Abstract
Plutella xylostella L. (diamondback moth) is a pest of cruciferous plants and has devastating effects on the yield and quality of cruciferous vegetables. Insect midgut protease plays an important role in the digestion of host plant nutrients and the detoxification of defensive compounds. The effects of host plants on the larval midgut protease activity and the performance of P. xylostella larvae were examined on six different host plants, including qingan 70, lvqiu 66, qingan 80, Brassica chinensis, broccoli and oilseed rape The results showed that P. xylostella larvae ingested all hosts, and the protease activities were significantly different at different instars. In the 3rd instar and early 4th instar, which were the important periods for digestion, 4 proteases activities of P. xylostella were greater than activity in other instars. Meanwhile, the activities of the high-alkaline trypsin and low-alkaline trypsin (important proteases in the insect midgut) were greatest when P. xylostella larvae ingested qingan 80. The relative host suitability, ranked from highest to lowest was: broccoli > oilseed rape = Brassica chinensis > qingan 70 > qingan 80 > lvqiu66. The intrinsic rate of increase indicated that P. xylostella had the best adaptability and food receptivity to the oilseed rape, whereas when P. xylostella larvae ingested Brassica chinensis, the activities of the trypsin were highest. This suggests Brassica chinensis in an appropriate host for P. xylostella that highly elevates the activities of trypsin that subsequently helps the insect to digest nutrients obtained from host plants. In practical application, we can reduce the harm to target plants by adjusting the plantation structure by optimally planting different hosts limit growth and reproduction to minimize pest outbreaks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proteases are involved in a variety of physiological and biochemical processes inside the midgut of insects, including breaking peptide bonds of proteins into shorter polypeptides and promoting food digestion and nutrient absorption. The midgut proteases can be classified into serine proteases, metalloproteinases (MMPs), cysteine proteases and aspartate proteases. Among these, the serine proteases take part mainly in the digestive processes. They normally play an important role in the digestive function by breaking peptide bonds of large proteins into smaller particles (Oppert 1999; Jiang and Kanost 2000; Li et al. 2004). Most serine proteases have an active center, the serine residue, at a conserved position (Ser-195). The function of serine protease can be fulfilled through three steps: firstly, the active center is activated by the nearby amino acid residues; secondly, activated hydroxyl and carbon atoms in the peptide bond react with nucleophilic reagents, and carbon on the acyl group is then esterified after the peptide bond breaks, resulting in the release of the N-terminus of the peptide bond; finally, the hydrolysis reaction, following the release of the C-terminus, completes the reaction (Bergmeyer et al., 1983). In lepidopterans, the larval midgut was found to contain several serine proteases, particularly trypsin and chymotrypsin (Milne and Kaplan 1993). The lepidopteran midgut has an alkaline environment where serine proteases are reported to have high level of activity (Pritehett et al. l981; Applebaum 1985; Broadway and Duffey 1986). The alkaline trypion refers to the enzyme capable of hydrolyzing protein peptide bond under alkaline conditions, and the optimal pH value is within the range of 9 to 11 (Berenbaum 1980). For instance, the optimal pH of trypsin in Trichoplusia ni Hübner (Lepidoptera: Noctuidae) and Pieris rapae L. (Lepidoptera: Pieridae) were reported as 8.5 and 8.0, respectively, and the optimal pH of chymotrypsin were reported to be 8.0 and 9.0, respectively (Broadway 1989).
Insects obtain a majority of their nutrients from host plants whilst they can synthesize some compounds, thus nutrition is important for the growth, reproduction and the daily energy supply of an organism (Pu 2007; Chapman 2013). As the content, types and ratio of nutrient material in different plants were different, so the host plants may influence the dynamics of pest populations, such as the larval survival of Cydia pomonella L. (Lepidoptera: Tortricidae) (Van Steenwyk et al. 2004). Previous studies showed that the chemical composition of host plants can affect the survival, development and reproduction of insects (Waldbauer 1968; Scriber & Slansky, 1981; Singh and Mullick 1997; Sarate et al. 2012). Many insects keep their nutrition balanced through the digestion activity of protease. The polyphagous insect, P. xylostella, obtains its nutrients from different host plants that can directly affect its growth, development and reproduction (Lu 2003). Although a number of studies have reported factors that influence the growth, development and reproduction of Plutella xylostella, the relationship between growth, development and midgut proteases activities remains unclear (Zhang et al. 1998; Chen & Liu, 2004; Duan et al. 2010; Chen et al. 2014).
