Abstract
The speed of toxic action of an insecticide is an indicator for control efficacy and has considerable practical importance. For agricultural pest control, fast-acting is an important feature for an insecticide to consistently reduce the amount of feeding damage. Butene-fipronil is a novel compound obtained via the structural modification of fipronil. However, information about the toxicity and speed of toxic action is still limited. In the present paper, we compared the toxic feature of butene-fipronil with seven other insecticides, of which imidacloprid and abamectin are slow-acting insecticides, and acephate, endosulfan, methomyl, α-cypermethrin and spinosad are fast-acting insecticides. We found that the contact and stomach toxicities of butene-fipronil were among the highest ever estimated to Leptinotarsa decemlineata and Drosophila melanogaster. The speed of toxic action of butene-fipronil was determined using median lethal time (LT50) at a dose (concentration) equivalent to LD80 values. For L. decemlineata, the values for butene-fipronil, imidacloprid, abamectin, acephate, endosulfan, methomyl, cypermethrin and spinosad were calculated to be 39.9, 36.5, 37.5, 20.2, 22.4, 23.8, 16.4 and 23.1 h, respectively. Those for D. melanogaster were 29.8, 31.5, 29.4, 14.0, 20.3, 18.1, 13.5, and 20.1 h, respectively. ANOVA analysis showed that butene-fipronil, imidacloprid, abamectin had similar LT50 values, whereas acephate, endosulfan, methomyl, spinosad and cypermethrin had comparable LT50 values. Thus, butene-fipronil belongs to slow-acting insecticides. Our results provide more empirical information for butene-fipronil potential application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, insecticides are classified as fast-, moderate- and slow-acting (Hannig et al. 2009) based on a most common criterion ‘time from exposure to mortality’ (Campbell 1926; Chang 1952; Bettini et al. 1958; Clinch and Ross 1970). Some insecticides cause the lethality within approximate 1 day (fast-acting), whereas some kill the pest several days later (slow-acting). The speed of toxic action of an insecticide is an indicator for control efficacy and has considerable practical importance (Cory et al. 1994; Sun et al. 2004). For agricultural pest control, fast-acting, especially the speed to cause cessation of feeding, is an important feature for an insecticide to consistently reduce the amount of feeding damage (Hannig et al. 2009). Baculoviruses, for example, exhibit slow speed of action in insect pest control (Moscardi 1999). Crop damage after application of a baculovirus spray can therefore be substantial, even if mortality in target insects is eventually high (Bianchi et al. 2000). Genetically modified baculoviruses show faster speed of action (Stewart et al. 1991; Inceoglu et al. 2006), and subsequently can provide improved protection of crops in comparison with wild type baculoviruses (Cory et al. 1994; Sun et al. 2004). In contrast, German cockroach (Blattella germanica) foraging individuals can translocate a slow-acting insecticide to other members of their aggregation, and conspecific individuals then contact the insecticide, or ingest insecticide-laden feces (coprophagy), other excretions, or dead and dying insects (necrophagy and cannibalism). Thus, slow-acting insecticides in palatable baits are most effective. For example, coprophagy by first instars is an important mechanism underlying the horizontal transmission of hydramethylnon, a slow-acting insecticide (Silverman et al. 1991; Kopanic and Schal 1997, 1999; Buczkowski et al. 2001). Similarly, termiticides are required to be slow-acting and non-repellent in order to achieve greater colony suppression or even elimination (Su et al. 1982, 1987, 1991, 1994; Su 1994; Su and Scheffrahn 1991; Yeoh and Lee 2007).
Butene-fipronil is a novel compound obtained via the structural modification of fipronil, and has a relatively low toxicity to fish (Niu et al. 2007a, c). Since butene-fipronil is a GABA-gated chloride channel blocking insecticide (Zhao and Salgado 2010), it has no obvious cross-resistance to other insecticides with different modes of action (Liu et al. 2009; Niu et al. 2007b, 2008). Up to now, butene-fipronil has been proven to exhibit high toxicity to some insect species in Lepidoptera, Hemiptera, Coleoptera, Orthoptera and Diptera (Niu et al. 2007a, c; Liu et al. 2009; Yuan et al. 2009; Li et al. 2009; Arain et al. 2014). However, the speed of action of butene-fipronil remains undetermined.
