Abstract
Competition for shared above-ground (e.g. light) and below-ground (e.g. water and nutrients) resources among neighbouring plants is one of the main processes that affect cropping systems. This study aimed to evaluate the effects of weed shading on growth and yield production of bean plants when grown in weedy condition without below-ground competition. The experiments were organized as a split plot design with three replications. The main plots consisted of the nitrogen application rates including of 0, 70, 140 and 280 kg N ha−1 and the sub plots consisted of barnyardgrass densities including 0 (D0), 2 (D2) and 20 (D20) plants m−2. The N application rates were 0 (N0), 25 (N25), 50 (N50), 100% (N100) and 200% (N200) of the recommended N rate on the basis of seed yield goal. Biomass of common bean decreased by 30% for N0, 41% for N50, 35% for N100 and 38% for N200, as weed pressure increased from D0 to D20.. Mean reduction in 100-seed weight, seed number and seed yield caused by high weed pressure was 11, 20 and 41% compared with weed free, respectively. The weed biomass and N uptake increased by 66 and 159% as the nitrogen dose increased from N0 to N200. The highest biomass and N uptake was obtained from N200 treatment. We conclude that agronomically optimum N rates for seed yield was obtained at 140 kg N ha−1 within the range of N fertilization rates used across all weed pressure levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The common bean (Phaseolus vulgaris L.) is an annual legume crop with high protein content which its production is important in areas where people are suffering from an insufficient of animal protein sources (Hillocks et al. 2006). The competitive ability of common bean is generally low as a consequence of short growing period, low height and lower nutrients acquired by root, for this reason weed control is one the critical operations in its production (Stagnari and Pisante 2011). Bean yield can reduce up to 83% by weed interference for above-ground and below groud resources, depending on the weed species, density, and duration of the interference (Aguyoh and Masiunas 2003; Saberali et al. 2012). Barnyardgrass is one of the main weed that affects the bean growth and yield in the field (Fennimore et al. 1984).
Nitrogen is the dominant nutrient added to increase seed yield and the level of N supply can affect leaf area index, leaf area duration, root extension, leaf chlorophyll and photosynthesis activity (Uhart and Andrade 1995; Ding et al. 2005). Bean plants have nitrogen fixation ability via rhizobium, but the amounts of nitrogen fixation are not considerable and variable depending on environmental conditions (Bliss 1993). Rhozobia are Gram negative, free living bacteria in the soil able to convert atmospheric N2 to NH3 only in an endo-symbiotic life association with the lateral roots of legumes (Cooper 2004). In a rhizobia-legume symbiotic relationship, atmospheric N fixation is carried out by the bacteria within the nodules and is absorbed by the plant. Several researches documented that well-managed nitrogen application may increase the competitive ability of crop more than weed (Cathcart and Swanton 2004; Evans et al. 2003). In practice, if an underground competition among neighbouring plants is prevented, then the effect of light competition on growth of competing plants can be defined.
It has also been reported that light has a great effect in determining crop feedback to nitrogen availability and plant nitrogen demand (Harbur and Owen 2004). Andersson and Lundegardh (1999) found that field horsetail (Equisetum arvense L.) responses to nitrogen decreased when plants were shaded. Lindquist and Mortensen (1999) showed that velvetleaf causes maize yield loss primarily through competition for light, suggesting that leaf area and plant height are important canopy characteristics that determine the weed-crop competition. Light competition can be affected by canopy architecture such as leaf area, vertical distribution of leaves and plant height (Spitters and Aerts 1983; Walker et al. 1988).
Barnyardgrass (Echinochloa crus-galli L.) is very competitive annual C4 grass in many parts of the world. Due to its unique ability to germinate and plant height and good branching, it is one of the most damaging weeds of summer crops (Sadeghloo et al. 2013). Barnyardgrass is a principal weed in many summer crops (such as common bean) and 42 countries all over the world (Holm et al. 1977; Fennimore et al. 1984). Common bean has a range of growth habits, ranging from determinate, short and erect types to fully prostrate vining or climbing types (Ehlers 1984). It has been reported that new common bean varieties with erect growth habit compared with semi-erect growth habits have a better shoot architecture, leaf chlorophyll, nitrogen assimilation, and distribution of branches, root length, mass and the number of basal roots (Wortmann 1993; Ramírez-Vallejo and Kelly 1998; Saberali et al. 2016). This study aimed to evaluate the interaction of barnyardgraas interference and nitrogen supply on common bean yield and yield components when below-ground resources (water and nitrogen) completely supply and competition exist only for above-ground resources (light).
