Abstract
Bemisia tabaci (Hemiptera: Aleyrodidae) is responsible for severe damage to horticultural and ornamental crops worldwide, mainly for its role as virus vector. In Italy, the B. tabaci Mediterranean (MED) and Middle East–Asia Minor 1 (MEAM1) cryptic species are widespread in the Southern regions as well as in Sicily and Sardinia. During the last two decades, MED populations progressively increased, in those areas where intensive farming is applied. The recent introduction of the begomovirus Tomato leaf curl New Delhi virus (ToLCNDV) prompted extensive surveys of both vector and symptomatic plants. In 2016 and 2017, monitoring activities were carried out in Lazio region (Central Italy) where begomovirus epidemics had never occurred before and the presence of B. tabaci was thought to be only occasional. ToLCNDV-infected zucchini plants were found in Southern Lazio together with whitefly populations belonging only to the MED cryptic species. The MED-Q2 haplotype was the most abundant, likely favored by high temperatures and intensive agricultural practices. Single and mixed populations of MED and MEAM1 were found in Central and Northern Lazio, suggesting that agro-ecological factors still limit MED outbreaks in these areas. This preliminary survey indicates that B. tabaci is well established in Lazio, making ToLCNDV potentially able to spread to the rest of the region as well as to the nearby regions of Central Italy that have similar climatic and cultural conditions. The northward spread of B. tabaci is a critical issue for viral disease epidemiology and the management of whitefly-transmitted viruses in Central Italy, and must be kept under strict surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is one of the most serious pest infesting a broad range of horticultural and ornamental crops worldwide. The phloem-feeding activity of B. tabaci reduces the plant vigour through sap removal and causes indirect damage, such as the abundant excretion of honeydew favouring the development of sooty moulds, and the transmission of plant viruses. This whitefly raised the status of the global agricultural threat primarily owing to its efficiency in transmitting more than 100 plant viruses, mostly belonging to the genus Begomovirus (family Geminiviridae) (Navas-Castillo et al. 2011; Polston et al. 2014).
Nowadays, B. tabaci is distributed in tropical, sub-tropical and temperate areas and its spread to new regions mainly occurred through the trade in ornamentals (EFSA Panel on Plant Health 2013). This large-scale dispersal enhanced adaptive processes and genetic differentiation, so that B. tabaci is actually considered as a complex of at least 36 cryptic species ranked in 11 major genetic groups (De Barro et al. 2011; Hu et al. 2011; Alemandri et al. 2012; Firdaus et al. 2013; Lee et al. 2013; Boykin and De Barro 2014; Lee et al. 2016). These species are morphologically indistinguishable and have been discriminated based on the mitochondrial cytochrome oxidase subunit 1 (COI) sequence variability (Dinsdale et al. 2010; De Barro et al. 2011). The cryptic species were originally identified as biotypes and differ in several biological traits, including host-plant range, induction of physiological plant disorders, insecticide resistance, adaptability to climatic conditions and virus transmission efficiency (Bedford et al. 1994; Brown et al. 1995; Perring 2001; Horowitz et al. 2005; Dalmon et al. 2008; Delatte et al. 2009). Most of the cryptic species are affiliated to continents, except the Mediterranean (MED) and Middle East–Asia Minor 1 (MEAM1), formerly referred to as biotype Q and B respectively, which are highly invasive because of their strong polyphagia, high reproductive rate, and high resistance to a large number of insecticides (EFSA Panel on Plant Health 2013). MED cryptic species now contains greater intra-specific variability, and four mitochondrial variants have been identified so far: Q1 and Q2, originally present in western and eastern Mediterranean countries respectively, and Q3 and ASL coming from Africa (Tsagkarakou et al. 2007; Chu et al. 2008; Gueguen et al. 2010).
In Europe, MED and MEAM1 are distributed almost everywhere on protected crops and are also present outdoors along the warm Mediterranean coasts (EFSA Panel on Plant Health 2013). MED and MEAM1 can co-exist in the same area, although significant shifts in the species ratio have been frequently observed. For instance, MED populations took advantage of high insecticide resistance and tolerance to high temperatures (Horowitz et al. 2005; Bonato et al. 2007; Horowitz and Ishaaya 2014), and became the predominant cryptic species in those Mediterranean regions where intensive farming is applied (Moya et al. 2001; Tsagkarakou et al. 2007; Dalmon et al. 2008; Parrella et al. 2012). On the other hand, MEAM1 has been more competitive than MED in insecticide-free areas, thanks to reproductive interference mechanisms that increase its mating efficiency (Pascual 2006; Crowder et al. 2010; Elbaz et al. 2010). Further changes in population dynamics were observed within the MED species, and the Q2 haplotype, that was initially reported only in eastern Mediterranean countries, recently settled also in Spain, France and Italy (Gauthier et al. 2014; Parrella et al. 2014; Terraz et al. 2014).