In this study, we determined the larval midgut protease activity of P. xylostella fed with different host plants. We hope that the findings of this study could improve our understanding about the relationship between protease activity, host plants and the growth and development of P. xylostella and determine a new way to control populations of this insect pest.
Materials and methods
Experimental insects and host plants
For this study, Plutella xylostella samples were obtained from a laboratory colony maintained at the College of Plant Protection, Northwest A&F University, Yangling, Shaanxi, China, and larvae were reared on Brassica oleracea (cv. Qingan 70) at 24 ± 2 °C, 70 ± 10% relative humidity (RH) and under a 15-h light:9-dark photoperiod. The adults were provided with 10% honey solution for supplementary adult feeding to improve the female oviposition.
The seeds of six host plants (Table 1) were producted by the College of Horticulture, Northwest A&F University, Yangling, Shaanxi, China, and the host plants were planted in the greenhouse (25 ± 2 °C, 75 ± 10% RH and under a 15-h light:9-dark photoperiod).
Reagents and instruments
Reagents: dimethyl sulfoxide (DMSO) azocasein, Nα-Benzoyl-DL-arginine-p-nitroanilide (BAρNA), Nα-P-Tosyl-L-arginine methyl ester hydrochloride (TAME), N-Benzoyl-L-Tyrosine Ethyl Ester (BTEE), NaCl, bovine serum albumin (BSA) were purchased from Shanghai Biotechnology Company.
Instruments: microplate reader (Sunrise, Tecan, Austria), refrigerated centrifuge (Hettich, Germary), thermostat incubation (Biotechnology Company, Shanghai).
P. xylostella larvae fed with different host plants
P. xylostella larvae (incubated the same number of days) ingested six different host plants. The group that ingested host plant qingan 70 and the host used in the laboratory colony served as the control group, served as the control group. There were 150 larvae in each experiment group, and the experiment was repeated 3 times.
Extraction of P. xylostella midgut proteases
The larval midgut proteases were extracted as described by Wang (1996). Healthy 2nd instar larvae of equal sizes were rapidly dissected on ice to collect the midgut, and the gut contents were flushed using 0.15 mol/L NaCl. Subsequently, the midgut was placed in a 1.5 mL centrifuge tube and rapidly homogenized over ice. The crude extract was centrifuged at 12,000×g for 15 min at 4 °C, and the supernatant was collected and stored at −20 °C. The same treatment was performed on 3rd, early 4th and late 4th instar larvae, respectively. Protein concentration in the extract was assayed using the method of Bradford (1976).
Determining the protease activity in the midgut of P. xylostella larvae
Total protease activity was determined as follows: A 20 mg/mL solution of azocasein, the substrate was prepared in NaCl (0.15 mol/L) and stored at 4 °C. Subsequently, the following substances were added to a 1.5 mL centrifuge tube: 100 μL azocasein solution, 10 μL midgut enzyme extract and 40 μL glycine/NaOH reaction buffer (0.1 mol/L, pH 11.0). The mixture was incubated at 30 °C for 3 h, and then 150 μL of 20% (v/v) trichloroacetic acid (pre-cooled, 4 °C) was added to terminate the reaction. The mixture was centrifuged at 12,000×g for 15 min at 4 °C to collect the supernatant, which was termed as the midgut protease extract in this study. Protease activity in the extract was determined by measuring absorbance at 415 nm using a plate reader (Wang 1996).