Materials and methods
Site description
The field experiments were conducted at the experimental station of Islamic Azad University of Sanandaj, Kurdistan (35° 11´ N, 46° 59′ E, 1400 m above sea level), Iran, in 2014 and 2015. The soil site is classified as inceptisols with a clay loam texture, pH of 7.34 and EC of 1.22 dSm−1. A soil analysis indicated that the available nitrogen to a depth of 0.6 m was 24 and 28 kg ha−1, available K was 277 and 269 mg kg−1, and available P was 6.9 and 6.4 mg kg−1 in 2014 and 2015, respectively.
Experimental design
The experimental design was arranged in split-plot based on randomized complete block design with three replications. The treatments included the rates of nitrogen fertilizers and barnyardgrass densities, which nitrogen treatments comprising the main plots and barnyardgrass densities treatments comprising the sub-plots. Nitrogen rates treatments consisted of 0, 70, 140 and 280 kg N ha−1. The N application rates were 0 (N0), 50 (N50), 100 (N100) and 200% (N200) of the recommended nitrogen rate based on bean seed yield goal. Barnyardgrass densities consisted of 0 (D0), 2 (D2) and 20 (D20) plants m−2. The bean: barnyardgrass ratios were 20:1 and 2:1 in low and high weed density, respectively, which could impose a low and high competition pressure on bean plants.
A determinate erect cultivar (cv. D81083) of common bean with 84 day growth period used in current studies. The urea fertilizer (46–0-0) was applied, with half of the total amount applied at planting and the rest applied at the early pod formation stage (R3, 44–46 days after planting). The N at rates of 140 kg ha−1 represented the amount of nitrogen that would meet the recommended rate based on seed yield goal 2.5 t in D81083 cultivar, where there is not any weed plants to compete with bean plants. The highest recommended nitrogen rate (280 kg ha−1) represented the amount of nitrogen that would meet demand for N in bean plants when compete with weeds. The area of each block was 640m2 (10 m wide × 64 m long) while main plots were of 200m2 (10 m wide × 20 m long) each. Spacing among the main plots were 2 m for easy movement of the implements between the plots. The field was moldboard plowing with average depth of 30 cm in the fall then two shallow disks followed by soil grubber and harrow in the March for seedbed preparation. Common bean seed was planted on May 28, 2014 and May 24, 2015 and thinned to the recommended plant density (40 plants m−2) when bean plants had two trifoliate leaves.
Barnyardgrass seed was acquired from a commercial supplier. Breaking seed dormancy of barnyardgrass was done by soaking them in concentrated sulfuric acid (98%) for 15 min, and then they were washed with distilled water (Sadeghloo et al. 2013). Target technique was used to avoid below-ground competition between bean and weed plants (McPhee and Aarssen 2001). In this technique root competition is typically prevented by surrounding the target roots with a deep tube, cylinder or pot. Therefore, the barnyardgrass sown in polyethylene grow-bag (50 × 15 × 15 cm) that had several drainage holes in bottom. The Plastic bags also pierced with several holes near the top ridge, which made it easier to get water when filed was irrigated. Plastic pots filled with field soil and placed into the holes which dug 15 cm away from the bean rows (Fig. 1). The holes arranged along the bean rows based on the target densities of either 2 or 20 barnyardgrass plants m−2. Barnyardgrass density was selected based on local weed densities that were recorded in common bean fields. The weed seeds were planted on the next day after bean planting (May 29, 2014 and May 25) at a high density and were thinned to one plant in each pot. All weeds other than barnyardgrass were removed by hand.
Soil volumetric water content was monitored daily for the top 30 cm of the soil using time-domain reflectometry (TDR, model 4593, Soil Moisture Equipment, Santa Barbara, USA), and the average soil water content based on the TDR readings of weed free treatments with the highest N application rate was used to irrigation scheduling. Because the number of available TDR access tube was limited, the treatments with maximum water requirement were used to avoid water stress in the bean plants. The soil was irrigated to field capacity using furrow irrigation when 40% of the available soil water (USDANRCS, 1997) was depleted in the top 0.3 m of the root zone.
