Abstract
Effects simultaneous and sequential inoculations of Meloidogyne incognita, Ralstonia solanacearum and Phomopsis vexans were studied on the growth, chlorophyll and carotenoid contents of eggplants grown in 25% fly ash and 25% sand mix soil. Plants grown in 25% fly ash mix soil had lesser plant growth than grown in 25% sand ash mix soil. Inoculation of M. incognita / R. solanacearum or P. vexans caused reduction in plant growth, chlorophyll and carotenoid contents in both types of soils but these pathogens in combination caused a greater reduction in than individual inoculation. Inoculation of M. incognita 20 days prior to R. solanacearum caused a greater reduction in plant growth than inoculation of M. incognita prior to P. vexans. Inoculation of P. vexans prior to R. solanacearum caused a lesser reduction in plant growth, chlorophyll and carotenoid contents than inoculation of P. vexans prior to M. incognita. Inoculation of R. solanacearum 20 days prior to M. incognita caused a greater reduction in plant growth, chlorophyll and carotenoid contents than inoculation of R. solanacearum prior to P. vexans. Galling and multiplication of M. incognita was higher in plants grown in 25% sand amended soil than with 25% fly ash soil. R. solanacearum and P. vexans had adverse effects on galling and nematode multiplication. Wilt and blight indices caused by R. solanacearum and P. vexans were 3 respectively. Wilt and blight indices were 4 when two pathogens were inoculated together.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eggplant (Solanum melongena L.) is an important crop of sub-tropics and tropics. In India, eggplant has been cultivated for the last 4000 years and its production is about 7.676 M mt with an average productivity of 16.3 mt / ha (www.ncpahindia.com/eggplant.php). Eggplant is known to suffer from number of pest and pathogens but important diseases include fruit and leaf blight caused by Phomopsis vexans (Sacc and Syd.) Harter, bacterial wilt caused by Ralstonia solanacearum (Smith) Yabuuchi et al. and root knot disease caused by Meloidogyne incognita (Kofoid and White) Chitwood (Khan and Siddiqui 2017). Root-knot nematodes are particularly damaging to vegetable crops in tropical and subtropical countries of the world and cause up to 80% losses in heavily infested fields (Sikora and Fernandez 2005). Ralstonia solanacearum causes wilt and is one of the most important plant pathogenic bacteria responsible for great economic losses world-wide (Hayward 1991). Similarly, Phomopsis vexans (teleomorph: Diaporthe vexans Gratz) is also a major pathogen, reduces yield and marketable value of the crop nearly 20–30% (Jain and Bhatnagar 1980; Kaur et al. 1985).
Soil texture largely determines soil moisture holding capacity and aeration and impacts the ability of nematodes to hatch, move through soil, locate and penetrate a host, and mate (Koenning and Barker 1995; Koenning et al. 1988). Soils that have good water holding capacity are less subject to nutrient loss through leaching (Lehmann and Schroth 2003). Soil texture is a reflection of particle size and small particles (clay and silt) have a larger surface area than sand particles allowing the soil to hold a greater quantity of water.
Fly ash is major solid waste as a coal combustion residue. Its recycling or safe disposal of solid industrial wastes have been identified including soil amendments in agricultural fields. Heavy metals (Fe, several micronutrients and toxic metals) are abundant in fly ash, with the deficiency of fixed C- and N-compounds (Martens et al. 1970; Parab et al. 2012). Several heavy metals are useful in minute quantities to plants which nonetheless are toxic at higher levels. Moreover, metal contents in fly ash cause the enhancement of crop growth in amended soils. Fly ash has been used in amending soils supporting several crops (Korcak 1995; Mishra et al. 2005, 2007; Samy et al. 2010), as a method of sustainable waste disposal and soil fertilization, worldwide (Dick et al. 2000).
Amendment of 25% fly ash in soil cause a significant increase in the growth, chlorophyll and carotenoid contents of eggplant over plants grown without fly ash (Khan and Siddiqui 2017). Similarly, carrot grown in sand mix soil showed a significant increase in root dry weight, chlorophyll and carotenoid contents compared to plants grown in 100% soil (Ahmad and Siddiqui 2017) therefore 25 sand mix soil may also increase the growth of brinjal.