The trypsin activity was determined using two specific substrates: BAρNA and TAME. BAρNA was dissolved in DMSO at a concentration of 20 mg/mL and stored at 4 °C. Then, 100 μL BAρNA, 10 μL midgut enzyme extract, and 90 μL Tris-HCl reaction buffer (0.1 mol/L, pH 10.5) were added to a 1.5 mL centrifuge tube and incubated at 30 °C for 20 min. Subsequently, 100 μL of 20% (v/v) trichloroacetic acid (pre-cooled, 4 °C) was added to terminate the reaction. The reaction mixture was centrifuged and 200 μL of the supernatant was used to determine the absorbance at 405 nm using a plate reader. TAME was dissolved in NaCl (0.15 mol/L) at a concentration of 2 nmol/L. Then, 100 μL TAME, 10 μL midgut enzyme extract and 90 μL of the Tris-HCl reaction buffer (0.1 mol/L, pH 8.5) were added to a 1.5 mL centrifuge tube and incubated at 30 °C for 20 min. The mixture was centrifuged, and the supernatant was used to measure the absorbance at 247 nm using a plate reader (Wirnt 1974).
The chymotrypsin activity was determined using the substrate BTEE dissolved in NaCl (0.15 mol/L) at a concentration of 1 mmol/L and stored at 4 °C. Then, in a 1.5 mL centrifuge tube, 100 μL BTEE solution, 10 μL midgut enzyme extract and 90 μL glycine/NaOH reaction buffer (0.1 mol/L) were added in a 1.5 mL centrifuge tube and incubated at 30 °C for 20 min. An aliquot of the supernatant was used to measure absorbance at 256 nm using a plate reader (Wirnt 1974).
Effect of the different host plants on the performance of P. xylostella
Plutella xylostella larvae ingested six different host plants, and the growth and development were determined under the same feeding conditions for each host. In each experiment, one hundred newly eggs were placed in the Petri dishes (circular, plastic, 9 cm in diameter) with host plant leaves. Hatching time was recorded for each egg. Each neonate larva was transferred to another Petri dish (circular, plastic, 9 cm in diameter) and numbered. The leaves in Petri dishes were changed every other day. When a larva transformed into a pupa, the time was recorded. The time was recorded when a pupa transformed into a female/male. A newly emerged male and female moth were put into paper cups and provided with 10% honey water to drink. The number of eggs and the time of death were recorded at 0800 h and 2000 h daily until emergence ceased, and there were three replicates per treatment. Subsequently, the parameters such as egg duration, larval duration, larval survival rate, pupal duration, pupation rate, pupal weight, adult duration and adult emergence rate for each treatment were computed based on the information that was recorded in the experiment.
Effect of the different host plants on the population parameter estimation of P. xylostella
In this study, using the above information, the following parameters were calculated: net reproduction rate (R0), mean generation Time (T), intrinsic rate of increase (rm), finite rate of increase (λ), and population doubling time (td).
- lx:
-
survival rate
- x:
-
time
- mx:
-
the number of eggs produced by per female.
Evaluation of host plant quality
Referring to the method of Yang (2013), in this study, we selected eight vital indicators for growth, reproduction and life table parameters of P. xylostella, such as the larval duration, generation duration, survival rate of larvae, pupation rate, fecundity, net reproduction rate, intrinsic rate of increase and population doubling time, according to its favorable degree (the appropriate degree) to the individual development of P. xylostella. Each of the eight indicators was given equal weight in the calculation of the index, ranked on a scale from highest (least suitable host) to lowest (most suitable host). The overall scores of the six host plants were compared, and the host plant with the lowest index was deemed the most appropriate host for P. xylostella.
Data analysis
The statistical differences between the control and treatment groups were analyzed by ANOVA (Analysis of Variance), while the treatment means were compared using the least significant difference (LSD) test at 5% significance level. To avoid pseudoreplication, each treatment and control had three sets of replicates. Data were analyzed statistically by using statistical software package SPSS 20.0, and GraphPad Prism 5 was used to create the figures.
