Abstract
The cotton mealybug, Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) is a devastating pest that cause rigorous damage to the number of crops through feeding, managed by using various insecticides. To assess the risk of resistance and design a strategy for resistance management, a field collected population of P. solenopsis was selected with deltamethrin in the laboratory for six generations to investigate the cost to its fitness and to examine cross resistance to different insecticides. Bioassay results at G8 showed that the deltamethrin selected population (Delta-SEL) developed a resistance ratio of 100-fold compared to that of the unselected population (UNSEL). The deltamethrin resistance population exhibited strong cross-resistance to acetamiprid and lambda-cyhalothrin, but no cross-resistance to profenofos when compared to that of the UNSEL. The relative fitness of the Delta-SEL population was 0.37, with considerably lower survival rates from crawler to second instar, fecundity, hatchability, number of next generation nymphs, net reproductive rate and biotic potential compared with that of the UNSEL. The cost of fitness associated with deltamethrin resistance was evident in the Delta-SEL population. The present study provided useful information for management strategies to overcome development of resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The mealybug, Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) is a devastating pest of cotton in different regions of the world such as Pakistan (Afzal et al . 2015b; Hodgson et al . 2008), India (Nagrare et al . 2009), China (YanPing et al . 2009), United State (Kumashiro et al . 2001), Taiwan and Thailand (Hodgson et al . 2008), due to its polyphagous nature. This pest has a wide host range and causes economic damage to several crops including cotton, ornamentals, medicinal plants and vegetables (Abbas et al . 2010; Nagrare et al . 2009; YanPing et al . 2009). Phenacoccus solenopsis causes severe damage to the cotton crop (Abbas et al . 2009; Wang et al . 2010), mainly by sucking plant sap and producing sugary material on which sooty mold develops, which ultimately blocks photosynthesis (Saeed et al . 2007). Attacked plants remain stunted and produce fewer bolls, leaves turn yellow, dry up and eventually fall off (Culik and Gullan 2005). For being the third largest exporter of cotton in the world, this pest is of major economic importance to the farming community of Pakistan (Abbas et al . 2010; Afzal et al . 2015b; Hodgson et al . 2008; Yousuf and Tayyib 2007)
In Indo-Pakistan subcontinent, the broad spectrum use of organochlorine, organophosphate and pyrethroid insecticides provides an ideal atmosphere for insecticide resistance development (Ahmad et al . 2007a; Kranthi et al . 2002). Deltamethrin, a pyrethroid insecticide is applied for the control of different pests of cotton and vegetables (Saddiq et al . 2014; Sayyed et al . 2005). Due to extensive use of this insecticide, resistance has been reported in many pests of cotton and vegetables such as diamondback moth, Plutella xylostella Linnaeus, armyworm, Spodoptera litura Fabricius, spotted bollworm, Earias vittella Fabricius and mealybug, P. solenopsis (Ahmad et al . 2007b; Jan et al . 2015; Saddiq et al . 2014; Sayyed et al . 2005).
Fitness costs associated with insecticide resistance are where the development of resistance to an insecticide is accompanied with high energetic cost or other significant disadvantages that diminish the insect’s fitness compared with its susceptible counterparts in the population (Kliot and Ghanim 2012; Mansoor et al . 2013). Roush and Daly (1990) pointed that either the relative fitness of resistant population increases or decreases. If relative fitness in resistant insect pests is decreased, the discontinued use of insecticides for which resistance has developed may reduce the occurrence of resistance genes and return the vulnerability of the insects to that insecticide (Roush and McKenzie 1987; Zaka et al . 2014). The pleiotropic effect related with resistance alleles may be manifested as a change in survival rates, percent eggs hatchability, fecundity (eggs laid per female), developmental time and pupal weights (Abbas et al . 2014b; Groeters et al . 1994). Fitness costs to insecticides have been documented in cotton and vegetable pests such as P. xylostella (Cao and Han 2006; Sayyed and Wright 2001; Sun et al . 2012), tobacco budworm, Heliothis virescens Fabricius (Sayyed et al . 2008), beet armyworm, Spodoptera exigua Hübner (Ishtiaq et al . 2014; Jia et al . 2009), and S. litura (Abbas et al . 2012, 2014b). Previously, we have studied the fitness cost in P. solenopsis to acetamiprid (Afzal et al . 2015b).
Better understanding of life history traits changed by costs of fitness, degree of dominance of these costs and ecological situations which affect these fitness costs can be helpful tools for developing strategies to manage resistance. The present study was planned for determination of life history parameters of unselected and deltamethrin-selected populations to see the fate of fitness.