Studies on interactions of pathogens are important as the studies of mono-pathogenic situation. Certain pathogens are destructive only when they occur in combination with other pathogens (Siddiqui et al. 2012). Mostly when a plant is infected with one pathogen its response to other pathogen is altered. These alterations exert significant influences upon disease development, etiology of pathogens involved and ultimately on disease control. Therefore, studies on interactions of pathogens are of prime importance for proper management of disease complexes on various crops (Siddiqui et al. 2012).
During the course of survey of plant pathogens of Aligarh, Uttar Pradesh, India, we found the concomitant occurrence of P. vexans, R. solanacearum and M. incognita in different eggplant fields with different soils. The pathogens were responsible for high yield losses with severe wilt blight and gall symptoms. Therefore, present study was carried out to observe the effect of sequential and simultaneous inoculation of R. solanacearum, M. incognita and P. vexans in fly ash mix soil and sand mix soil on the growth, chlorophyll and carotenoid contents of eggplant.
Materials and methods
The physico-chemical characters of the soils used in the treatments were determined before sowing seed (Table 1). Loam soil was obtained from a field of Department of Botany, A.M.U. Aligarh and sand from a nearby river. Sand: loam soil was mixed in 25:75 (v /v) and autoclaved at137.9 kPa for 20 min. Similarly, fly ash was dried in the sun for 10 days while field soil which was collected and autoclaved at 137.9 kPa for 20 min. Later, fly ash and soil were mixed in 25: 75 ratio (v /v). Clay pots (30 cm dia.) were filled with 3.5 kg soil (25% fly ash with 75% soil and 25% sand with 75% soil) of both types separately. Soil samples were passed through a 2 mm sieve before analyses and porosity and water holding capacity by hydrometry; pH, conductivity and cation exchange capacity (CEC) using soil: distilled water in pH and conductivity meters; and sulphur content determined. Organic carbon was estimated following Nelson and Sommers (1972), zinc, manganese, copper, and iron were determined following Chopra and Kanwar (1982); nitrogen was determined with the Kjeldahl digestion (Nelson and Sommers 1972); and phosphorus by phosphomolybdic blue colorimetry (Jackson 1958).
Seeds of eggplant, cv. Navkiran, were surface sterilized by dipping in 0.1% sodium hypochlorite solution for 1 min and then washed 3 times with sterile distilled water. Five seed were sown in clay pots (30 cm dia.) filled with 3.5 kg soil as described above. Two-weeks after emergence plants were thinned to 1 per pot. Plants grown in each soil mix were inoculated with pathogens as described below. Pots were arranged on a glasshouse bench at 30 °C and watered daily with each pot receiving 200 mL water.
The fungus P. vexans was isolated from infected eggplant leaves exhibiting blight symptoms following surface sterilization with 0.1% sodium hypochlorite solution as described for seeds above. Leaves were cut into small pieces and placed aseptically in Petri dishes containing potato dextrose agar (PDA) medium at 25 °C for 15 days. The identity of the fungus was confirmed as P. vexans using microscopic morphological characteristics. For obtaining sufficient inoculum, P. vexans was inoculated with a sterile inoculation needle in to Richard’s liquid medium (Riker and Riker 1936) containing: 10 g potassium nitrate; 5 g potassium dihydrogen phosphate; 2.5 g magnesium sulphate; 0.02 g ferric chloride; 50 g sucrose, and 1000 mL distilled water. The Richards liquid medium was prepared and filtered through muslin cloth, sterilized in an autoclave at 103.4 kPa for 15 min in 250 mL Erlenmeyer flasks containing 80 mL of liquid medium. The fungus was inoculated in each flask, as stated above, which were incubated at 25 ± 1 °C for about 15 days. After sufficient fungal growth the liquid medium was filtered through Whatman filter paper No. 1. The fungal mycelia mat on the filter paper was washed with distilled water and excess water and nutrients removed with blotting paper. The inoculum was prepared by mixing 10 g of fungal mycelium in 100 mL of distilled water and the mix blended (10,000 rpm) for 30 s in a Waring blender. Twenty mL of the suspension, containing 2 g fungus, was used for inoculation of seedlings.