Colorado potato beetle Leptinotarsa decemlineata is a notorious defoliator of potato throughout most of the northern Xinjiang Uygur autonomous region in China, and it often causes extremely large potato yield loss each year (Jiang et al. 2010, 2011, 2012; Shi et al. 2012, 2013; Lu et al. 2011). The control of this pest has mainly relied on insecticides (Alyokhin 2009; Alyokhin et al. 2008). This inevitably leads to the development of resistance to various insecticides such as organophosphates, carbamates, pyrethroids, and neonicotinoids (Alyokhin 2009; Alyokhin et al. 2008; Zichová et al. 2010; Mohamadi et al. 2010; Jiang et al. 2010, 2011, 2012; Shi et al. 2012; Lu et al. 2011; Malekmohammadi et al. 2012; Rinkevich et al. 2012; Szendrei et al. 2012). Moreover, Drosophila melanogaster is easy to care for, breeds quickly, and lays many eggs, it is an excellent model system to assess toxicities of insecticides (Arain et al. 2014). In order to test the speed of action of butene-fipronil in different representative insects and under different mode of actions, we selected a coleopteran and a dipteran, and determined the contact toxicity in L. decemlineata and stomach toxicity in D. melanogaster, in comparison to seven other insecticides. Among them acephate, endosulfan, methomyl, α-cypermethrin and spinosad were fast-acting compounds, whereas abamectin and imidacloprid were slow-acting chemicals (Hannig et al. 2009; Franc and Bouhsira 2009).
Material and methods
Experimental animals
Post-diapause L. decemlineata adults were collected from potato fields in the spring at Urumqi (43.82 N, 87.61E), Xinjiang Uygur autonomous region in China. Insects were routinely reared in an insectary at 28 ± 1 °C under a 14 h:10 h light–dark photoperiod and 50–60% relative humidity, with potato foliage at vegetative growth or young tuber stages in order to assure sufficient nutrition. The newly-eclosed fourth-instar larvae and adults at least 7 days after emergence were used in the experiments.
D. melanogaster Canton-S (CS), w 1118 and Oregan flies were raised on standard cornmeal/molasses/agar medium under controlled temperature (25 ± 1 °C), photoperiod (12 h light/12 h dark) and relative humidity (about 50%). The 5 day-old adults were used in the experiments.
Insecticides
Technical grade butene-fipronil, abamectin, acephate, chlorpyrifos, methomyl, α-cypermethrin, imidacloprid and spinosad were selected (Table 1). These chemicals were kept in a refrigerator between the experimental sessions.
Bioassays
For L. decemlineata, a topical application was used to assess the contact toxicity in the fourth instar larvae and the adults. Insecticides were dissolved individually in analytical-grade acetone, and five concentrations within a mortality range of 0–100% based on preliminary assays were used. Larvae were treated individually with 0.22 μL of insecticide solution, which was applied to the dorsal abdominal segment using a 10-μL microsyringe connected to a microapplicator (Hamilton Company, Reno, NV). Similarly, adults were treated individually with 1.1 μL of insecticide solution, which was applied to the ventral area of the abdomen using a 50-μL microsyringe. Each control larva or adult received 0.22 or 1.1 μL of acetone. Three replications of 10 individuals per concentration were performed. After treatment, the test insects were placed in Petri dishes (9 cm in diameter and 1.5 cm in height) containing fresh potato foliage and kept under environmental conditions outlined for beetle rearing (Shi et al. 2012).