Material and methods
Insects
The P. solenopsis population was collected from various parts of the Multan region of Punjab, Pakistan, in April 2010. The insects were reared on China rose, Hibiscus rosasinensis L. leaves in plastic jars (22×13 cm) at 27 ± 2 °C and 65 ± 5 % relative humidity with a 14:10 h light: dark photoperiod. Fresh leaves of China rose were changed every two days (Saddiq et al . 2014). Susceptible counterpart (UNSEL) was generated by rearing without insecticide exposure parallel to the resistant population in the laboratory.
Insecticides
Commercial-grade formulated insecticides used for bioassays included deltamethrin (Decis super® 10EC, Bayer Crop Science, Pakistan), lambda-cyhalothrin (Karate® 2.5EC, Syngenta, Pakistan), profenofos (Curacron® 500EC, Syngenta, Pakistan) and acetamiprid (Mospilon® 20WP, Dow Agro-Sciences, Pakistan).
Concentration response bioassays
Bioassays on second instar nymphs of P. solenopsis of all populations were carried out by using a leaf-dip method (Saddiq et al . 2014). Five concentrations of each insecticide were prepared as serial dilutions for each insecticide. Fresh China rose leaves were dipped in each concentration for 10 seconds and air-dried at room temperature for 1–1.5 hour. Five second instar nymphs were introduced in each Petri dish, and each treatment was repeated 5 times. A total of 25 nymphs were used for each concentration. For the control, China rose leaves dipped in tap water (without insecticides) were presented to the nymphal instars. Mortality was assessed after 48 h exposure to deltamethrin, lambda-cyhalothrin, and profenofos and 72 h exposure to acetamiprid. Nymphs were considered dead if they failed to move after a gentle touch with a fine brush (Afzal et al . 2015b).
Selection with deltamethrin
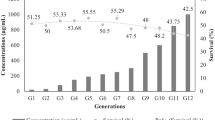
The field collected population designated as Field Pop was divided into two subpopulations at second generation (G2). One population was left unexposed, designated as UNSEL while second one was continuously selected with different concentrations of deltamethrin (21.21, 60.00, 120.47, 181.71, 334.06 μgmL-1) for six consecutive generations. An average of 170 nymphs were selected per generation. After 48 hours exposure to deltamethrin, mortality was determined and the surviving nymphs were used as parents of the next generation.
Life history parameters
Beginning with the 8th generation, 75 newly hatched crawlers were collected randomly from each of the UNSEL and Delta-SEL populations for the experiment. The young crawlers were weighed and reared in plastic jars (14 × 9 cm) at 27 ± 2 °C and 65 ± 5 % RH with a 14:10 h light: dark photoperiod. The nymphs were fed daily on fresh China rose leaves and observed for different life history parameters. For each population, the jars were set as three replicates. Nymphal mortality was observed daily. Nymphal duration and time of development (DT) from egg to adult female and male were recorded.
A total of 17 females and 5 males for UNSEL and 22 females and 3 males were used for egg laying. Small numbers of males were due to low proportion in the population. The fecundity was recorded as no of eggs/female until death and the hatchability was recorded according to Liu and Han (2006) as Hatchability = All neonates / All eggs. The net reproductive rate (Ro) was estimated according to Jia et al . (2009) as Ro=Nn+1 / Nn.Where Nn is the neonate numbers of parental generation and Nn+1 is the neonate numbers of next generation. The relative fitness was calculated according to Abbas et al . (2012) as Relative fitness = Ro of the Delta-SEL / Ro of the UNSEL
Estimation of realized heritability
Following, the method of Falconer et al . (1996) and Tabashnik (1992), the realized heritability (h 2) of resistance to deltamethrin and other insecticides was estimated as follows:
In the above equation, R (selection response) was estimated as follows:
Final LC50 means the LC50 of population after six generations of continuous selection, initial LC50 means the LC50 of field population before selection and n means the number of generations selected with deltamethrin.
Whereas, S (Selection differential) was calculated as follows:
Where i mean intensity of selection calculated according to following formula:
Where p is the average percentage of surviving rate (Tabashnik and McGaughey 1994).
σp means phenotypic standard deviation calculated as follows:
The number of generations required for a ten-fold increase in the LC50 of deltamethrin and other insecticides were calculated as follows:
Statistical Analysis
The concentration response data was analyzed by probit analysis (Finney 1971) with Polo Software (Software 2005), to assess the LC50 values, their standard errors, slopes and 95% fiducial limits (FL). Mortality was corrected by Abbott’s formula (Abbott 1925), whenever necessary. Resistance Ratio (RR) and its 95% CL were calculated by dividing the LC50 value and its 95% FL of the Delta-SEL strain divided by the LC50 of the UNSEL strain. Resistance ratio were considered significantly different if the 95% CL did not include the value of 1 which is for UNSEL strain (Robertson and Preisler 1992).