Eggplants exhibiting typical symptoms of bacterial wilt were collected from a production field. Stems of infected eggplant was cut obliquely at the base and placed in sterile distilled water. Stem pieces showing milky white ooze in water were used for isolation of the pathogen on nutrient agar medium. Colonies on nutrient agar were identified as R. solanacearum. Later, nutrient agar plates were streaked separately with this pure colony of R. solanacearum and incubated at 30 °C for 24 h. Single colonies from a 24-h-old pure culture of R. solanacearum were inoculated separately into nutrient broth liquid medium (5 g·L−1 peptone; 1 g·L−1 meat extract; 2 g·L−1 yeast extract; 5 g·L−1 sodium chloride; pH 7.0) in flasks and incubated at 30 °C for 72 h. Bacterial cell density in the concentrated suspension was determined following Sharma (2001) and 1.2 × 105 colony-forming units (cfu)·mL−1 was adjusted by adding sterilized water. Twenty mL of the suspension was used to inoculate each seedling.
The nematode M. incognita was collected from eggplant roots and multiplied on roots of previously uninfected eggplants using a single egg mass per plant. Large numbers of egg masses from heavily infected eggplant roots were hand-picked with sterilized forceps. The egg masses were washed with distilled water and placed in a small sieve (9 cm dia with 1-mm pore size) containing crossed layers of tissue paper. The sieve was placed in a Petri plate containing distilled water deep enough to contact the egg masses. A number of these assemblies were kept in an incubator running at 25 ± 1 °C to obtain the required number of second-stage juveniles needed for inoculation. Second-stage juveniles were collected after hatching from the Petri plates every 24 h, fresh water was added, and the process repeated. An average of 5 counts was made to determine the density of juvenile nematodes in the suspension. The volume of the nematode suspension was adjusted so that each mL contained 200 ± 5 nematodes. Twenty mL of this suspension (4000 freshly hatched juveniles) was added to each pot around an eggplant seedling.
The soil around roots was carefully removed and suspensions of M. incognita, R. solanacearum and P. vexans uniformly poured around roots and the soil replaced. In control pots, a similar amount of water was used to inoculate the plant. There were 16 treatments of pathogens and these treatments were inoculated in 2 soil types (fly ash mix soil and sand mix soil) i.e. 16 × 2 = 32 treatments (Table 4). Each treatment was replicated 5 times, 32 × 5 = 160 pots and pots were arranged in a complete factorial design. Experiment was conducted in 2015 and 2016 and results of both experiments were almost similar. Polled data of both experiments are presented.
Plants were harvested 120 days after inoculation. Data on plant length, plant fresh weight, plant dry weight, chlorophyll and carotenoid contents were recorded. Wilt and blight indices and number of galls, and nematode population were recorded. Plant length was measured from the top of the first leaf to the end of the root. Excess water was removed by blotting before weighing to determine plant fresh weight. Plants were cut with a knife above the root collar to separate shoots and roots which were kept in envelopes at 60 °C for 2–3 days before dry weight determination. To obtain nematode counts a 250 g subsample of well-mixed soil from each treatment was processed by Cobb’s sieving and decanting technique followed by Baermann funnel extraction (Southey 1986). The suspension was collected after 24 h and numbers of nematodes counted in 5 aliquots of 1 mL of suspension from each sample. The means of 5 counts were used to calculate the population of nematodes·kg−1 soil. To estimate numbers of juveniles, eggs and females inside roots, a 1 g subsample of roots was macerated in a Waring blender and counts made from the suspension obtained. Numbers of nematodes present in roots were calculated by multiplying the number of nematodes present in 1 g of root by the total weight of root. Wilt and blight symptoms were observed on leaves. Wilt and blight index were determined by scoring the severity of disease on visual observations of disease symptoms. Disease rating was on a scale from 0 to 5 where 0 = no disease (no wilt/blight symptoms observed); 1 = wilt/blight symptoms up to 12.5% on shoot; 2 = wilt/blight symptoms 12.6 to 25% on shoot; 3 = wilt/blight symptoms 25.1 to 37.5% on shoot; 4 = wilt/blight symptoms 37.6 to 50% on shoot, and 5 = more than 50% wilt / blight symptoms on shoot. Chlorophyll content and carotenoids in fresh leaves were estimated following the method of Mackinney (1941).