Results
Effects of the different host plants on the midgut protease activity in P. xylostella larvae
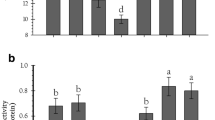
P. xylostella larvae ingested six different host plants and the activity of total proteases, high-alkaline trypsin, low-alkaline trypsin and chymotrypsin were measured in different instars (Fig. 1). P. xylostella larval ingested lvqiu 66 showed that the total protease activities was the highest among the six experiment groups. Moreover, the activity of all treatment groups was the highest in 3rd instar (F = 10.74, df = 5, 12, p < 0.05) and 4th early instar (F = 21.21, df = 5, 12, p < 0.05) (Fig. 1a). The high-alkaline trypsin activities of P. xylostella larvae ingested different host plants were significantly different in 2nd instar (F = 34.67, df = 5, 12, p < 0.05), 3rd instar (F = 23.56, df = 5, 12, p < 0.05), 4th early instar (F = 21.07, df = 5, 12, p < 0.05) and 4th late instar (F = 41.03, df = 5, 12, p < 0.05). The high-alkaline trypsin activity was greatest when P. xylostella larvae ingested the qingan 80, and the lowest when the larvae ingested broccoli (Fig. 1b). The low-alkaline trypsin activities in all the experimental treatments were the highest in the 3rd instar larvae (F = 4.72, df = 5, 12, p < 0.05) (Fig. 1c). In the 2nd instar (F = 36.81, df = 5, 12, p < 0.05) and 3rd instar larvae (F = 2.66, df = 5, 12, p = 0.15), the chymotrypsin activity was the highest when P. xylostella ingested qingan 80, however in the early 4th instar (F = 16.91, df = 5, 12, p < 0.05) and late 4th instar larvae(F = 5.00, df = 5, 12, p < 0.05), the chymotrypsin activities were the highest when P. xylostella larvae ingested Brassica chinensis (Fig. 1d).
Effects of host plants on the activity of proteases in Plutella xylostella larvae. a Total protease. b High-alkaline trypsin. c Low-alkaline trypsin. d Chymotrypsin. The dotted line indicates the control group. Columns represent means of three independent values and their SEM. P1: cabbage-lvqiu 66; P2: cabbage-qingan 70; P3: cabbage-qingan 80; P4: brassica chinensis; P5: broccoli; P6: oilseed rape
Effect of different host plants on the performance of P. xylostella
P. xylostella larvae were provisioned with different host plants in order to examine host performance (Table 2). Generation duration (F = 14.29, df = 5, 12, p < 0.05) of P. xylostella that ingested qingan 70, lvqiu66, qingan 80, Brassica chinensis, broccoli and oilseed rape were significantly different. The egg duration (F = 1.571, df = 5, 12, p = 0.24) and the pupal duration (F = 0.797, df = 5, 12, p = 0.57) of P. xylostella on different hosts did not change significantly. The larval duration (F = 17.33, df = 5, 12, p = 0.03) of P. xylostella showed significant difference among the six treatments, was longest (10.48 days) when ingesting oilseed rape. The adult duration (F = 28.56, df = 5, 12, p < 0.05) of P. xylostella showed significant difference, and was longest (16.63 days) when larvae ingested Brassica chinensis.
The larval survival rate is an important parameter that affecting the population dynamics of P. xylostella. In Table 3, the larval survival rates showed significant differences when P. xylostella ingested different host plants (F = 10.29, df = 5, 12, p = 0.02), and was greatest on the broccoli and the qingan 70 treatment groups, followed by oilseed rape and Brassica chinensis. The pupation rate (F = 44.87, df = 5, 12, p < 0.05) and the fecundity (F = 25.10, df = 5, 12, p = 0.04) were greatest when P. xylostella ingested oilseed rape. Additionally, the emergence rate of P. xylostella was was greatest when larvae ingested qingan 70, and lowest when larvae ingested the qingan 80.
The intrinsic rate of increase of the insect population indicates the adaptability and voracity of the insects to its host. In Table 4, the intrinsic rate of increase rm (F = 82.27, df = 5, 12, p = 0.02) was highest when P. xylostella ingested the broccoli, followed by the Brassica chinensis and the oilseed rape. The net reproduction rate R0 (F = 1240, df = 5, 12, p < 0.05) was highest when P. xylostella ingested the Brassica chinensis, with an average fecundity of 386.38 offspring per female, indicating that P. xylostella has the potential to erupt on the Brassica chinensis, followed by the broccoli. At the same time, the population doubling time td (F = 224.9, df = 5, 12, p < 0.05) and the mean generation time T (F = 1146, df = 5, 12, p = 0.03) were lowest when P. xylostella ingested broccoli. The finite rate of increase λ(F = 67.61, df = 5, 12, p < 0.05) was the largest when P. xylostella ingested the broccoli.