For D. melanogaster, a method described previously (Wang et al. 2013) was used to determine insecticide stomach toxicity to the adults. Insecticides were dissolved in and serially diluted two fold with acetone to obtain several solutions. An aliquot of 200 μL of test solution (treatment) was mixed with 10 g of hot conventional artificial diet to prepare artificial diets demonstrating five different concentrations within a mortality range of approximately 0–100% based on preliminary assays. Acetone was used as negative control. Thirty newly emerged flies were housed in a rearing vial (3 cm in diameter and 12 cm in height) containing 10 g of standard cornmeal-sucrose-yeast agar diet for a period of 4 days. And then, 90 adults in three replicates were transferred into 3 vials (3 cm in diameter and 12 cm in height) containing the same quantity of one of the toxic diets or acetone control diet prepared above. The adults were kept under environmental conditions outlined for the fly rearing.
To compare the speed of toxic action of the eight insecticides, the median lethal time (LT50) of these insecticides at a dose (concentration) equivalent to LD80 values were calculated.
Data analysis
The mortalities were assessed daily after treatment. Control mortality was typically less than 10%. Abbott’s formula was used to correct the data for control mortality (Abbott 1925). Probit analysis was used to calculate the concentrations needed to cause 50% mortality, their 95% fiducial limits and the slope of the line relating probit mortality to the log dose by POLO Plus logit probit software (LeOra Software Company, Petaluma, CA, USA). For median lethal time (LT50) values, we gave the data as means ± SD, and were analyzed by ANOVA followed by the Tukey-Kramer test, using SPSS for Windows (SPSS, Chicago, IL, USA).
Results
Toxicity of eight insecticides to L. decemlineata and D. melanogaster
Ninety six hours after insecticide treatment to L. decemlineata by the topical application, the LD50 values for the eight selected insecticides in the fourth instars ranged from 0.002(0.002-0.003) to 0.691(0.371–1.014) μg/individual. According to the relative toxicity index (RTI, determined by comparing butene-fipronil LD50 value with the LD50 value of other insecticide), the eight insecticides could be classified into three groups with relatively least (RTI > 10.0), medium (5.0 < RTI < 10.0) and high (RTI < 5.0) toxicity to the larvae. Acephate, methomyl, and endosulfan fell into the first category; α-cypermethrin and spinosad belonged to the second group; whereas butene-fipronil, abamectin, and imidacloprid were placed into the third group (Table 2).
The toxicities of insecticides to the L. decemlineata adults also differ widely among the eight insecticides. The LD50 values ranged from 0.010 (0.008–0.014) to 2.046 (0.766–4.504) μg/individual. Butene-fipronil, abamectin, imidacloprid, and α-cypermethrin were most toxic, with the RTI less than 5, spinosad showed a moderate toxic effect, with the RTI of 7.5. In contrast, acephate, methomyl, and endosulfan were least toxic, with the RTI more than 10 (Table 2).
For the D. melanogaster adults, the LC50 values for the eight insecticides ranged from 0.05(0.04-0.06) to 20.88(18.31–23.21) μg/g. From the mean LC50 values in CS, w 1118 and Oregan strains, the order of toxicities from the most toxic to the least toxic was spinosad, α-cypermethrin, abamectin, butene-fipronil, imidacloprid, endosulfan, methomyl and acephate (Table 3).
Comparison of LT50 values
In order to determine the speed of toxic action of the eight insecticides, the LT50 values of butene-fipronil, imidacloprid, abamectin, acephate, endosulfan, methomyl, cypermethrin and spinosad at a dose (concentration) equivalent to LD80 values were compared.
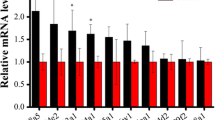
For L. decemlineata, the values for butene-fipronil, imidacloprid, abamectin, acephate, endosulfan, methomyl, cypermethrin and spinosad were calculated to be 39.9, 36.5, 37.5, 20.2, 22.4, 23.8, 16.4 and 23.1 h, respectively. ANOVA analysis showed that butene-fipronil, imidacloprid, abamectin had similar LT50 values, whereas acephate, endosulfan, methomyl, spinosad and cypermethrin had comparable LT50 values (Figs. 1 and 2).