Pooled data of both experiments was used for statistical analysis. Tukey’s test was used to test significance of soil analysis and other data. The other data i.e. plant length, fresh plant weight, shoot and root dry weight, chlorophyll and carotenoid contents were analysed with a 2 factorial (pathogens × types of soil) ANOVA. Nematode galling and nematode population were analysed by single factor (soil types). Graphs of nematode population and number of galls per root system were prepared using Sigma Plot™ and bars with different alphabets are significantly different at P ≤ 0.05 using Tukey’s test.
Results
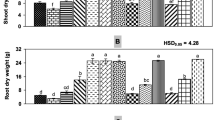
Effect of pathogens / types of soil on plant length, plant fresh weight, shoot and root dry weight, chlorophyll and carotenoid pigments was significant at P ≤ 0.05 (ANOVA not shown). Effects of interactions of pathogens and types of soil on plant length, plant fresh weight, shoot dry weight was significant while interaction effect was non-significant on root dry weight, chlorophyll and carotenod at P ≤ 0.05.
Sand mix soils (25%) was found better for plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid than 25% fly ash amended soil (Table 2). Inoculation of single pathogen or their inoculation in combination of two caused less reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid than one pathogen was inoculated first and second pathogen 20 days later (Table 3). Inoculation of M. incognita first and other pathogen 20 days later caused a greater reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents than inoculation of R. solanacearum and other pathogen 20 days later. Inoculation of P. vexans first and inoculation of other pathogen 20 days later caused less reduction in plant length, plant fresh weight, shoot dry weight, chlorophyll and carotenoid contents than R. solanacearum was inoculated first and other pathogen 20 days later (Table 3).
Effect of pathogens interaction in fly ash amended soil
Inoculation of R. solanacearum and P. vexans caused a significant reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents over uninoculated control (Table 4). Inoculation with R. solanacearum caused a greater reduction in plant length, plant fresh weight, shoot dry weight and chlorophyll content than caused by P. vexans. Simultaneous inoculation of R. solanacearum with P. vexans resulted in a greater reduction in plant length, plant fresh weight and shoot dry weight than by R. solanacearum or P. vexans alone. Significant reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents was caused by M. incognita over uninoculated control. However, inoculation of M. incognita prior to R. solanacearum caused a greater reduction in plant length and plant fresh weight than M. incognita was inoculated prior to P. vexans. Inoculation of P. vexans 20 days prior to R. solanacearum caused a lesser reduction in plant length, plant fresh weight, shoot dry weight, chlorophyll and carotenoid contents than by inoculation of P. vexans prior to M. incognita. Similarly, inoculation of R. solanacearum 20 days prior to M. incognita caused a greater reduction in plant length, plant fresh weight, shoot dry weight, chlorophyll and carotenoid contents than inoculation of R. solanacearum prior to P. vexans (Table 4).
Effect of pathogens interaction in sand amended soil.
Inoculation of R. solanacearum and P. vexans caused a significant reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents over uninoculated control (Table 4). Inoculation with R. solanacearum caused a greater reduction in plant fresh weight, shoot dry weight and chlorophyll content than caused by P. vexans. Simultaneous inoculation of R. solanacearum with P. vexans resulted in a greater reduction in plant length, plant fresh weight and shoot dry weight than by R. solanacearum or P. vexans alone. Inoculation of M. incognita caused a significant reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents over uninoculated control. However, inoculation of M. incognita prior to R. solanacearum caused a similar reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents as by inoculation of M. incognita prior to P. vexans. Inoculation of P. vexans 20 days prior to R. solanacearum caused a lesser reduction in plant length, plant fresh weight, shoot dry weight, chlorophyll and carotenoid contents than by inoculation of P. vexans prior to M. incognita. Similarly, inoculation of R. solanacearum 20 days prior to M. incognita caused a greater reduction in plant length, plant fresh weight, shoot dry weight, chlorophyll and carotenoid contents than inoculation of R. solanacearum prior to P. vexans (Table 4).
Effect of two types of soils
Plants grown in sand amended soil without pathogens were generally better in plant growth than plants grown in fly ash amended soil (Table 4). Inoculation of pathogens individually in both fly ash and sand amended soil resulted in almost similar effect on plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents. However, inoculation of pathogens in combinations and in sequential inoculations in sand amended soil caused a greater reduction in plant length, plant fresh weight, shoot dry weight, root dry weight, chlorophyll and carotenoid contents than in fly ash amended soil (Table 4).