In Italy, B. tabaci was not regarded as an important pest prior to the introduction of the begomoviruses Tomato yellow leaf curl virus (TYLCV) and Tomato yellow leaf curl Sardinia virus (TYLCSV) (Rapisarda and Tropea Garzia 2002; Davino et al. 2006). These viruses caused severe economic losses to tomato crops in 1990s and 2000s, and led to an extensive survey of the vector distribution. To date, B. tabaci is known to be present mainly in the Southern regions and in the main islands Sicily and Sardinia. Both MED and MEAM1 cryptic species have been recorded in these areas, together with the minor species Italy (formerly referred to as biotype T) and Ru, which are present in uncultivated areas (Simón et al. 2003; Parrella et al. 2012, 2016). A progressive increase of MED infestations as well as a displacement of MEAM1 has been observed over the past fifteen years (Bosco et al. 2006; Simón et al. 2007; Cavalieri and Rapisarda 2008), not only in greenhouses but also in field cultivations and on weeds (Parrella et al. 2012). Furthermore, recent samplings carried out in the Campania region, where the MED-Q2 genetic variant was absent until 2008, showed that Q2 progressively spread and became predominant over Q1 (Parrella et al. 2014).
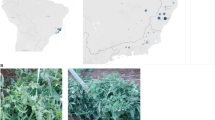
New concerns about the whitefly infestations in Italy have arisen from the recent introduction of a new B. tabaci-transmitted begomovirus, the Tomato leaf curl New Delhi virus (ToLCNDV). This bipartite ssDNA virus originated from India and was unknown in the Mediterranean basin until 2012, when it was detected for the first time on zucchini squashes in Spain (Juárez et al. 2014). The begomovirus-like symptoms observed on infected zucchini included curling, vein swelling, and severe mosaic in young leaves, short internodes, and fruit skin roughness. The virus further spread on tomato crop in Spain (Ruiz et al. 2015), and new outbreaks have been reported on cucurbits in Tunisia and Sicily in 2015 (Mnari-Hattab et al. 2015; Panno et al. 2016), and in Sardinia in 2016 (Luigi et al. 2016). The rapid spread of the disease immediately prompted monitoring activities to assess the presence of both ToLCNDV and B. tabaci in the mainland regions of Southern and Central Italy. The present study reports the infestation status of B. tabaci between September 2016 and October 2017 in Lazio region (Fig. 1), where the intensive cucurbit cultivations in both protected and open-field conditions make this area highly susceptible to ToLCNDV establishment.
Materials and methods
Sample collection
Different sites at seven coastal localities in Lazio region were monitored for the presence of B. tabaci and ToLCNDV symptomatic plants. The localities are spread over the three main areas devoted to the horticulture in this region: Agro Pontino (Southern Lazio), Agro Romano (Central Lazio), and the coast of the Viterbo district (Northern Lazio) (Fig. 1). The survey was carried out from September 2016 to October 2017 on cucurbit plants (Cucurbita pepo L., Cucumis melo L., Citrullus lanatus (Thunb.) Matsum. & Nakai and Cucumis sativus L.) and solanaceous plants (Solanum lycopersicum L. and Capsicum annuum L.) cultivated in both open fields and greenhouses; a field of Brassica oleracea L. was also inspected for the presence of B. tabaci (Table 1). Whitefly adults were collected by means of a mouth aspirator and stored in 99% ethanol at 4 °C until the laboratory analyses. Each sample consisted of 5–30 individuals of B. tabaci coming from different sampling sites and from different host plants cultivated in greenhouses or open fields. At those sites with ToLCNDV symptomatic zucchini squashes, both whitefly and host plant samples were collected and analysed for virus detection.
Whitefly species identification
The collected specimens were first examined through a stereo-microscope to exclude any contamination with other whitefly species, namely Trialeurodes vaporariorum (Westwood). Total genomic DNA was then extracted from single individuals of B. tabaci according to Delatte et al. (2005) for cryptic species and haplotype identification. Five to ten B. tabaci specimens per each collection sample were analysed.
The identification of MEAM1 and MED cryptic species was carried out by multiplex TaqMan® Real-Time PCR assay using the species-specific primers and probes (BEM COI MEAM1 and BEM COI MED sets) designed on the mitochondrial cytochrome oxidase 1 (COI) gene by Cavalieri et al. (2014). Real-Time PCR was performed in a final volume of 25 μl using 2X Applied Biosystems® TaqMan® Universal PCR Master Mix (ThermoFisher Scientific), 600 nM of each primer, 200 nM of each probes and 1 μl of DNA. The thermo-cycling conditions consisted of an initial cycle at 50 °C for 2 min, followed by 10 min at 95 °C and 40 cycles at 95 °C for 15 s and 60 °C for 60 s. Real-Time PCR assays were carried out in a Applied Biosystems 7500Fast Thermal Cycler supported by 7500 Software v.2.0.4 (ThermoFisher Scientific). Thresholds cycles (Ct) and baselines were automatically calculated by the system software. The samples were assigned to MEAM1 and MED cryptic species when the Ct ranges were 21–28 and 18–29 for BEM COI MEAM1 and BEM COI MED probes respectively.