Median lethal time (LT50) value (hour) of butene-fipronil, compared with seven insecticides in L. decemlineata adults. A topical application was used to assess the contact toxicity in the adults. Adults were treated individually with 1.1 μL of insecticide solution. The mortalities were assessed at an interval of 8 h. The value indicate LT50 (±SD). Different letters indicate significant difference at P value <0.05
Median lethal time (LT50) value (hour) of butene-fipronil, compared with seven insecticides in the D. melanogaster adults. A diet exposure bioassay was used to determine stomach toxicities of insecticides. See the legend in Fig. 1 for further explanation
For D. melanogaster, the LT50 values for butene-fipronil, imidacloprid, abamectin, acephate, endosulfan, methomyl, cypermethrin and spinosad were respectively 29.8, 31.5, 29.4, 14.0, 20.3, 18.1, 13.5, and 20.1 h. ANOVA analysis revealed that the insecticides could be classified into three groups. Butene-fipronil, imidacloprid, abamectin showed the largest LT50 values, followed by acephate, endosulfan, methomyl, and spinosad, whereas cypermethrin exhibited the shortest LT50 value (Figs. 1 and 2).
Thus, butene-fipronil belongs to slow-acting insecticides.
Discussion
Insects are major biotic factors that cause losses in agricultural production. Continuous development of new insecticides (Hainzl and Casida 1996) with selective properties and specific action (Brévault et al. 2013) is necessary for their control. Butene-fipronil is a novel compound that has a relatively low toxicity to fish (Niu et al. 2007a). In the present paper, the toxicity of butene-fipronil to the coleopteran L. decemlineata and the dipteran D. melanogaster was determined, along with seven other insecticides. Among the eight insecticides, butene-fipronil belongs to the phenylopyrazoles and endosulfan is a polychlorocycloalkane. Both of them block insect GABA-gated chloride channels (Zhao and Salgado 2010). Abamectin is 16-membered macrocyclic lactones produced during fermentation by the soil microorganism, Streptomyces avermitilis (Gouamene-Lamine et al. 2003). The mechanisms of action for abamectin are also involved in GABA- and glutamate-gated chloride channel (Kass et al. 1980; Wolstenholme and Rogers 2005; Crump and Omura 2011; Moreno et al. 2010; Fritz et al. 1979). Neonicotinoid imidacloprid is a selective nicotinic acetylcholine receptor (nAchR) agonist (Casida and Durkin 2013). Spinosad is a macrocyclic lactone derived from the fungus Saccharopolyspora spinosa (Mertz and Yao 1990). It is an allosteric activator of insect nAChRs (Thompson et al. 2000; Kirst 2010). Acephate is an organophosphate and methomyl is a methylcarbamate. The toxicity of acephate and methomyl is attributable to inhibition of acetylcholinesterase (AChE) (Casida and Durkin 2013). Cypermethrin is a pyrethroid. It acts on axonal neurotransmission at insect voltage-gated sodium channel to block sodium transport (Casida and Durkin 2013).
Our results showed that the contact and stomach toxicities of butene-fipronil were among the highest ever estimated to L. decemlineata and D. melanogaster. The LD50 values of butene-fipronil for contact toxicity were 0.002 and 0.017 μg/individual to the L. decemlineata fourth instars and adults respectively. Comparably, the average LD50 values for fipronil in the L. decemlineata fourth instars and the adults were 0.012 and 0.023 μg/individual (Shi et al. 2012). Consistent with our results, butene-fipronil has been proven to exhibit high toxic to insect species in Lepidoptera, Hemiptera, Coleoptera and Orthopteran (Niu et al. 2007a, c; Liu et al. 2009; Yuan et al. 2009; Li et al. 2009). Thus, butene-fipronil is a powerful insecticide for the control of L. decemlineata.
In order to test the speed of toxic action of butene-fipronil, we selected five fast-acting compounds acephate, endosulfan, methomyl, α-cypermethrin and spinosad, and two slow-acting chemicals abamectin and imidacloprid (Hannig et al. 2009; Franc and Bouhsira 2009). We evaluated the speed of toxic action based on the most common criterion ‘time from exposure to mortality’ (Hannig et al. 2009). Our results revealed that butene-fipronil belongs to slow-acting insecticides.