Traits measurement
Green leaf area was determined using a leaf area meter (AAM-9, Hayashi Denko, Tokyo, Japan). Chlorophyll meter readings (SPAD) were collected using a hand-held dual wavelength meter (SPAD 502, Chlorophyll meter, Minolta Camera Co., Ltd., Japan) at the flowering stage. Measurements were taken midway along the uppermost leaf from four representative plants at the center of two rows (rows 3 and 4) within each plot. Two destructive plant samples were cut at ground level, one during and the other one at the end of the growing season in each experimental unit to quantify crop and weed growth. At the sampling date, whole plants of both species were removed from 2.0 m2 areas lying across the center two rows of each plot by clipping plants at the soil surface. Seed yield was adjusted to 9% moisture content. Biomass of barnyardgrass and common bean was calculated by oven-dried above ground biomass for 48 h at 70 °C and weighed.
The all dried plant materials including the vegetative and reproductive parts for each species were combined, ground to pass through a 1-mm sieve, and analyzed for total N content using Kjeldahl procedures described by Chattha et al. (2015). In this method 2.0 g of oven dried grinded plant material was mixed with 25 ml of concentrated H2SO4 and 5 g digestion mixture (K2SO4:CuSO4:FeSO4 in the ratio of 20:2:1) and digested the material on the gas heater in Kjeldahl digestion flask until the light green color was appeared, cooled it and made the volume up to 250 ml. put 10 ml of that diluted solution in micro Kjeldahl apparatus and added 25 ml of 40% NaOH solution. Put a receiving flask containing 10 ml of 2% boric acid solution and indicator (methyl red) in such a way that the deli very tube after coming through condenser dipped into it. Opened the steam generator plug and let the content of distillation tube be boiled until whole ammonia was liberated. After that it was titrated it against standard N/10 H2SO4 solutions (Chattha et al. 2015). The nitrogen concentration was reported as N yield (kg ha−1) of the seed and dry biomass.
Statistical analysis
A combined analysis was operated after checking for homogeneity of variances. Data collected was statistically analyzed by ANOVA using the GLM procedure in the Statistical Analysis System (SAS Institute 2002). The least significant difference (LSD) was used to compare means of traits (p < 0.05). Indeed, if a significant interaction was found in the ANOVA, slicing was used and the differences in the means of one factor are tested separately on each level of the other factor (Gomez and Gomez 1984; Clewer and Scarisbrick 2001). Because the year by factor interaction was not significant, the mean data for two years are presented.
Results
Leaf chlorophyll and leaf area index
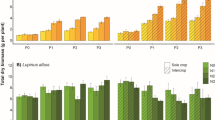
The leaf area index (LAI) and leaf chlorophyll was affected by nitrogen application rate (N) (P < 0.01), weed density (D) (P < 0.01) and interaction of N × D (P < 0.01) (Table 1). The regression analysis showed that the best fit between leaf chlorophyll and N application rate was linear across all weed densities (Fig. 2.a). These regression models were significant and could explain 81–99% of variation in leaf chlorophyll content across the weed densities. The models also showed that bean leaf chlorophyll under high weed density was significantly more responsive to increasing N supply compared to that under weed-free condition (Table 2). In all N treatments, the leaf chlorophyll decreased under weed-infested compared to weed free treatment. For example, chlorophyll content decreased by 11% for N0 and 20% for N200, under high weed density compared with weed-free conditions (Fig. 2.a).
Regression analysis showed that LAI increased linearly as N application rate increased from 0 to 280 kg ha−1, across all weed pressure levels (Fig. 2.b). The regression analysis showed that the response rate of LAI to N supply under weed-free condition was significantly greater than that under weed-infested condition (Table 2). These regression models were significant and could explain 82–96% of variation in LAI across all weed densities. Regardless of nitrogen application level, LAI was decreased as weed density increased. LAI was reduced with high weed density compared with weed-free by 10, 3, 9 and 23% in N0, N50, N100 and N200 treatments, respectively (Fig.2.b).