The DNA obtained from B. tabaci MED specimens was further subjected to PCR-RFLP assays in order to discriminate between Q1 and Q2 haplotypes, following the protocol suggested by Parrella et al. (2012). The discriminating 866-bp COI region was amplified using the primers C1-J-2195 and TL2-N-3014 (Simon et al. 1994). The PCR was performed in 25 μl reaction volume containing: 2X PCR Master Mix (Promega), 2 mM MgCl2, 0.5 μM of each primer and 1 μl of DNA. The thermo-cycling conditions consisted of an initial denaturation cycle at 94 °C for 5 min, 35 cycles at 95 °C for 30 s, 45 °C for 45 s and 72 °C for 1 min and a final cycle at 72 °C for 10 min. Amplified DNA was analyzed by electrophoresis onto a 1% (w/v) agarose gel and then digested with the restriction enzyme ApoI according to the manufacturer’s instructions (New England BioLabs). The restriction fragments were separated by electrophoresis onto 2% (w/v) 1× TBE agarose gels (1.5% MetaPhor™ agarose for resolution of small nucleic acids +0.5% SeaKem® LE Agarose; Lonza) at 70 V for 2 h and stained with ethidium bromide.
COI amplicons representative of MEAM1, MEDQ1 and MEDQ2 haplotypes were purified (through Amicon® Ultra-0.5 Centrifuge Filter Devices, Merck KGaA) and sequenced in both directions. The sequences have been deposited in GenBank (National Centre for Biotechnology Information, NCBI) with the accession number MF447847 for MEAM1, MF447849 for MEDQ1, and MF447848 for MEDQ2.
Detection of ToLCNDV in insect vector and host plants
The virus detection in both insects and plants was performed by PCR assay. Insect DNA was extracted as described above from 5 to 10 single specimens collected at those sites where symptomatic plants were present. Total Nucleic Acids (TNA) of symptomatic plants were extracted using REAL Total RNA from Tissues and Cells Kit (Durviz S.L.): fresh plant tissues were ground in 1:10 (w/v) of 0.1 M PO4 buffer using plastic extraction bags (Bioreba), then 100 μl of ground tissue were added to 650 μl of Lysis solution provided in the kit, and TNA purified following the manufacturer’s instructions.
Diagnostic PCR was carried out using the primer pair ToLCNDV-B2F / ToLCNDV-B2R (Mizutani et al. 2011), which amplifies about 1100 bp of the ToLCNDV genome B. Two μl of plant TNA or one μl of insect DNA were added to the reaction mixture containing 1X GoTaqG2 amplification buffer (Promega), 2.5 mM dNTPs, 2.5 μM of each primer, 1.25 U GoTaqG2 (Promega) in a final volume of 25 μl, and amplification was carried out as follows: initial denaturation cycle at 95 °C for 5 min, 35 cycles at 95 °C for 45 s, 53 °C for 45 s and 72 °C for 1 min, and a final elongation cycle at 72 °C for 10 min. Amplified DNA was analyzed by electrophoresis onto a 1.2% (w/v) agarose gel.
Results
Presence and distribution of B. tabaci species complex
In Lazio, specimens of B. tabaci were collected in all the inspected localities. Most of the samples were from protected or open-field cucurbit cultivations, and to a lesser extent from protected solanaceous crops (Table 1). These populations often co-existed with T. vaporariorum. In Agro Pontino, B. tabaci was widespread in both protected and open-field cultivations of zucchini squashes. On the contrary, the whitefly was sporadically present in the nearby tomato greenhouses and was absent in watermelon crops. A total of fifteen and ten samples were collected in this area in 2016 and 2017, respectively. All the specimens belonged to the MED cryptic species, and the RFLP analyses showed that thirteen samples included both the MED haplotypes Q1 and Q2 whereas only Q2 was found in the remaining twelve samples (Table 1). The restriction fragments, even if small (44 bp), could be clearly detected by agarose gel electrophoresis, avoiding the use of polyacrilamide gel (Fig. 2). In Central Lazio, a farm located in Fiumicino was monitored for the presence of B. tabaci in September and November 2016 as well as in May, June and October 2017. In both years, whitefly specimens were found only during the autumn, when the production was limited to cucurbit crops. Although the samplings were limited, specimens representative of MEAM1 cryptic species and MED-Q1 haplotype were collected on zucchini and cucumber plants. In Northern Lazio, high population levels of B. tabaci were found on cucurbit and solanaceous crops cultivated in both protected and open-field conditions in Pescia Romana, and all the collected specimens belonged to MEAM1. Few MEAM1 specimens were also sampled in a field of B. oleracea in Tarquinia.