Disease index
Wilt and blight indices were 3 when R. solanacearum and P. vexans were inoculated singly in fly ash or sand amended soils (Table 4). Indices were 4 when R. solanacearum and P. vexans were inoculated together or any one of these was inoculated with M. incognita (Table 4).
Galling and nematode population
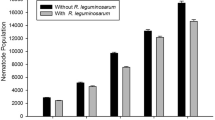
Root galling and nematode multiplication was higher in plant grown in sand amended soil than in fly ash amended soil (Figs. 1 and 2). Similarly, galling and nematode multiplication was high in plant where M. incognita was inoculated alone. Galling and nematode multiplication was reduced in plant where M. incognita was inoculated with R. solanacearum or P. vexans. Inoculation of R. solanacearum or P. vexans prior to M. incognita caused maximum reduction in galling and nematode multiplication (Figs. 1 and 2).
Discussion
Eggplant requires loose, rich soil, preferably with some sand. The soil should be well drained to prevent root forking and stunting of root growth. The best growth of eggplant, greater chlorophyll and carotenoid contents is in soil mixes with optimum pore space and pH, or aeration (Black 1973). The soil analysis of 25% sand mix soil indicated less nutrients in sand amended soil. Eggplant likely grew better in sand amended soil because of optimum pH, aeration and pore space. Growth, chlorophyll and carotenoid contents of eggplant in 25% fly ash amended soil was good apparently due to the availability of a greater amount of utilizable plant nutrients as revealed by the chemical analysis of the soil. A greater amount of potassium, sulphur, manganese and copper etc. present in the fly ash amended soil have been absorbed by the roots and utilized by the plant that led to proper growth of egg plant. The nutrients from fly ash have been reported to be beneficial for the plant growth and yield of rice (Sarangi et al. 1997), wheat, chickpea (Dubey et al. 1982) and tomato (Khan and Khan 1996).
Greater availability of nutrients in fly ash-amended soil enabled the plants to grow better and may inhibit invasion of M. incognita. Higher uptake of boron and potassium etc. helped the plants in building natural defences against the nematode (Kirkpatrick et al. 1964; Francois 1984; Khan et al. 1997). Therefore, galling and nematode multiplication was reduced in fly ash amended soil than in sand amended soil. The substantial decline in the galling and nematode population indicates that the fly ash caused direct inhibitory effect on the survival and multiplication of M. incognita. Amendment of 25% fly ash had no apparent effect on the blight and wilt disease indices of eggplant. Moreover, there was no substantial decline in the soil population of the P. vexans or R. solanacearum (data not shown) in fly amended soil indicates that the fly ash caused no direct inhibitory effect on the survival and multiplication of the fungal / bacterial pathogens in the soil.
Nematodes can move more freely in sandy soil therefore reduction in plant growth caused by nematodes is often greater in sandy soils (Ravichandra 2014). Plant growth and chlorophyll and carotenoid contents are affected by nematodes in soil mixes (Trudgill and Phillips 1997). Mechanisms by which soil suppress pathogens, although not well understood, can involve biotic (soil microflora) and/or abiotic factors (soil physicochemical properties); these may vary with the pathogen. Fluctuations in soil moisture content might confound the principles and relationships between plants and soil types and may influence microbial communities (Garbeva et al. 2004). Similarly, different pathogens also require different types of soil for their proper multiplication and survival. Interaction studies of different pathogens and soil mixes provide a better understanding on their effects on plant growth and productivity.
Inoculations of M. incognita, R. solanacearum and P. vexans in combinations and particularly inoculation of M. incognita prior to bacterial or fungal pathogen caused a greater damage to eggplant. Interactions between these pathogens may have both direct and indirect effects on disease severity. The direct effect includes physical interactions of pathogens in the rhizosphere and occupancy of same infection site inside the root. The direct interactions of pathogens inside host plants at the same infection site generally had antagonistic effect on pathogen multiplication (Siddiqui et al. 2012). Indirect effects of interactions via plant response, such as breaking of disease resistance, and modification of host substrate had synergistic effects on disease severity.