In this experiment, the host quality of the six plant varieties was comprehensively evaluated. According to the index, the smaller the number, the better the quality of the host. Brocolli was the most superior host (Index = 12, Table 5) for P. xylostella followed by oilseed rape. In summary, the host suitability can be ranked as follows: broccoli > oilseed rape = Brassica chinensis > qingan 70 > qingan 80 > lvqiu 66 (Table 5).
Discussion
The diverse plant hosts had different effects on the activities of total protease, high-alkaline trypsin, low alkaline trypsin and chymotrypsin in the midgut of larval P. xylostella. In this study, when P. xylostella larvae ingested different hosts, the activities varied greatly, in this study,the total protease activity of P. xylostella larvae that ingested lvqiu 66 was higher than the other experimental treatments, and the activity of all treatment groups was the highest in 3rd instar and 4th early instar, and lowest in 4th later instar. In other studies, the activities of the proteases increased from the 3rd to the 5th instar and then decreased at the onset of pupation in the late 5th instar (Kipgen and Aggarwal 2014). As insects get closer to the pupal stage, they eat little, consequently leading to a decline of protease activities. According to Zhao et al. (2017), the high-alkaline trypsin is an important digestive protease in the P. xylostella larval midgut, and in this study, the high-alkaline trypsin activity was greatest when P. xylostella larvae ingested the qingan 80, and lowest when the larvae ingested the broccoli, that is, when P. xylostella larvae ingested the host plant qingan 80 and lvqiu 66 with lower suitability, the activity of the high-alkaline trypsin were great, that high-activity can help P. xylostella larvae to ingest the host plant and get the nutrient for developing.
The nutrient levels of host plants have great influences on the growth and development of insects, and the protein content of the host has a significant effect on survival rate and fecundity of P. xylostella population (Wakisaka et al. 1992). Among the bean cultivars tested, red kidney bean (Phaseolus vulgaris) Sayyad was the most unsuitable host for Helicoperva armigera Hübner (Lepidoptera: Noctuidae) Namin et al. (2014). Although Orgyia postica Walker (Lepidoptera: Erebidae) was found to complete its life cycle when the larvae ingested six different plants, the female pupal weight, survival rates, fecundity and adult longevity were significantly different. Syzygium samarangense (Myrtaceae) and Mangifera indica (Anacardiaceae) were reported to be suitable host plants for O. postica. Insects that ingested these hosts had higher population trend indexes (Zhu et al. 2005). Our study confirmed that the effects of 6 host plants on the generation cycle of P. xylostella were significantly different, and our results were consistent with those reported by Zhang et al. (2013) and Mu (2015). In this study, the qualities of the six host plants was comprehensively evaluated, the appropriate degree (rank from highest suitability to lowest) of the host plants was: broccoli > oilseed rape > Brassica chinensis > qingan 70 > qingan 80 > lvqiu 66. The general result of the insects performing best on broccoli and worst on lvqiu66 is in agreement with Mu (2015).
Namin et al. (2014) showed that the efficiency of converting ingested and digested food to smaller usable peptides through protease activity was the highest in 5th instar H. armigera larvae when they ingested red kidney beans (Akhtar and Naz) but the lowest when they ingested white kidney bean (Pak). The trypsin activity of P. xylostella larvae that ingested broccoli was the lowest among different instars, indicating that the trypsin activity may be linked to the growth and development of P. xylostella. Together, our results indicated that the host plants, probably with different nutrient level, and pests with different protease activity levels, may jointly affect the growth and development of insects.