Total biomass
Analysis of variance showed that the bean biomass was affected by N (P < 0.01), D (P < 0.01) and there was interaction effects of N × D (Table 1). Bean biomass production increased non-linearly with an increase in N rate, across all weed densities (Fig. 2.c). The non-linear model was not significant under high weed density, but could explain the great variation in bean biomass (R2 = 0.85). The regression analysis showed that the responses rate of bean biomass to N supply under no and low weed pressure were significantly greater than that under high weed pressure (Table 2). Bean biomass loss associated with weed competition for N0, N50, N100 and N200 treatments were 30, 41, 35 and 38% under high weed pressure compared with weed-free, respectively (Fig.2.c).
Nitrogen accumulation
Nitrogen accumulation was significantly influenced by N (P < 0.01), D (P < 0.01) and there was interaction effects of N × D (Table 1). Bean nitrogen accumulation increased linearly with an increase in N rate across all weed densities (Fig. 2.d). These models were statistically significant, and could explain 90–98% of variation in nitrogen accumulation across all weed densities. The regression analysis showed that the response rate of nitrogen accumulation to N supply under weed-free condition was significantly greater than that under high weed density (Table 2). N accumulation was reduced with high weed density compared with weed-free by 44, 42, 39 and 42% in N0, N50, N100 and N200 treatments, respectively (Fig. 2.d).
Seed yield and yield components
Analysis of variance revealed that seed number, 100-seeds weight and seed yield were significantly affected by N, D and interaction of N × D (Table 1). The response of seed weight to N application rate differed among weed densities. The regression analysis showed that the best fit between seed weight and N application rate was quadratic under no and low weed density and linear under high weed density (Fig. 2.d). The 100-seed weight was significantly reduced under high weed pressure. A linear regression model was determined to be the best model to explain the relation between seed number and N application rate, across all weed densities (Fig. 2.e). The response rates of seed number to N supply were similar across weed densities (Table 2). Furthermore, bean seed yield increased non-linearly with an increase in N rate across all weed densities (Fig. 2.g). These models were statistically significant, and could explain 99% of variation in seed yield across all weed densities. The response rates of seed yield to N supply under no and low weed density were significantly greater than that under high weed density (Table 3). According to these models, bean yield increased linearly as N application rate increased from 0 to 140 kg ha−1, and seems to reach a plateau as N application rate increased beyond 140 kg ha−1. The responses of seed number and seed yield to weed pressure level were depended on N application rate. For example, seed yield was reduced with high weed density compared with weed-free by 35 and 39% in N0 and N200 treatments, respectively (Fig. 2.g). Mean reduction in seed weight, seed number and seed yield caused by high weed pressure was 11, 20 and 41% compared with weed free, respectively (Fig. 2.e, f, g).
Barnyardgrass biomass and N accumulation
Barnyardgrass biomass and N accumulation were affected by N and D. The N × D interaction was not significant (Table 4). Weed biomass and N uptake increased linearly with an increase in N rate (Fig. 3). Linear model for weed N uptake was statistically significant, and the models could explain 87 and 76% of variation in weed N uptake and biomass production, respectively (Table 3). The weed biomass and N uptake increased by 66 and 159% as the nitrogen rate increased from N0 to N200. Weed biomass and weed N uptake of barnyardgrass significantly increased by increased weed density.
Discussion
In this study, the barnyardgrass was sown in polyethylene pots, to prevent or minimize below-ground competition for water and nitrogen, and potted weed plant arranged along the common bean rows with different density. By using the physical separation of shoot competition from root competition, we were able to study the effect of light competition on common bean growth and yield in absence of competition for nutrients and water. The increase of barnyardgrass density and N application rate caused an increased growth and N uptake in barnyardgrass. Previous studies have shown that the competitive ability of weeds increased with increasing weed density (Bosnic and Swanton 1997) and soil fertility (Blackshaw and Brandt 2008).