Presence of ToLCNDV in insect vectors and host plants
In Agro Pontino, ToLCNDV symptoms were observed on zucchini squash plants cultivated in ten out of the fourteen sites inspected in 2016, and in eight out of the ten sites in 2017. Symptomatic plants were present in all the four localities within this area (Terracina, Sabaudia, Capratica di Fondi and Salto di Fondi), and were found in both greenhouses and open fields. Almost all the collected symptomatic leaf samples tested positive for ToLCNDV in PCR analyses (detection rate: 80%; Table 2). No symptoms ascribable to ToLCNDV were observed on both tomato and watermelon plants cultivated nearby the infected zucchini squashes in Agro Pontino. Likewise, no symptoms were observed on cucurbit and solanaceuos crops in Central and Northern Lazio.
Among the B. tabaci samples collected from the infected cultivations in Agro Pontino, only few individuals tested positive to ToLCNDV in 2016 as well as in 2017 (detection rate: 10%; Table 2). The virus-positive specimens belonged to both MED-Q1 and -Q2 haplotypes.
Discussion
In Lazio, the main horticultural cultivations are located in the Agro Pontino area, where zucchini squash, cucumber, melon, watermelon, tomato and pepper crops are intensively grown in both greenhouses and open fields. Other few important sites with melon, watermelon and tomato crops are present in Agro Romano and Viterbo districts. In all these areas, viral diseases are responsible for serious damages and for crop yield reductions. The most common infections on cucurbits are due to the aphid-transmitted viruses causing leaf mosaic and yellowing, and often fruit deformation (i.e. Watermelon mosaic virus, Zucchini yellow mosaic virus, Cucumber mosaic virus, Cucurbit aphid-borne yellows virus) whereas Tomato spotted wilt virus transmitted mainly by Frankliniella occidentalis (Pergande) is the most dangerous virus on solanaceous. No outbreaks associated with begomoviruses on tomato have been recorded in Lazio so far, even in 1990s and 2000s when TYLCSV and TYLCV infections seriously damaged the tomato productions in Sicily, Sardinia and in other regions of Southern Italy (Parrella et al. 2005; Davino et al. 2006; Comes et al. 2009; Nannini et al. 2009). The TYLCSV and TYLCV epidemics were strictly related to the distribution of B. tabaci occurring in Italy at that time. Indeed, the survey carried out between 1992 and 1995 by Bosco and Caciagli (1998) reported that the open-field presence of B. tabaci in Italy was limited to the two main islands and to the Southern Italian regions. A small and isolated population was also found in the North-western coast (Liguria region), in a very restricted area with a specific warm microclimate. No populations were found to be established in Lazio nor in the rest of Central Italy at that time. The absence of begomovirus infections probably did not push for further extensive surveys in Lazio, and T. vaporariorum was thought to be the only whitefly commonly present in this region. Limited numbers of records reported in 2000s assumed an occasional presence of B. tabaci restricted to the Agro Pontino district (Bosco et al. 2006; EFSA Panel on Plant Health 2013). The present study carried out in 2016 and 2017 to investigate the presence of the newly introduced ToLCNDV outlines a different map of both begomovirus and vector spread.
ToLCNDV-symptomatic zucchini plants were identified at several sites in the Agro Pontino area since September 2016. The symptoms were never seen in Lazio before and corresponded to the typical symptoms previously described for the ToLCNDV infections occurred in zucchini squash cultivations in Spain and Italy (Juárez et al. 2014; Panno et al. 2016; Luigi et al. 2016). The presence of the virus was confirmed by PCR diagnosis in most of the symptomatic samples. Almost at the same time, ToLCNDV infections were recorded in the adjacent Campania region. The ToLCNDV infections in Lazio and Campania emerged shortly after the previous Italian outbreaks occurred in Sicily in October 2015 and in Sardinia at the beginning of August 2016. Since their emergence, the ToLCNDV epidemics rapidly got serious in the islands as well as in Campania, where the vector B. tabaci is well-established. The spread of the disease appeared to be slightly slower in Agro Pontino: a 2–5% symptom incidence was observed in the field, and no evident increases were recorded between 2016 and 2017. The control measures adopted by the farmers to maintain low levels of vector density probably contribute to limit the ToLCNDV epidemics in this area. Anyway, B. tabaci was found in all the ToLCNDV-infected zucchini cultivations in Agro Pontino, providing the first evidence that begomoviruses and B. tabaci co-exist in Lazio. Among the whitefly samples collected at the infected sites, only few individuals were found to be positive to the virus, probably because of the low ToLCNDV disease pressure in Agro Pontino. These viruliferous specimens belonged to both the MED genetic variants, providing the evidence that also the haplotype Q2 is involved in ToLCNDV transmission, after that the virus was found in association with indigenous Asian B. tabaci species in India and with MED-Q1 in Spain (Maruthi et al. 2007; Janssen et al. 2017).