Meloidogyne spp. wound roots allowing other pathogens to become established (Stewart and Schindler 1956; Siddiqui et al. 2012). Synergistic effects of nematode, fungal and bacterial interactions have also been reported by others (Partridge 2008; Mallesh et al. 2009). Inoculation of nematodes with other plant pathogens increased disease severity by pre-disposing plants to pathogens. Fungi and mainly bacteria depend on wounds as an infection court (Goodman et al. 1967) and these wounds are provided by nematodes feeding on roots (Sitaramaiah and Sinha 1984a, b). Inoculation of nematodes with P. vexans caused less damage to growth than caused by R. solanacearum. This is possible because individually P. vexans is less pathogenic to eggplant than R. solanacearum. Moreover, inoculation of pathogenic bacterium together with fungus caused less damage to eggplant growth than either of them was inoculated with nematodes. Nematodes aggravated disease by wounding the roots and allow bacterial and fungal pathogens to enter the plant and these are less adapted for penetrating the host’s epidermis (Pitcher 1965). On the other hand, inoculation of bacterium with fungus together had inhibitory effect on each other and was unable to aggravate damage. Root-knot nematode induced physiological and / biochemical changes in hosts when inoculated with R. solanacearum / P. vexans and aggravated damage. Modifications in the host substrate due to nematode infestation provide an advantage to other pathogens. Creation of an infection court is one way in which nematode modifies a host to enhance infection by additional pathogens. Changes in biochemistry of the host are probably the most important factors favoring disease complexes involving nematodes (Slack 1963).
P. vexans and R. solanacearum adversely affected multiplication of M. incognita. Adverse effect of fungus and bacterium on nematode multiplication as observed in the present findings has also been observed by others (Lucas et al. 1955; Johnson and Powell 1969). The contents of giant cells degenerated following bacterial / fungal invasion, leaving virtually empty cells resulting into the death of root-knot nematodes. Swain et al. (1987) observed inhibitory effect of bacterium on M. incognita. Inoculation of M. incognita alone produces more galls and egg-masses compared to its association with bacterium or fungus. It may be due to the reason that establishment of bacterial / fungal pathogen induces certain changes in root system which are not favourable for nematodes (Bhagawati et al. 1996; Hazarika 2003; Hussain and Bora 2009).
The sand mix soil was better for the growth of eggplant than fly ash amended soil. Inoculation of M. incognita, R. solanacearum and P. vexans together or pre and post inoculation of these pathogens aggravate the damage. Aggravated damage to plant growth, chlorophyll and carotenoid contents was more in sand mix soil than in fly ash amended soil.
References
Ahmad, L., & Siddiqui, Z. A. (2017). Effects of Meloidogyne incognita, Alternaria dauci and Fusarium solani on carrot in different types of soil. Acta Phytopathologica et Entomologica Hungarica, 52, 39–48.
Bhagawati, B., Gogi, R., & Phukan, P. N. (1996). Interaction of Meloidogyne incognita and Pseudomonas solanacearum on jute. Indian Journal of Nematology, 26, 259–261.
Black, C. A. (1973). Soil plant relationships. New Delhi: Wiley.
Chopra, S. L., & Kanwar, J. S. (1982). Analytical agricultural chemistry. New Delhi: Kalyani.
Dick, W. A., Hao, Y. L., Stehouwer, R. C., Bigham, J. M., Wolfe, W. E., Adriano, D., Beeghly, J. H. and Haefner, R. J. (2000). Beneficial uses flue gas desulphurization by-products: Examples and case studies of land application. In: J. M. Bartels and W a. Dick (eds): Land Application of Agricultural, Industrial and Municipal by-products. Soil science Society of America, Madison, USA: American Society of Agronomy, pp. 505-536.
Dubey, P. S., Pawar, K., & Trivedi, L. (1982). Effect of fly ash deposition on photosynthetic pigment and dry matter production of wheat and gram. Agro-Ecosystems, 8, 137–140.
Francois, L. E. (1984). Effect of excess boron on tomato yield, fruit sizeand vegetable growth. Journal of the American Society for Horticultural Science, 109, 322–324.
Garbeva, P., Veen, J. A. V., & Elsas, J. D. V. (2004). Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annual Review of Phytopathology, 42, 243–270.