The results showed negative effect of barnyardgrass competition on LAI, Leaf chlorophyll common bean biomass production and N accumulation depended on the N application rate and barnyardgrass density. In all weed pressure levels, bean leaf area and leaf chlorophyll increased linearly with an increase in N rate application. The regression analysis showed that the response rate of LAI to N supply under weed-free condition was significantly greater than that under weed-infested condition. Previous studies have shown that there was a decrease in LAI and leaf chlorophyll under weed competition compared with weed-free conditions (Tollenaar et al. 1994; Saberali et al. 2016). The effect of N fertility on increasing LAI and leaf chlorophyll content can improve the ability of bean plants to intercept greater light than weeds and suppress competing weeds by shading effect (Tollenaar et al. 1994; Liebman and Gallandt 2002). Wortmann (1993) showed that LAI is the most important bean plant trait for improving weed suppression. Furthermore, the negative effect of barnyardgrass competition on LAI and Leaf chlorophyll was lower with an N application compared with no N application. Previous studies have shown that there was a decrease in LAI and leaf chlorophyll under weed competition compared with weed-free conditions (Tollenaar et al. 1994; Saberali et al. 2016). Nitrogen application improved LAI and leaf and leaf chlorophyll of common bean under weed competition conditions (Tollenaar et al. 1994; Saberali et al. 2016).
Common bean N accumulation and biomass production, respectively, increased linearly and nonlinear as N rate increased from 0 to 280 kg ha−1. The regression analysis showed that the responses rate of biomass production to N supply under no and low weed pressure were significantly greater than that under high weed pressure (Table 2). Saberali et al. (2016) showed that common bean N assimilation and biomass production improved with N application under low weed infested condition compared with no N application, but reduceded with N application at a high weed infested condition. In this study, N application increased the N accumulation and biomass production of bean plants in both low and high weed pressure level. Our finding showed that only aboveground competition for light, without any belowground competition for nitrogen and water, could not cause a negative response to nitrogen application under high weed pressure.
Studies have shown that above-ground plant performance is often mediated by below-ground nutrient acquisition (Wilson 1988). Radiation interception was significantly correlated with LAI, leaf N concentration and LAD (Sinclair and Horie 1989; Liebman et al. 1995), and asymptotically these traits are affected by N deficiency resulting from weed competition (Tollenaar et al. 1994; Saberali et al. 2016). Furthermore, water stress associated with weed competition might also be limiting factors for N uptake and N2 fixation (Corre-Hellou and Crozat 2005).
The negative effect of barnyardgrass interference on common bean seed yield, seed m−2 and seed weight increased with increasing weed density, and as N application rate increased. The greater light competition in high weed density compared with low weed density, justifying the greater common bean seed reduction in the presence of competing weeds (Tollenaar et al. 1994). Seed yield and yield components showed a positive response to N application under no, low and high weed pressure, whereas it has been shown crop seed yield generally decreased with increasing N application rate under high weed competition (Liebman and Gallandt 2002; Saberali et al. 2016). The responses of seed yield and seed number to N fertilization rate were curvilinearly and linearly, respectively, across all weed densities. The responses of seed weight to N fertilization rate were differed among various weed density. It has reported seed and pod number in bean were strongly affected by weed competition (Saberali et al. 2016; Woolley et al. 1993).
Agronomically optimum N rates for seed yield was obtained at 140 kg N ha−1 within the range of N fertilization rates used across all weed pressure levels. Bean seed yield increased 21% as N application rate increased from N0 to N100, when bean plants experienced the high weed competition with no below-ground competition. Whereas under full competition (above- and below-ground competition), the seed yield in the current common bean variety decreased by 55% in N100 treatment compared with N0 treatment when bean plants experienced high redroot pigweed competition (Saberali et al. 2012). It was found that the increasing effects of both above- and below-ground competition as productivity increases (Nicotra and Rodenhouse 1995). Wilson (1988) found that below-ground competition usually affected the balance between the competing species more than above-ground competition.
Our results showed that the higher aboveground competitive ability of barnyardgrass compared with common bean caused a maximum yield reduction of 40–43% under high N application rate where two species compete only for light. Regardless of weed density, growth and seed yield of common bean plants showed a positive response to the N application in the absence of a below-ground competition, suggests that lower access of common bean to N pool in presence of high weed pressure caused a negative response to the N application, as was observed by Saberali et al. (2012). Our finding supports that above-ground plant performance is mediated by below-ground nutrient acquisition. In conclusion, our results showed that the effect of weed interference on bean dry matter production and seed yield was larger under low than under high soil N levels. Consequently, using an optimum N supply level would be a critical part to develop a sustainable weed management systems with rely less on herbicide.
References
Aguyoh, J. N., & Masiunas, J. B. (2003). Interference of redroot pigweed (Amaranthus retroflexus) with snap beans. Weed Science, 51, 202–207. https://doi.org/10.1614/0043-1745(2003)051[0202:IORPAR]2.0.CO;2.