The presence of B. tabaci was not restricted to Southern Lazio, but was extended to Central and Northern parts of the region at all the inspected sites. This wide diffusion leads to suppose that the occurrence of B. tabaci infestations is not occasional in Lazio and insect populations could have been well-established in the region. As well, the occurrence of outdoor populations suggests that the whitefly can spread from greenhouse to open fields more frequently than it was previously thought (Bosco and Caciagli 1998). Since there are many host plant species available for this polyphagous pest, the adaptation to outdoor conditions is influenced only by temperatures. Laboratory experiments correlating developmental rates of the cryptic species MEAM1 with climatic parameters (monthly and annual mean temperatures and number of frost days per winter) recorded at different localities in late 1980s suggested that the outdoor establishment of B. tabaci in Italy is limited to the warmer areas south of 41°N (Bosco and Caciagli 1998). This would place the Lazio region just out of the range of establishment, but likely exposed to invasion. Prediction models have proved that increases in average temperatures would expand the northern boundaries of the open-field presence of B. tabaci in Europe (Gilioli et al. 2014). Thus, the northward shift of B. tabaci observed in Lazio may have been favored by the steady increase of both winter and summer temperatures occurring in the Mediterranean basin in the last two decades as a consequence of the global climate warming (IPCC, 2014).
The present study showed that both MED and MEAM1 cryptic species were present in Lazio and were differently distributed along a South-to-North route. In Agro Pontino, no MEAM1 specimens were found and the MED-Q2 haplotype was prevailing, being present in all the sampling sites with or without MED-Q1 in 2016 as well as in 2017. The disappearance of MEAM1 and the progressive predominance of MED-Q2 have already been reported in the Campania region, not far from Agro Pontino (Parrella et al. 2014). Both the climatic conditions and the intensive agricultural practices likely favored the MED-Q2 establishment in these areas. Indeed, the MED cryptic species, and in particular the Q2 haplotype that originated from hot eastern Mediterranean countries (Israel, Cyprus and Turkey), is known to be more adapted than MEAM1 to the high temperatures that can be reached during the summer, especially under the plastic greenhouses (Bonato et al. 2007). MED is also more tolerant to insecticides and can better perform under the pressure of intensive chemical treatments (Horowitz and Ishaaya 2014). The haplotype identification showed that only MED-Q1 was present in Agro Romano and co-existed with MEAM1 whereas MED-Q2 appeared to be not established yet. The spread of MED populations was stopped in Northern Lazio, where the lower temperatures and less intensive agricultural practices were likely to favor the establishment of MEAM1 only. In presence of these favorable agro-climatic conditions, it is known that MEAM1 populations also take advantage of their higher reproductive efficiency that allow to grow faster than MED populations until their exclusion (Crowder et al. 2010).
This study allows to roughly define a first map of B. tabaci species and haplotype distribution in Lazio region, relative to ToLCNDV epidemics. To date, the virus spread appears to be limited to the Southern Lazio but the presence of the vector throughout the region makes ToLCNDV potentially able to spread to the other areas as well as to the nearby regions of Central Italy that have similar climatic and cultural conditions. Further concerns arise from possible TYLCSV and TYLCV epidemics on tomato that could also be favored by the fact that the absence of infections in Central Italy has induced many growers to not cultivate resistant tomato varieties in the last years. Therefore, the present survey points out the importance to keep under close surveillance possible new ToLCNDV (and begomoviruses in general) outbreaks in the rest of Lazio and Central Italy as already occurred in Southern Italy and in the main islands. Likewise, further investigations of the northward spread of B. tabaci in other regions of Central Italy would be essential to implement the viral disease management, in terms of both control and prevention. Moreover, more detailed information on the cryptic species distribution as well as on their relative virus-transmission efficiency would improve the knowledge of ToLCNDV disease epidemiology in Italy.
References
Alemandri, V., De Barro, P., Bejerman, N., Argello Caro, E. B., Dumón, A. D., Mattio, M. F., Rodriguez, S. M., & Truol, G. (2012). Species within the Bemisia tabaci (Hemiptera: Aleyrodidae) complex in soybean and bean crops in Argentina. Journal of Economic Entomology, 105(1), 48–53.
Bedford, I. D., Briddon, R. W., Brown, J. K., Rosell, R. C., & Markham, P. G. (1994). Geminivirus transmission and biological characterisation of Bemisia tabaci (Gennadius) biotypes from different geographic regions. Annals of Applied Biology, 125(2), 311–325.
