.
Kasap, İ., Aktuğ, Y., & Atlıhan, R. (2003). Avcı Böcek Chrysoperla carnea (Stephens) (Neuroptera:Chrysopidae)‘nın bazı biyolojik özellikleri üzerine araştırmalar. Yüzüncü Yil Üniversitesi Ziraat Fakultesi Tarim Bilimleri Dergisi, 13, 49–53.
Khan, J., Haq, E., Javed, H. I., Mahmood, T., Rasool, A., Akhtar, N., & Abid, S. (2013). Bıologıcal parameters and predatory potential of Chrysopa carnea (Neuroptera: Chrysopidae) feding on wheat aphid Schızaphis graminum under laboratory conditions. Pakistan Journal of Agricultural Research, 26, 4.
Khuhro, N. H., Chen, H. Y., Zhang, Y., Zhang, L. S., & Wang, M. Q. (2012). Effect of different prey species on the life history parameters of Chrysoperla sinica (Neuroptera: Chrysopidae). European Journal of Entomology, 109, 175–180.
Klingen, N. S., Johansen, N. S., & Hofsvang, T. (1996). The predation of Chrysoperla carnea (Neurop., Chrysopidae) on eggs and larvae of Mamestra brassicae (Lep., Noctuidae). Journal of Applied Entomology, 120(1–5), 363–367.
Kişmir, A., & Şengonca, Ç. (1981). Anisochrysa (Chrysoperla) carnea (Stephens) (Neuroptera: Chrysopidae)‘nın kitle üretim yönteminin geliştirilmesi üzerinde çalışmalar. Türkiye Entomoloji Derneği, 5(1), 35–43.
Kok, L.T. (1999). Biological Control for the public. https://www.ento.vt.edu/~kok/Biological_Control/BC_html.htm. Accessed 09.01.2019
Liu, T., & Chen, T. (2001). Effects of three aphid species (Homoptera: Aphididae) on development, survival and predation of Chrysoperla carnea (Neuroptera: Chrysopidae). Applied Entomology and Zoology, 36, 361–366.
Momen, F. M., & Abdel-Khalek, A. (2008). Effect of the tomato rust mite Aculops lycopersici (Acari: Eriophyidae) on the development and reproduction of three predatory phytoseiid mites. International Journal of Tropical Insect Science, 28(01), 53.
Obrycki, J. J., Hamid, M. N., Sajap, A. S., & Lewis, L. C. (1989). Suitability of corn insect pests for development and survival of Chrysoperla carnea and Chrysopa oculata (Neuroptera: Chrysopidae). Environmental Entomology, 18(6), 1126–1130.
Polat-Akköprü, E., & Atlıhan, R. (2014). Two-sex life table and predation rate of Chrysoperla carnea (Stephen) (Neuroptera: Chrysopidae) fed on Panaphis juglandis (Goeze) (Hemiptera: Callaphididae). Entomological Society of America Annual Meeting 2014.
Polat-Akköprü, E., & Atlıhan, R. (2016). Combining insect life table and predation rate for IPM and biological control: A study of Chrysoperla carnea (Stephen) (Neuroptera:Chrysopdiae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). 2016 International Congress of Entomology, https://doi.org/10.1603/ICE.2016.91797.
Polat-Akköprü, E., Atlihan, R., Okut, H., & Chi, H. (2015). Demographic assessment of plant cultivar resistance to ınsect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. Journal of Economic Entomology, 108(2), 1–10.
Reichelderfer, K. (1980). Economic Feasibility of Biological Control of Crop Pests. In: Beltsville Symposia in Agricultural Research (ed.) (1980): Biological control in crop production. London/ Torondo: Osmun. Pg. 403–417.
Saljoqi, A. R., Muhammad, G., Huma, Z., Ahmad, B., Zada, H., Rehman, S., Nadeem, M., & Salim, M. (2015). Screening of various irrigated wheat varieties against Angoumois grain moth, Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae). Journal of Entomology and Zoology Studies, 3, 36–41.
Sattar, M., Abro, G. H., & Syed, T. S. (2011). Effect of different hosts on biology of Chrysoperla carnea (Stephens)(Neuroptera: Chrysopidae) in laboratory conditions. Pakistan Journal of Zoology, 43(6).
Sharov, A. (2012). Population ecology, Virginia Tech, VA, USA. http://home.comcast.net/~sharov/popechome/welcome.html. Accessed 02.02.2019.
Silva, G. A., Carvalho, C. F., & Souza, B. (2002). Aspectos biológicos de Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) alimentada com lagartas de Alabama argillacea (Hubner, 1818) (Lepidoptera: Noctuidae). Ciências e Agrotecnologia, 26, 682–698.
Takalloozadeh, M. H. (2015). Effect of different prey species on the biological parameters of Chrysoperla carnea (Neuroptera: Chrysopidae) in laboratory conditions. Journal of Crop Protection, 4(1), 11–18.
The Food and Agriculture Organization Corporate Statistical Database (2018). Walnut production quantities by country. (http://faostat3.fao.org, accessed 24 May. 2019).
Toros, S., Yaşar, B., Özgökçe, M. S., & Kasap, İ. (1996). Van İlinde Aphidoidea (Homoptera) üst familyasına bağlı türlerin saptanması üzerinde çalışmalar. Türkiye 3. Entomoloji Kongresi Bildirileri. 24-28 Eylül 1996, Ankara, 549-556.
Tuik (2018) Türkiye İstatistik Kurumu. Ceviz üretim miktarı. http://www.tuik.gov.tr/Start.do (accessed 05 May 2019).
Tuan, S. J., Lee, C. C., & Chi, H. (2014). Population and damage projection of Spodoptera litura (F.) on peanuts (Arachis hypogaea L.) under different conditions using the age-stage, two-sex life table. Pest Management Science, 70, 805–813.
Van Emden, H. F., & Service, M. W. (2004). Pest and vector control. Cambridge: University Press.
Yarımbatman, A., & Atlıhan, R. (2008). Farklı Sıcaklık Koşullarında “Chrysoperla carnea” nın Gelişme ve Üremesinin İncelenmesi (yüksek lisans tezi, basılmamış) YYÜ, Fen Bilimleri Enstitüsü, Van.
Yu, J. Z., Chi, H., & Chen, B. H. (2013a). Comparsion of the life table and predation rates of Harmonia dimidiate (F.) (Coleptera: Coccinellidae) fed on Aphis gossypii glover (Hemiptera: Aphididae) at different temperatures. Biological Control, 64, 1–9.
Yu, L., Chen, Z., Zheng, F., Shı, A., Guo, T., Yeh, B., Chi, H., & Xu, Y. (2013b). Demographic analysis, a comparison of the jackknife and bootstrap methods, and predation projection: A case study of Chrysopa pallens (Neuroptera: Chrysopidae). Journal of Economic Entomology, 106(1), 1–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yılmaz, M., Polat Akköprü, E. Predation rate linked to life table of Chrysoperla carnea (Stephen) (Neuroptera: Chrysopidae) fed on small walnut aphid (Chromaphis juglandicola) (Kalt.) (Hemiptera: Aphididae): with population and predation projections. Phytoparasitica 49, 217–228 (2021). https://doi.org/10.1007/s12600-020-00834-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-020-00834-3