Andersson, T. N., & Lundegardh, B. (1999). Field horsetail (Equisetum arvense) effects of potassium under different light and nitrogen conditions. Weed Science, 47, 47–54.
Blackshaw, R. E., & Brandt, R. N. (2008). Nitrogen fertilizer rate effects on weed competitiveness is species dependent. Weed Science, 56, 743–747. https://doi.org/10.1614/ws-08-065.1.
Bliss, F. A. (1993). Breding common bean for improved biological nitrogen fixation. Plant and Soil 152, 71–79.
Bosnic, A. C., & Swanton, C. J. (1997). Influence of barnyardgrass (Echinochloa crus-galli) time of emergence and density on corn (Zea mays). Weed Science, 45, 276–282.
Cathcart, R. J., & Swanton, C. J. (2004). Nitrogen management will influence threshold values of green foxtail (Setaria viridis) in corn. Weed Science, 51, 975–986. https://doi.org/10.1614/p2002-145.
Chattha, M. U., Sana, M. A., Munir, H., Ashraf, U., Haq, I., & Zamir, S. (2015). Exogenous application of plant growth promoting substances enhances the growth, yield and quality of maize (Zea mays L.). Plant Knowledge Journal, 4(1), 1–6.
Clewer, A. G., & Scarisbrick, D. H. (2001). Practical statistics and experimental design for plant and crop science. Chichester: Wiley.
Cooper, J. (2004). Multiple responses of rhizobia to flavonoids during legume root infection. In J. A. Callow (Ed.), Advances in botanical research: Incorporating advances in plant pathology (pp. 1–62). Academic Press.
Corre-Hellou, G., & Crozat, Y. (2005). N2 fixation and N supply in organic pea (Pisum sativum L.) cropping systems as affected by weeds and peaweevil (Sitona lineatus L.). European Journal of Agronomy, 22, 449–458.
Ding, L., Wang, K. J., Jiang, G. M., Biswas, D. K., Xu, H., Li, L. F., & Li, Y. H. (2005). Effects of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years. Annals of Botany, 96, 925–930. https://doi.org/10.1093/aob/mci244.
Ehlers, J.D. (1984). Assessment and utilization of exotic cowpea germplasm to improve blackeye pea, Vigna unguiculata (L.) Walp. Ph.D. dissertation, University of California, Davis, CA.
Evans, S. P., Knezevic, S. Z., Lindquist, J. L., Shapiro, C. A., & Blankenship, E. E. (2003). Nitrogen application influences the critical period for weed control in corn. Weed Science, 51, 408–417. https://doi.org/10.1614/0043-1745(2003)051[0408:naitcp]2.0.co;2.
Fennimore, S. A., Mitich, L. W., & Radosevich, S. R. (1984). Interference among bean (Phaseolus vulgaris) cultivar red kidney, barnyardgrass (Echinochloa crus-galli), and black nightshade (Solanum nigrum). Weed Science, 32, 336–342.
Gomez, K. A., & Gomez, A. A. (1984). Statistical procedures for agricultural research. An international Rice research institute book. 2nd edn. New York: Wiley.
Harbur, M. M., & Owen, M. D. K. (2004). Light and growth rate effects on crop and weed responses to nitrogen. Weed Science, 52, 578–583. https://doi.org/10.1614/ws-03-115r1.
Hillocks, R. J., Madata, C. S., Chirwa, R., Minja, E. M., & Msolla, S. (2006). Phaseolus bean improvement in Tanzania. Euphytica., 150, 215–231. https://doi.org/10.1007/s10681-006-9112-9.
Holm, L. G., Pancho, J. V., Herberger, J. P., & Plucknett, D. L. (1977). The World's worst weeds. Honolulu: Univ. Press of Hawaii.
Liebman, M., & Gallandt, E. R. (2002). Differential responses to red clover residue and ammonium nitrate bycommon bean and wild mustard. Weed Science, 50, 521–529. https://doi.org/10.1614/0043-1745(2002)050[0521:drtrcr]2.0.co;2.
Liebman, M., Corson, S., Rowe, R. J., & Halteman, W. A. (1995). Dry bean responses to nitrogen fertilizer in two tillage and residue management systems. Agronomy Journal, 87, 538–546.