Bonato, O., Lurette, A., Vidal, C., & Fargues, J. (2007). Modelling temperature-dependent bionomics of Bemisia tabaci (Q-biotype). Physiological Entomology, 32(1), 50–55.
Bosco, D., & Caciagli, P. (1998). Bionomics and ecology of Bemisia tabaci (Sternorrhyncha: Aleyrodidae) in Italy. European Journal of Entomology, 95(4), 519–527.
Bosco, D., Loria, A., Sartor, C., & Cenis, J. L. (2006). PCR-RFLP identification of Bemisia tabaci biotypes in the Mediterranean Basin. Phytoparasitica, 34(3), 243–251.
Boykin, L. M., & De Barro, P. J. (2014). A practical guide to identifying members of the Bemisia tabaci species complex: And other morphologically identical species. Frontiers in Ecology and Evolution, 2(45), 1–5.
Brown, J. K., Frohlich, D. R., & Rosell, R. C. (1995). The sweetpotato or silverleaf whiteflies: Biotypes of Bemisia tabaci or a species complex? Annual Review of Entomology, 40(1), 511–534.
Cavalieri, V., & Rapisarda, C. (2008). Indagini molecolari sui biotipi di Bemisia tabaci in Sicilia (Hemiptera: Aleyrodidae). Bollettino di Zoologia Agraria e di Bachicoltura, 40(2), 145–154.
Cavalieri, V., Manglli, A., Tiberini, A., Tomassoli, L., & Rapisarda, C. (2014). Rapid identification of Trialeurodes vaporariorum, Bemisia tabaci (MEAM1 and MED) and tomato-infecting criniviruses in whiteflies and in tomato leaves by real-time reverse transcription-PCR assay. Bulletin of Insectology, 67(2), 219–225.
Chu, D., Wan, F., Tao, Y., Liu, G., Fan, Z., & Bi, Y. (2008). Genetic differentiation of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotype Q based on mitochondrial DNA markers. Insect Science, 15, 115–123.
Comes, S., Pacella, R., Fanigliulo, A., & Crescenzi, A. (2009). Further spreading of Tomato yellow leaf curl Sardinia virus in southern Italy. Acta Horticulturae, 808, 199–202.
Crowder, D. W., Sitvarin, M. I., & Carrière, Y. (2010). Plasticity in mating behaviour drives asymmetric reproductive interference in whiteflies. Animal Behaviour, 79(3), 579–587.
Dalmon, A., Halkett, F., Granier, M., Delatte, H., & Peterschmitt, M. (2008). Genetic structure of the invasive pest Bemisia tabaci: Evidence of limited but persistent genetic differentiation in glasshouse populations. Heredity, 100(3), 316–325.
Davino, S., Napoli, C., Davino, M., & Accotto, G. P. (2006). Spread of Tomato yellow leaf curl virus in Sicily: Partial displacement of another geminivirus originally present. European Journal of Plant Pathology, 114(3), 293–299.
De Barro, P. J., Liu, S. S., Boykin, L. M., & Dinsdale, A. B. (2011). Bemisia tabaci: A statement of species status. Annual Review of Entomology, 56, 1–19.
Delatte, H., Reynaud, B., Granier, M., Thornary, L., Lett, J. M., Goldbach, R., & Peterschmitt, M. (2005). A new silverleaf-inducing biotype Ms of Bemisia tabaci (Hemiptera: Aleyrodidae) indigenous to the islands of the south-west Indian Ocean. Bulletin of Entomological Research, 95(1), 29–35.
Delatte, H., Duyck, P. F., Triboire, A., David, P., Becker, N., Bonato, O., & Reynaud, B. (2009). Differential invasion success among biotypes: Case of Bemisia tabaci. Biological Invasions, 11(4), 1059–1070.
Dinsdale, A., Cook, L., Riginos, C., Buckley, Y. M., & De Barro, P. (2010). Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Annals of the Entomological Society of America, 103(2), 196–208.
EFSA Panel on Plant Health. (2013). Scientific opinion on the risks to plant health posed by Bemisia tabaci species complex and viruses it transmits for the EU territory. EFSA Journal, 11(4), 3162 Available online: www.efsa.europa.eu/efsajournal.
Elbaz, M., Lahav, N., & Morin, S. (2010). Evidence for pre-zygotic reproductive barrier between the B and Q biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Bulletin of Entomological Research, 100(5), 581–590.
Firdaus, S., Vosman, B., Hidayati, N., Jaya Supena, E. D., Visser, R. G. F., & van Heusden, A. W. (2013). The Bemisia tabaci species complex: Additions from different parts of the world. Insect Science, 20(6), 723–733.
Gauthier, N., Clouet, C., Perrakis, A., Kapantaidaki, D., Peterschmitt, M., & Tsagkarakou, A. (2014). Genetic structure of Bemisia tabaci MED populations from home-range countries, inferred by nuclear and cytoplasmic markers: Impact on the distribution of the insecticide resistance genes. Pest Management Science, 70(10), 1477–1491.