Conclusions and future perspectives
In this study, we researched the effect of host plants and protease activities on the growth and development of P. xylostella larvae. We found that the high-alkaline trypsin can help insects to digest nutrients which were obtained from host plants B. chinensis, and it was related to the parameters of the growth and development of P. xylostella. Our study may provide potential basis for forecasting the population dynamics and protease activities of P. xylostella in other cruciferous plants and for making recommendations to growers to change the plantation structure of host plants in the field in order to minimize outbreaks.
References
Applebaum, S. W. (1985). 7 – Biochemistry of digestion. Regulation Digestion Nutrition Excretion, 279–311.
Berenbaum, M. (1980). Adaptive significance of midgut ph in larval lepidoptera. American Naturalist, 115(1), 138–146.
Bergmeyer, H. U., Bergmeyer, J., Grassl, M. (1983). Methods of enzymatic analysis: V.2: Samples, reagents, assessment of results /−3rd ed. Verlag Chemie.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(s 1–2), 248–254.
Broadway, R. M. (1989). Characterization and ecological implications of midgut proteolytic activity in larvalpieris rapae andtrichoplusia ni. Journal of Chemical Ecology, 15(7), 2101–2113.
Broadway, R. M., & Duffey, S. S. (1986). The effect of dietary protein on the growth and digestive physiology of larval Heliothis zea and Spodoptera exigua. Journal of Insect Physiology, 32(8), 673–680.
Chapman, R. F. (2013). The insects. Cambridge: University Press.
Chen, F. Z., & Liu, S. S. (2004). Effects of low and subzero temperature on a Plutella xylostella laboratory population. Chinese Journal of Applied Ecology, 15(1), 99~102.
Chen H. F., Chen Q. H., Huang S. J. 2014. Influences of sublethal concentration of metaflumizone on growth, development and fecundity of Plutella xylostella, (8), 46–48,52.
Duan, Y., Wu, R. H., Wu, Y. Q., Jiang, Y. L., Zhao, M. Q. (2010). Effects of LED illumination on the biology of Plutella xylostella. Journal of henan agricultural sciences, 89(1), 80–82.
Jiang, H., & Kanost, M. R. (2000). The clip-domain family of serine proteinases in arthropods. Insect Biochemistry and Molecular Biology, 30(2), 95–105.
Kipgen, L., & Aggarwal, K. K. (2014). Gut protease profiles of different instars of Helicoverpa armigera (Lepidoptera: Noctuidae). International Journal of Tropical Insect Science, 34(3), 172–178.
Li, H., Oppert, B., Higgins, R. A., Huang, F. N., Zhu, K. Y., and Buschman, L. L. (2004). Comparative analysis of proteinase activities of Bacillus thuringiensis-resistant and susceptible Ostrinia nubilalis (Lepidoptera: Crambidae). Insect Biochem. Mol. Biol. 34, 753–762.
Lu, L. H., He, Y. R., & Pang, X. F. (2003). Effect of cruciferous vegetables on natural populations of the diamondback moth, Pllutella xylostella L. (Lepidoptera: Plutellidae). Acta Ecologica Sinica, 23(12), 2624–2630.
Milne, R., & Kaplan, H. (1993). Purification and characterization of a trypsin-like digestive enzyme from spruce budworm (choristoneura fumiferana) responsible for the activation of delta-endotoxin from bacillus thuringiensis. Insect Biochemistry and Molecular Biology, 23(6), 663–673.
Mu, F., Sun, L., Duan, F., Shen, C., Wan, F. (2015). Influences of three host plants on the traits of experimental population of diamondback moth Plutella xylostella (lepidoptera: Putellidae). Journal of Plant Protection, 42(3), 289–296.
Namin, F. R., Naseri, B., Razmjou, J. (2014). Nutritional performance and activity of some digestive enzymes of the cotton bollworm, Helicoverpa armigera, in response to seven tested bean cultivars. Journal of insect science, 14(93), 1–18.
Oppert, B. (1999). Protease interactions with Bacillus thuringiensis insecticidal toxins. Archives of Insect Biochemistry & Physiology, 42(1), 1–12.