Lindquist, J. L., & Mortensen, D. A. (1999). Ecophysiological characteristics of four maize hybrids and Abutilon theophrasti. Weed Research, 39, 271–285.
McPhee, C. S., & Aarssen, L. W. (2001). The separation of above- and below-ground competition in plants. A review and critique of methodology. Plant Ecology, 152, 119–136.
Nicotra, A. B., & Rodenhouse, N. L. (1995). Intraspecific competition in Chenopodium album varies with resource availability. American Midland Naturalist Journal, 134, 90–98.
Ramírez-Vallejo, P., & Kelly, J. D. (1998). Traits related to drought resistance in common bean. Euphytica, 99, 127–136.
Saberali, S. F., Modarres-Sanavy, S. A. M., Bannayan, M., Baghestanic, M. A., Rahimian Mashhadid, H., & Hoogenboom, G. (2012). Dry bean competitiveness with redroot pigweed as affected by growth habit and nitrogen rate. Field Crop Research, 135, 38–45. https://doi.org/10.1016/j.fcr.2012.06.016.
Saberali, S. F., Modarres-Sanavy, S. A. M., Bannayan, M., Aghaalikhani, M., Haghayegh, G., & Hoogenboom, G. (2016). Common bean canopy characteristics and N assimilation as affected by weed pressure and nitrogen rate. The Journal of Agricultural Science, 154, 598–611. https://doi.org/10.1017/s0021859615000477.
Sadeghloo, A., Asghari, J., & Ghaderi-Far, F. (2013). Seed germination and seedling emergence of velvetleaf (Abutilon Theophrasti) and barnyardgrass (Echinochloa Crus-Galli). Planta Daninha, 31, 259–266. https://doi.org/10.1590/S0100-83582013000200003.
SAS Institute. (2002). The SAS system for Microsoft windows. Release 8.2. Cary, NC.
Sinclair, T. R., & Horie, H. (1989). Leaf nitrogen, photosynthesis, and crop radiation use efficiency: A review. Crop Science, 29, 90–98.
Spitters, C. J. T., & Aerts, R. (1983). Simulation of competition for light and water in crop-weed associations. Aspects of Applied Biology, 4, 467–483.
Stagnari, F., & Pisante, M. (2011). The critical period for weed competition in French bean (Phaseolus vulgaris L.) in Mediterranean areas. Crop Protection, 30, 179–184. https://doi.org/10.1016/j.cropro.2010.11.003.
Tollenaar, M., Nissanka, S. P., Aguilera, A., Weise, S. F., & Swanton, C. J. (1994). Effect of weed interference and soil nitrogen on four maize hybrids. Agronomy Journal, 86, 596–601. https://doi.org/10.2134/agronj1994.00021962008600040004x.
Uhart, S. A., & Andrade, F. H. (1995). Nitrogen deficiency in maize. I. Effects on crop growth, development, dry matter partitioning, and kernel set. Crop Science, 35, 1376–1383.
U.S. Department of Agriculture. (1997). Summary Report: National Resources Inventory (revised December 2000), Natural Resources Conservation Service, Washington, DC, and Statistical Laboratory, Iowa State University, Ames, Iowa, 89 pp.
Walker, G. K., Blackshaw, R. E., & Dekker, L. (1988). Leaf area and competition for light between plant species using direct sunlight transmission. Weed Technology, 2, 159–165.
Wilson, J. B. (1988). Shoot competition and root competition. Journal of Applied Ecology, 25, 279–296.
Woolley, B. L., Michaels, T. E., Hall, M. R., & Swanton, C. J. (1993). The critical period of weed control in white bean (Phaseolus vulgaris). Weed Science, 41, 180–184.
Wortmann, C. S. (1993). Contribution of bean morphological characteristics to weed suppression. Agronomy Journal, 85, 840–843. https://doi.org/10.2134/agronj1993.00021962008500040012x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saberali, S.F., Mohammadi, K. The above-ground competition between common bean (Phaseolus vulgaris L.) and barnyardgrass (Echinochloa crus-galli L.) affected by nitrogen application. Phytoparasitica 47, 451–460 (2019). https://doi.org/10.1007/s12600-019-00745-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-019-00745-y