Gilioli, G., Pasquali, S., Parisi, S., & Winter, S. (2014). Modelling the potential distribution of Bemisia tabaci in Europe in light of the climate change scenario. Pest Management Science, 70(10), 1611–1623.
Gueguen, G., Vavre, F., Gnankine, O., Peterschmitt, M., Charif, D., Chiel, E., Gottlieb, Y., Ghanim, M., Zchori-Fein, E., & Fleury, F. (2010). Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Molecular Ecology, 19(19), 4365–4378.
Horowitz, A. R., & Ishaaya, I. (2014). Dynamics of biotypes B and Q of the whitefly Bemisia tabaci and its impact on insecticide resistance. Pest Management Science, 70(10), 1568–1572.
Horowitz, A. R., Kontsedalov, S., Khasdan, V., & Ishaaya, I. (2005). Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Archives of Insect Biochemistry and Physiology, 58(4), 216–225.
Hu, J., de Barro, P., Zhao, H., Wang, J., Nardi, F., & Liu, S. S. (2011). An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS One, 6(1), e16061.
IPCC Intergovernmental Panel on Climate Change (2014). Climate change 2014: Synthesis report. In Core Writing Team, R.K. Pachauri & L.A. Meyer (Eds.), Fifth assessment report of the intergovernmental panel on climate change. Geneva (Switzerland), 151 pp. Available online: www.ipcc.ch/pdf/assessment-report/ar5/syr/SYR_AR5_FINAL_full_wcover.pdf.s
Janssen, D., Simon, A., Crespo, O., & Ruiz, L. (2017). Genetic population structure of Bemisia tabaci in Spain associated with Tomato leaf curl New Delhi virus. Plant Protection Science, 53(1), 25–31.
Juárez, M., Tovar, R., Fiallo-Olivé, E., Aranda, M. A., Gosálvez, B., Castillo, P., Moriones, E., & Navas-Castillo, J. (2014). First detection of Tomato leaf curl New Delhi virus infecting zucchini in Spain. Plant Disease, 98(6), 857.
Lee, W., Park, J., Lee, G. S., Lee, S., & Akimoto, S. I. (2013). Taxonomic status of the Bemisia tabaci Complex (Hemiptera: Aleyrodidae) and reassessment of the number of its constituent species. PLoS One, 8(5), e63810.
Lee, W., Kim, C. S., Lee, K. Y., & Lee, G. S. (2016). The JpL species of the Bemisia tabaci Complex in Korea: Detection by an extensive field survey and analysis of COI sequence variability. Journal of Asia-Pacific Entomology, 19(1), 23–29.
Luigi, M., Manglli, A., Valdes, M., Sitzia, M., Davino, S., & Tomassoli, L. (2016). Occurrence of Tomato leaf curl New Delhi virus infecting zucchini in Sardinia (Italy). Journal of Plant Pathology, 98(3), 695.
Maruthi, M. N., Rekha, A. R., & Muniyappa, V. (2007). Pumpkin yellow vein mosaic disease is caused by two distinct begomoviruses: Complete viral sequences and comparative transmission by an indigenous Bemisia tabaci and the introduced B-biotype. EPPO Bulletin, 37(2), 412–419.
Mizutani, T., Daryono, B. S., Ikegami, M., & Natsuaki, K. (2011). First report of Tomato leaf curl New Delhi virus infecting cucumber in central java, Indonesia. Plant Disease, 95, 1485.
Mnari-Hattab, M., Zammouri, S., Belkadhi, M. S., Bellon Doña, D., ben Nahia, E., & Hajlaoui, M. R. (2015). First report of Tomato leaf curl New Delhi virus infecting cucurbits in Tunisia. New Disease Reports, 31, 21.
Moya, A., Guirao, P., Cifuentes, D., Beitia, F., & Cenis, J. L. (2001). Genetic diversity of Iberian populations of Bemisia tabaci (Hemiptera: Aleyrodidae) based on random amplified polymorphic DNA-polymerase chain reaction. Molecular Ecology, 10(4), 891–897.
Nannini, M., Testa, M., Dellacroce, C., & Accotto, G. P. (2009). Occurrence of TYLCD and its vector Bemisia tabaci in Sardinia (Italy): A study on weeds and volunteers. Acta Horticulturae, 808, 193–198.
Navas-Castillo, J., Fiallo-Olivé, E., & Sànchez-Campos, S. (2011). Emerging virus diseases transmitted by whiteflies. Annual Review of Phytopathology, 49, 219–248.