It is well known that L. decemlineata consumes a small amount of foliage before fourth-instar. However, approximately 40 cm2 of potato leaves are consumed mainly by fourth instars. Moreover, close to 10 cm2 of foliage per day are consumed during the adult stage (Ferro et al. 1985). In the summer, the L. decemlineata larvae progressed through four distinct instars, with approximate periods of the first-, second-, third-, and fourth-instar stages of 2, 2, 2 and 4 days, respectively. According to our results, butene-fipronil should be used at the neonate or the second-instar larval stage. The slow-acting butene-fipronil then kills the larvae before the fourth-instar larval stage, to effectively protect potato foliage from L. decemlineata.
References
Abbott, W. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265–267.
Alyokhin, A. (2009). Colorado potato beetle management on potatoes: current challenges and future prospects. Fruit, Vegetable and Cereal Science and Biotechnology, 3, 10–19.
Alyokhin, A., Baker, M., Mota-Sanchez, D., Dively, G., & Grafius, E. (2008). Colorado potato beetle resistance to insecticides. American Journal of Potato Research, 85, 395–413.
Arain, M. S., Hu, X.-X., & Li, G.-Q. (2014). Assessment of toxicity and potential risk of butene-fipronil using Drosophila melanogaster, in comparison to nine conventional insecticides. Bulletin of Environmental Contamination and Toxicology, 92, 190–195.
Bettini, S., Boccacci, M., & Natalizi, G. (1958). A comparative study on the speed of action of some halogen-containing thiol alkylating agents on resistant house flies. Journal of Economic Entomology, 51, 880–882.
Bianchi, F., Joosten, N. N., Vlak, J. M., & Van Derwerf, W. (2000). Greenhouse evaluation of dose- and time-mortality relationships of two nucleopolyhedroviruses for the control of beet armyworm, Spodoptera exigua, on chrysanthemum. Biological Control, 19, 252–258.
Brévault, T., Heuberger, S., Zhang, M., Ellers-Kirk, C., Ni, X., Masson, L., Li, X., Tabashnik, B. E., & Carrière, Y. (2013). Potential shortfall of pyramided transgenic cotton for insect resistance management. Proceedings of the National Academy of Sciences of the United States of America, 110, 5806–5811.
Buczkowski, G., Kopanic, R. J., Jr., & Schal, C. (2001). Transfer of ingested insecticides among cockroaches: effects of active ingredient, bait formulation, and assay procedures. Journal of Economic Entomology, 94, 1229–1236.
Campbell, F. (1926). Speed of toxic action of arsenic in the silkworm. The Journal of General Physiology, 9, 433–443.
Casida, J. E., & Durkin, K. A. (2013). Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annual Review of Entomology, 58, 99–117.
Chang, S.-C. (1952). The speed of toxic action on the pea aphid of several insecticides. Journal of Economic Entomology, 45, 370–372.
Clinch, P., & Ross, J. (1970). Laboratory assessment of the speed of action on honey bees of orally dosed insecticides. New Zealand Journal of Agricultural Research, 13, 717–725.
Cory, J. S., Hirst, M. L., Williams, T., Hails, R. S., Goulson, D., Green, B. M., Carty, T. M., Possee, R. D., Cayley, P. J., & Bishop, D. H. L. (1994). Field trial of a genetically improved baculovirus insecticide. Nature, 370, 138–140.
Crump, A., & Omura, S. (2011). Ivermectin,“Wonder drug” from Japan: the human use perspective. Proceedings of the Japan Academy, 87, 13–28.
Ferro, D., Logan, J., Voss, R., & Elkinton, J. (1985). Colorado potato beetle (Coleoptera: Chrysomelidae) temperature-dependent growth and feeding rates. Environmental Entomology, 14, 343–348.
Franc, M., & Bouhsira, E. (2009). Evaluation of speed and duration of efficacy of spinosad tablets for treatment and control of Ctenocephalides canis (Siphonaptera: Pulicidae) infestations in dogs. Parasite, 16, 125–128.