Pritehett, D. W., Young, S. Y., Geren, C. R. (1981). Proteolytic activity in the digestive fluid of larvae of trichoplusia ni. Insect Biochemistry, 11(5), 523–526.
Pu, M.W. (2007). Evaluation of host plant quality and host selection behavior of Spodoptera litura (Fabricius). Yangzhou university.
Sarate, P. J., Tamhane, V. A., Kotkar, H. M., Ratnakaran, N., Susan, N., & Gupta, V. S. (2012). Developmental and digestive flexibilities in the midgut of a polyphagous pest, the cotton bollworm, Helicoverpa armigera. Journal of Insect Science, 12(1), 238–240.
Scriber, J. M., & Slansky, F. (1981). The nutritional ecology of immature insects. Annual Review of Entomology, 26(1), 183–211.
Singh, A. K., & Mullick, S. (1997). Effect of leguminous plants on the growth and development of gram pod borer, Helicoverpa armigera. Indian Journal of Entomology, 59(2), 209–214.
Van Steenwyk, R. A., Fouche, C. F., Collier, T. R. (2004). Seasonal susceptibility of ‘Bartlett’ pears to codling moth (Lepidoptera:Tortricidae) infestation and notes on diapause induction. Journal of Economimc Entomology, 97(3), 976–980.
Wakisaka, S., Tasukuda, R., Nakasui, F. (1992). Effect of natural enemies, rainfall, temperature and host plants on suvrival and reproduction of the diamond back moth. In: Talekar NS ed. Management of Diamond back moth and other crucifer pests: Proceedings of the second international workshop. Taiwan: Asian Vegetable Research and Development Center, 15–26.
Waldbauer, G. P. (1968). The consumption and utilization of food by insects. Advances in Insect Physiology, 5(C), 229–288.
Wang, C. (1996). Partial characterization of protease activity in the midgut of helicoverpa armigera larvae. Acta Entomologica Sinica.
Wirnt, R. (1974). Trypsin, measurements with N-a-ptoluenesulfonyl-l-arginine methyl ester as substrate, 1013–1024.
Yang, X. F. (2013). Effect of host plant factors on occurrence and growing development and oviposition preference of Grapholita molesta Busck.
Zhang G.M., Liu S. S., Lou Z.Y. 1998. Effect of Bacillus thuringiensis on the feeding, development and fecundity of Plutella xylostella, 14(2):58–61.
Zhang, H. C., Gong, L. J., Peng, P., & Yang, Z. X. (2013). The difference of laboratory reproduction of feeding on three kinds host plants. Journal of Hunan Agricultural University (Natural Sciences), 39(1), 70–73.
Zhao, A. P., Zhan, E. L., Sun, C., Liu, T. X., & Li, Y. P. (2017). Effects of Cry1Ac toxin on proteases and carboxylesterase activities in the larvael midgut of Plutella xylostella. Journal of Plant Protection, 44(5), 713–720.
Zhu, J.H., Zhu, W., Zhang, F.P. (2005). Effects of different food plants on development and reproduction of orgyia postica (lepidoptera: Lymantriidae). Entomological Journal of East China.
Acknowledgements
We thank the anonymous reviewers for valuable comments on the manuscript. This research was supported by National Natural Science Foundation of China (Grant No. 31871971, 31772503), National Key R&D Program of China (Grant No. 2017YFD0200900, Agricultural Science and Technology Innovation Projection in Shaanxi Province (Grant No. 2016NY-058).
Author information
Authors and Affiliations
Contributions
Zhao AP and Yuan XQ participated in the design of the study, performed the experiments and data analysis, generated the Figures and Tables, and helped draft the manuscript; Hu Di, Li Y and Leng CM helped Zhao AP to perform the experiments; Li YP , Yuan XQ and Wang P conceived the study, designed the study, coordinated the study and drafted the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicting interests with regard to the work as carried out and reported in this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, A., Yuan, X., Hu, D. et al. The effect of host plant on the development and larval midgut protease activity of Plutella xylostella (Lepidoptera: Plutellidae). Phytoparasitica 47, 475–483 (2019). https://doi.org/10.1007/s12600-019-00746-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-019-00746-x