Panno, S., Iacono, G., Davino, M., Marchione, S., Zappardo, V., Bella, P., Tomassoli, L., Accotto, G. P., & Davino, S. (2016). First report of Tomato leaf curl New Delhi virus affecting zucchini squash in an important horticultural area of southern Italy. New Disease Reports, 33, 6.
Parrella, G., Scassillo, L., Crescenzi, A., & Nappo, A. G. (2005). Tiping of tomato yellow leaf curl viruses and their vector in Italy. Communications in Agricultural and Applied Biological Sciences, 71(3PtB), 1229–1236.
Parrella, G., Scassillo, L., & Giorgini, M. (2012). Evidence for a new genetic variant in the Bemisia tabaci species complex and the prevalence of the biotype Q in southern Italy. Journal of Pest Science, 85(2), 227–238.
Parrella, G., Nappo, A. G., Manco, E., Greco, B., & Giorgini, M. (2014). Invasion of the Q2 mitochondrial variant of Mediterranean Bemisia tabaci in southern Italy: Possible role of bacterial endosymbionts. Pest Management Science, 70(10), 1514–1523.
Parrella, G., Cozzolino, A., Manco, E., Stinca, A., Formisano, G., & Giorgini, M. (2016). Identificata in Campania la specie Italy (= biotipo T) del complesso di specie Bemisia tabaci. Protezione delle Colture, 4, 28–30.
Pascual, S. (2006). Mechanisms in competition, under laboratory conditions, between Spanish biotypes B and Q of Bemisia tabaci (Gennadius). Spanish Journal of Agricultural Research, 4(4), 351–354.
Perring, T. M. (2001). The Bemisia tabaci species complex. Crop Protection, 20(9), 725–737.
Polston, J. E., De Barro, P., & Boykin, L. M. (2014). Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Management Science, 70(10), 1547–1552.
Rapisarda, C., & Tropea Garzia, G. (2002). Tomato yellow leaf curl Sardinia virus and its vector Bemisia tabaci in Sicilia (Italy): Present status and control possibilities. OEPP/EPPO Bulletin, 32, 25–29.
Ruiz, M. L., Simón, A., Velasco, L., García, M. C., & Janssen, D. (2015). First report of Tomato leaf curl New Delhi virus infecting tomato in Spain. Plant Disease, 99(6), 894.
Simon, C., Frati, F., Beckenbach, A., Crespi, B., Liu, H., & Flook, P. (1994). Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America, 87, 651–701.
Simón, B., Cenis, J. L., Demichelis, S., Rapisarda, C., Caciagli, P., & Bosco, D. (2003). Survey of Bemisia tabaci (Hemiptera: Aleyrodidae) biotypes in Italy with the description of a new biotype (T) from Euphorbia characias. Bulletin of Entomological Research, 93(3), 259–264.
Simón, B., Cenis, J. L., & De La Rúa, P. (2007). Distribution patterns of the Q and B biotypes of Bemisia tabaci in the Mediterranean basin based on microsatellite variation. Entomologia Experimentalis et Applicata, 124(3), 327–336.
Terraz, G., Gueguen, G., Arnó, J., Fleury, F., & Mouton, L. (2014). Nuclear and cytoplasmic differentiation among Mediterranean populations of Bemisia tabaci: Testing the biological relevance of cytotypes. Pest Management Science, 70(10), 1503–1513.
Tsagkarakou, A., Tsigenopoulos, C. S., Gorman, K., Lagnel, J., & Bedford, I. D. (2007). Biotype status and genetic polymorphism of the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) in Greece: Mitochondrial DNA and microsatellites. Bulletin of Entomological Research, 97(1), 29–40.
Acknowledgements
The authors are grateful to Società cooperativa “Il Chiarone” (Montalto di Castro, Viterbo, Italy), Società cooperativa agricola “Mediana” (Terracina, Latina, Italy), Società cooperativa agricola “Orto-Sole” (Torrimpietra, Roma, Italy), Syngenta® Italia and Enza Zaden Italia for helping in sample collection. This work was supported by the project “EMERAMB, Emergent viruses and virus vectors in Mediterranean Basin crops” that is funded through the ARIMNet2 2015 Call. ARIMNet2 (2014-2017) is an ERA-NET coordinated by INRA (France); it has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 618127.
Funding
This work was supported by the project “EMERAMB, Emergent viruses and virus vectors in Mediterranean Basin crops” that is funded through the ARIMNet2 2015 Call. ARIMNet2 (2014–2017) is an ERA-NET coordinated by INRA (France); it has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 618127.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bertin, S., Luigi, M., Parrella, G. et al. Survey of the distribution of Bemisia tabaci (Hemiptera: Aleyrodidae) in Lazio region (Central Italy): a threat for the northward expansion of Tomato leaf curl New Delhi virus (Begomovirus: Geminiviridae) infection. Phytoparasitica 46, 171–182 (2018). https://doi.org/10.1007/s12600-018-0649-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-018-0649-7