Fritz, L. C., Wang, C. C., & Gorio, A. (1979). Avermectin B1a irreversibly blocks postsynaptic potentials at the lobster neuromuscular junction by reducing muscle membrane resistance. Proceedings of the National Academy of Sciences, 76, 2062.
Gouamene-Lamine, C. N., Sup Yoon, K., & Marshall Clark, J. (2003). Differential susceptibility to abamectin and two bioactive avermectin analogs in abamectin-resistant and-susceptible strains of Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Pesticide Biochemistry and Physiology, 76, 15–23.
Hainzl, D., & Casida, J. E. (1996). Fipronil insecticide: novel photochemical desulfinylation with retention of neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 93, 12764–12767.
Hannig, G. T., Ziegler, M., & Marçon, P. G. (2009). Feeding cessation effects of chlorantraniliprole, a new anthranilic diamide insecticide, in comparison with several insecticides in distinct chemical classes and mode‐of‐action groups. Pest Management Science, 65, 969–974.
Inceoglu, A. B., Kamita, S. G., & Hammock, B. D. (2006). Genetically modified baculoviruses: a historical overview and future outlook. Advances in Virus Research, 68, 323–360.
Jiang, W., Wang, Z., Xiong, M., Lu, W., Liu, P., Guo, W., & Li, G. (2010). Insecticide resistance status of Colorado potato beetle (Coleoptera: Chrysomelidae) adults in northern Xinjiang Uygur autonomous region. Journal of Economic Entomology, 103, 1365–1371.
Jiang, W.-H., Guo, W.-C., Lu, W.-P., Shi, X.-Q., Xiong, M.-H., & Li, G.-Q. (2011). Target site insensitivity mutations in the AChE and LdVssc1 confer resistance 3 to pyrethroids and carbamates in Leptinotarsa decemlineata in northern 4 Xinjiang Uygur autonomous region. Pesticide Biochemistry and Physiology, 100, 74–81.
Jiang, W.-H., Lu, W.-P., Guo, W.-C., Xia, Z.-H., Fu, W.-J., & Li, G.-Q. (2012). Chlorantraniliprole susceptibility in Leptinotarsa decemlineata in the north Xinjiang Uygur autonomous region in China. Journal of Economic Entomology, 105, 549–554.
Kass, I., Wang, C., Walrond, J., & Stretton, A. (1980). Avermectin B1a, a paralyzing anthelmintic that affects interneurons and inhibitory motoneurons in Ascaris. Proceedings of the National Academy of Sciences, 77, 6211–6215.
Kirst, H. A. (2010). The spinosyn family of insecticides: realizing the potential of natural products research. The Journal of Antibiotics, 63, 101–111.
Kopanic, R. J. J., & Schal, C. (1997). Relative significance of direct ingestion and adult-mediated translocation of bait to German cockroach nymphs (Dictyoptera: Blattellidae). Journal of Economic Entomology, 90, 1073–1079.
Kopanic, R. J. J., & Schal, C. (1999). Coprophagy facilitates horizontal transmission of bait among cockroaches (Dictyoptera: Blattellidae). Environmental Eotomology, 28, 431–438.
Li, S.-Y., Liu, X., Gao, C.-F., Bo, X.-P., Su, J.-Y., Wang, Y.-H., Yu, L., Yan, X., Shen, J.-L., Yang, J., & Tao, L.-M. (2009). Laboratory screening of alternatives to highly toxic insecticides for controlling the white backed plant hopper, Sogatella furcifera and resistance risk assessment to imidacloprid in rice. Chinese Journal of Rice Science, 23, 79–84.
Liu, S., Niu, H., Xiao, T., Xue, C., Liu, Z., & Luo, W. (2009). Does phenoloxidase contributed to the resistance? Selection with butane-fipronil enhanced its activities from diamondback moths. The Open Biochemistry Journal, 3, 9–13.
Lu, W.-P., Shi, X.-Q., Guo, W.-C., Jiang, W.-H., Xia, Z.-H., Fu, W.-J., & Li, G.-Q. (2011). Susceptibilities of Leptinotarsa decemlineata (Say) in the north xinjiang Uygur autonomous region in China to two biopesticides and three conventional insecticides. Journal of Agricultural and Urban Entomology, 27, 61–73.