Goodman, R. N., Király, Z. and Zaitlin, M. (1967). The Biochemistry and Physiology of Infectious Plant Disease. Z. Király And M. Zaitlin (Eds.) van Nostrand, Princeton, New Jersey. 354 pp.
Hayward, A. C. (1991). Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annual Review of Phytopathology, 29, 65–87.
Hazarika, K. (2003). Interrelationship of Meloidogyne incognita and Pseudomonas solanacearum on jute and management of the disease complex caused by them. Ph.D. (nematology) thesis, submitted to Assam Agricultural University Johrat-13, India.
Hussain, Z., & Bora, B. C. (2009). Interrelationship of Meloidogyne incognita and Ralstonia solanacearum complex in brinjal. Indian Journal of Nematology, 39, 41–45.
Jackson, M. L. (1958). Soil chemical analysis. Englewood Cliffs: Prentice hall.
Jain, M. R., & Bhatnagar, M. K. (1980). Efficacy of certain chemicals in the control of fruit rot of brinjal. Pesticides, 14, 27–28.
Johnson, H. A., & Powell, N. T. (1969). Influence of root-knot nematodes on bacterial wilt development in flue-cured tobacco. Phytopathology, 59, 486–491.
Kaur, S., Kaur, R., Kaur, P., & Singh, D. (1985). Studies on wilt and fruit rot of brinjal caused by Fusarium semitectum. Indian Phytopathology, 38, 736–738.
Khan, M. R., & Khan, M. W. (1996). Effect of fly ash on plant growth and yield of tomato. Environmental Pollution, 92, 105–112.
Khan, M., & Siddiqui, Z. A. (2017). Effects of fly ash amendments, Ralstonia solanacearum, Meloidogyne incognita and Phomopsis vexans on the growth of Solanum melongena. Acta Phytopathologica et Entomologica Hungarica. https://doi.org/10.1556/038.52.2017.017.
Khan, M. R., Khan, M. W., & Singh, K. (1997). Management of root-knot disease of tomato by the application of fly ash in soil. Plant Pathology, 46, 33–43.
Kirkpatrick, J. D., Mai, W. F., Parker, K. G., & Fisher, E. G. (1964). Effect of phosphorus and potassium nutrition of sour cherry on the soil population levels of five plant parasitic nematodes. Phytopathology, 54, 706–712.
Koenning, S. R., & Barker, K. R. (1995). Soybean photosynthesis and yield as influenced by Heterodera glycines, soil type and irrigation. Journal of Nematology, 27, 51–62.
Koenning, S. R., Anand, S. C., & Wrather, J. R. (1988). Effect of within-field variation in soil texture on Heterodera glycines and soybean yield. Journal of Nematology, 12(5), 373–380.
Korcak, R. F. (1995). Utilization of coal combustion by-product in agriculture and horticulture. In D. L. Karlen, R. J. Wright, & W. D. Kemper (Eds.), Agricultural utilization of urban and industrial by-products (pp. 107–130). Madison: ASA Special Publication.
Lehmann, J., & Schroth, G. (2003). Nutrient leaching. In G. Schroth & F. L. Sinclair (Eds.), Trees, crops and soil fertility (pp. 151–165). Wallingford: CAB International.
Lucas, G. B., Sasser, J. N., & Kelman, A. (1955). The relationship of root-knot nematodes to Granville wilt resistance in tobacco. Phytopathology, 45, 537–540.
Mackinney, G. (1941). Absorption of light by chlorophyll solutions. The Journal of Biological Chemistry, 140, 315–322.
Mallesh, S. B., Lingraju, S., Byadgi, A. S., Hegde, Y. R., Mokashi, A. N., & Krishnaraj, P. U. (2009). Bioefficacy of rhizobacteria on root-knot / wilt disease complex in coleus and ashwganda. Karnataka Journal of Agricultural Sciences, 22, 1116–1120.
Martens, D. C., Schnappinger, M. G., & Zelazny, L. W. (1970). The plant availability of potassium in fly ash. Proceedings of the Soil Science Society of America, 34, 453–456.
Mishra, M., Sahu, R. K., & Padhy, R. N. (2005). Growth, yield, metabolism and elemental status of green gram (Phaseolus aureus) and til (Sesamum indicum) grown in soils amended with fly ash. Fresenius Environmental Bulletin, 14, 559–564.