Malekmohammadi, M., Hejazi, M., Mossadegh, M., Galehdari, H., Khanjani, M., & Goodarzi, M. (2012). Molecular diagnostic for detecting the acetylcholinesterase mutations in insecticide-resistant populations of Colorado potato beetle, Leptinotarsa decemlineata (Say). Pesticide Biochemistry and Physiology, 104, 150–156.
Mertz, H., & Yao, R. C. (1990). Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. International Journal of Systematic Bacteriology, 40, 34–39.
Mohamadi, M., Mossadegh, M., Hejazi, M., Goodarzi, M., Khanjani, M., & Galehdari, H. (2010). Synergism of resistance to phosalone and comparison of kinetic properties of acetylcholinesterase from four field populations and a susceptible strain of Colorado potato beetle. Pesticide Biochemistry and Physiology, 9, 254–262.
Moreno, Y., Nabhan, J. F., Solomon, J., Mackenzie, C. D., & Geary, T. G. (2010). Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proceedings of the National Academy of Sciences, 107, 20120–20125.
Moscardi, F. (1999). Assessment of the application of baculoviruses for control of Lepidoptera. Annual Review of Entomology, 44, 257–289.
Niu, H.-T., Yan, L., Zong, J.-P., Wei, S.-J., & Luo, W.-C. (2007a). A comparison on toxicity of fipronil and butene-fipronil against diamondback moth larvae in laboratory. Agrochemicals Research and Application, 11, 28–30.
Niu, H.-T., Zong, J.-P., Wang, H.-Y., Wei, S.-J., Zhu, X.-F., & Luo, W.-C. (2007b). Resistance selection of Plutella xylostella to butene-fipronil and its growth fitness. Chinese Journal of Pesticide Science, 9, 245–250.
Niu, H. T., Luo, W. C., Jiang, G. Q., & Zhu, X. F. (2007c). Bioactivity of butene-fipronil and its field efficacy against diamondback moth, Plutella xylostella (L.). Acta Phytophylacica Sinica, 34, 316–320.
Niu, H.-T., Luo, W.-C., Zong, J.-P., Wei, S.-J., Wang, H.-Y., & Pan, Z.-X. (2008). Realized heritability of resistance to butene-fipronil in diamondback moth, Plutella xylostella. Acta Phytophylacica Sinica, 35, 165–168.
Rinkevich, F. D., Su, C., Lazo, T., Hawthorne, D., Tingey, W., Naimov, S., & Scott, J. G. (2012). Multiple evolutionary origins of knockdown resistance (kdr) in pyrethroid-resistant Colorado potato beetle, Leptinotarsa decemlineata. Pesticide Biochemistry and Physiology, 104, 192–200.
Shi, X.-Q., Xiong, M.-H., Jiang, W.-H., Wang, Z.-T., Guo, W.-C., Xia, Z.-H., Fu, W.-J., & Li, G.-Q. (2012). Efficacy of endosulfan and fipronil and joint toxic action of endosulfan mixtures against Leptinotarsa decemlineata (Say). Journal of Pest Science, 85, 519–526.
Shi, X.-Q., Guo, W.-C., Wan, P.-J., Zhou, L.-T., Ren, X.-L., Tursun, A., Fu, K.-Y., & Li, G.-Q. (2013). Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Research Notes, 6, 93.
Silverman, J., Vitale, G. I., & Shapas, T. J. (1991). Hydramethylnon uptake by Blattella germanica (Orthoptera: Blattellidae) by coprophagy. Journal of Economic Entomology, 84, 176–180.
Stewart, L. M. D., Hirst, M., Ferber, M. L., Merryweather, A. T., Cayley, P. J., & Possee, R. D. (1991). Construction of an improved baculovirus insecticide containing an insect-specific toxin gene. Nature, 352, 85–88.
Su, N.-Y. (1994). Field evaluation of a hexaflumuron bait for population suppression of subterranean termites (Isoptera: Rhinotermitidae). Journal of Economic Entomology, 87, 389–397.
Su, N.-Y., & Scheffrahn, R. H. (1991). Laboratory evaluation of two slow-acting toxicants against Formosan and eastern subterranean termites (Isoptera: Rhinotermitidae). Journal of Economic Entomology, 84, 170–175.
Su, N.-Y., Tamashiro, M., Yates, J. R., & Haverty, M. I. (1982). Effect of behavior on the evaluation of insecticides for prevention of or remedial control of the Formosan subterranean termite. Journal of Economic Entomology, 75, 188–193.
Su, N.-Y., Tamashiro, M., & Haverty, M. I. (1987). Characterization of slow-acting insecticides for the remedial control of the Formosan subterranean termite (Isoptera: Rhinotermitidae). Journal of Economic Entomology, 80, 1–4.
Su, N.-Y., Paul, B. M., & Schffrahn, R. H. (1991). Suppression of foraging populations of the Formosan subterranean termite (Isoptera: Rhinotermitidae) by field applications of a slow-acting toxicant bait. Journal of Economic Entomology, 84, 1525–1531.
Su, N.-Y., Tokoro, M., & Scheffrahn, R. H. (1994). Estimating oral toxicity of slow-acting toxicants against subterranean termites (Isoptera: Rhinotermitidae). Journal of Economic Entomology, 87, 398–401.
Sun, X. L., Wang, H. L., Sun, X. C., Chen, X. W., Peng, C. M., Pan, D. M., Jehle, J. A., Van Derwerf, W., Vlak, J. M., & Hua, Z. (2004). Biological activity and field efficacy of a genetically modified Helicoverpa armigera single-nucleocapsid nucleopoly hedrovirus expressing an insect-selective toxin from a chimeric promoter. Biological Control, 29, 124–137.
Szendrei, Z., Grafius, E., Byrne, A., & Ziegler, A. (2012). Resistance to neonicotinoid insecticides in field populations of the Colorado potato beetle (Coleoptera: Chrysomelidae). Pest Management Science, 68, 941–946.
Thompson, G. D., Dutton, R., & Sparks, T. C. (2000). Spinosad-a case study: an example from a natural products discovery programme. Pest Management Science, 56, 696–702.
Wang, S.-P., Hu, X.-X., Meng, Q.-W., Muhammad, S. A., Chen, R.-R., Li, F., & Li, G.-Q. (2013). The involvement of several enzymes in methanol detoxification in Drosophila melanogaster adults. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 166, 7–14.
Wolstenholme, A., & Rogers, A. (2005). Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology, 131, S85–S95.
Yeoh, B.-H., & Lee, C.-Y. (2007). Tunneling responses of the Asian subterranean termite, Coptotermes gestroi in termiticide-treated sand (Isoptera: Rhinotermitidae). Sociobiology, 50, 457–468.
Yuan, Z.-J., Wang, X.-T., Hao, X.-M., Lai, Z.-W., & Deng, X.-P. (2009). Formulation development of butene-fipronil 20% WG. Agrochemicals Research and Application, 13, 14–17.
Zhao, X., & Salgado, V. L. (2010). The role of GABA and glutamate receptors in susceptibility and resistance to chloride channel blocker insecticides. Pesticide Biochemistry and Physiology, 97, 153–160.
Zichová, T., Kocourek, F., Salava, J., Nad’ová, K., & Stará, J. (2010). Detection of organophosphate and pyrethroid resistance alleles in Czech Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) populations by molecular methods. Pest Management Science, 66, 853–860.
Acknowledgements
This research was supported by the National Basic Research Program of China (973 Program, No. 2010CB126200), the National Natural Sciences Foundation of China (31272047 and 31360442) and a nationally special fund of China for agri-scientific research in the public interest (201103026).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Arain, M.S., Wan, PJ., Shakeel, M. et al. Toxicity of butene-fipronil, in comparison with seven other insecticides, in Leptinotarsa decemlineata and Drosophila melanogaster . Phytoparasitica 45, 103–111 (2017). https://doi.org/10.1007/s12600-016-0560-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-016-0560-z