Mishra, M., Sahu, R. K., & Padhy, R. N. (2007). Growth, yield, metabolism and elemental status of rice (Oryza sativa L.) grown in fly ash amended soils. Ecotoxicology, 16, 271–278.
Nelson, D. W., & Sommers, L. F. (1972). A simple digestion procedure for estimation of total nitrogen in soil and sediments. Journal of Environmental Quality, 1, 423–425.
Parab, N., Mishra, S., & Bhonde, S. R. (2012). Prospects of bulk utilization of fly ash in agriculture for integrated nutrient management. Bulletin of the National Institute of Ecology, 23, 31–46.
Partridge, J. E. (2008). Bacterial wilt of alfalfa. Department of plant pathology: University of Nebraska-Lincoln http://nudistance.unl.edu/homer/disease/agron/alfalfa/AlfBacWi.html.
Pitcher, R. S. (1965). Interrelationships of nematodes and other pathogens of plants. Helminthologia Abstracts, 34, 1–17.
Ravichandra, N. G. (2014). Horticultural nematology. New Delhi: Springer.
Riker, A. J., & Riker, R. S. (1936). Introduction to research on plant diseases. New York: John’s Swift Co..
Samy, N. T., Mishra, M., Sahu, R. K., & Padhy, R. N. (2010). Growth, yield and metabolism of rice (Oryza sativa L.) during repeated applications of fly ash on soil. Advanced Food Science, 32, 110–117.
Sarangi, P. K., Mishra, T. K., & Mishra, P. C. (1997). Soil metabolism, growth and yield of Oryza sativa L. in fly ash amended soil. Indian Journal of Environmental Sciences, 1, 17–24.
Sharma, P. D. (2001). Microbiology. Meerut: Rastogi and Company.
Siddiqui, Z. A., Nesha, R., Singh, N. and Alam, S. (2012). Interactions of plant parasitic nematodes and plant pathogenic bacteria. In (ed. D. K. Maheshwari) Bacteria in Agrobiology. Plant Probiotics. Springer-Verlag: Berlin Heidelberg. Pp 251-267, ISBN 978-3-642-27515-9.
Sikora, R. A., & Fernandez, E. (2005). Nematode parasites of vegetables. In M. Luc, R. A. Sikora, & J. Bridge (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (2nd ed., pp. 319–392). Wallingford: CABI publishing.
Sitaramaiah, K., & Sinha, S. K. (1984a). Histological aspects of Pseudomonas and root-knot nematode wilt complex in brinjal. Indian Journal of Nematology, 14, 175–178.
Sitaramaiah, K., & Sinha, S. K. (1984b). Interaction between Meloidogyne javanica and Pseudomonas solanacearum on brinjal. Indian Journal of Nematology, 14, 1–5.
Slack, D. A. (1963). Introduction. Symposium of interrelationships between nematodes and other agents causing plant diseases. Phytopathology, 53, 27–47.
Southey, J. F. (1986). Laboratory methods for work with plant and soil nematodes. Fisheries and Food, Her Majesties Stationary Office, London: Ministry of Agric.
Stewart, R. N., & Schindler, A. F. (1956). The effect of some ectoparasitic and endoparasitic nematodes on the expression of bacterial wilt in carnations. Phytopathology, 46, 219–222.
Swain, P. K., Rath, J. C., & Mishra, S. K. (1987). Interaction between Meloidogyne incognita and Pseudomonas solanacearum on brinjal. Indian Journal of Nematology, 17, 61–71.
Trudgill, D. L., & Phillips, M. S. (1997). Nematode population dynamics, threshold levels and estimation of crop losses. Food and Agriculture Organization, Plant Production and Protection Paper-144, Rome.
Acknowledgements
The first author thanks University Grant Commission, New Delhi for the award of Junior Research fellowship to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, M., Siddiqui, Z.A. Effect of simultaneous and sequential inoculations of Meloidogyne incognita, Ralstonia solanacearum and Phomopsis vexans on eggplant in sand mix and fly ash mix soil. Phytoparasitica 45, 599–609 (2017). https://doi.org/10.1007/s12600-017-0613-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-017-0613-y