For comparison of the life history traits of the strains, the two sample t test was used with Statistix version 8.1, Analytical software (Anonymous 2005). Means were considered significantly different when P values were ≤ 0.05.
Results
Response of UNSEL, Field Pop and Delta-SEL populations of P. solenopsis to various insecticides
The toxicities of deltamethrin, profenofos and acetamiprid in the Field Pop were not significantly different (95% FL overlap) when compared with that of the UNSEL and produced a resistance ration of 3.18-fold, 4.78-fold and 9.72-fold, respectively. The toxicity of lambda-cyhalothrin in the Field Pop was significantly different (95% FL did not overlap) when compared with that of the UNSEL and exhibited a resistance ration of 8.15-fold (Table 1).
The toxicities of all tested insecticides on the Delta-SEL were significantly lower than that of the UNSEL. The toxicities of deltamethrin and acetamiprid in the Delta-SEL were significantly different (95% FL did not overlap) when compared with the Field Pop while the toxicities of lambda-cyhalothrin and profenofos were similar (95% FL overlap). After 7 generations of selection, the Delta-SEL population developed a resistance ratio of 100-fold to deltamethrin when compared with the UNSEL (Table 1).
Cross-resistance to other insecticides in the Delta-SEL population
The Delta-SEL population at G8 was used to assess the cross-resistance against different insecticides. Results indicated that the Delta-SEL population showed strong evidence of cross-resistance against acetamiprid (75-fold) and lambda-cyhalothrin (19-fold) but lack of cross-resistance to profenofos (95% FL overlap, RR = 2.03-fold), when compared to the UNSEL (Table 1).
Fitness parameters
The survival rate from crawler to 2nd instar nymph of the Delta-SEL strain was significantly lower in comparison with the UNSEL strain (df = 4, t = 12.37, P < 0.001). The male nymphal duration (df = 4, t = 0.45, P = 0.68) and female nymphal duration (df = 4, t = -0.40, P = 0.71) of the Delta-SEL strain did not significantly differ from that of the UNSEL strain. There were also no significant difference in developmental time (DT) from egg to adult female (df = 4, t = -0.64, P = 0.56) and male (df = 4, t = 0.78, P = 0.48) of the Delta-SEL strain compared with the UNSEL strain. The number of eggs laid per female of the Delta-SEL strain was significantly lower in comparison with the UNSEL strain (df = 4, t = 4.47, P = 0.01). There were significant difference in percent hatching (df = 4, t = 3.34, P = 0.03) of the Delta-SEL strain compared with the UNSEL strain. The biotic potential of the Delta-SEL strain was significantly lower (df = 4, t = 3.12, P = 0.04) compared with that of the UNSEL strain. The number of next generation crawlers of the Delta-SEL and UNSEL strains were 1874 and 5021, respectively. The net reproductive rate of the Delta-SEL and UNSEL strains was 79.74 and 212.17, respectively. The relative fitness of the Delta-SEL strain was 0.37 compared with the UNSEL strain (Table 2).
Realized heritability
After six generations of continuous selection with deltamethrin, the LC50 value increased from 21 - 658 μgmL-1 and the slope increased from 1.37 - 1.75 in the Delta-SEL population of P. solenopsis. The values estimated for realized heritability (h 2) to deltamethrin resistance and cross-resistance to lambda-cyhalothrin, profenofos and acetamiprid were 0.84, 0.16, -0.18 and 0.37, respectively in P. solenopsis (Table 3). The number of generations required for a ten-fold increase in LC50 of deltamethrin, lambda-cyhalothrin, profenofos and acetamiprid were expected to be 4, 17, -17 and 7, respectively (reciprocal of R; Table 3).
Discussion
The field population (G2) exhibited a 3.18-fold resistance to widely used pyrethroid insecticide, deltamethrin before selection. After six generations of experimental exposure, the Delta-SEL population of P. solenopsis developed 100-fold resistance compared to that of the UNSEL suggesting that the selection had marked effect on the development of resistance. The increase in LC50 value of an insecticide due to constant exposure to the same group of insecticides is known as cross-resistance (Tabashnik et al . 1987). Cross-resistance among the same and different group of insecticides is a normally observed event (Abbas et al . 2014a). However, low cross-resistance among pyrethroid group of insecticides in many insects is documented. For example, Ahmad et al . (2007b) found that deltamethrin selected population of S. litura showed low cross-resistance against cypermethrin (17-fold) and no cross-resistance against organophosphate insecticides. Similarly, the deltamethrin selected population of P. xylostella showed low cross-resistance against spinosad (10-fold), and no cross-resistance against fipronil (1-fold) and indoxacarb (2-fold) (Sayyed et al . 2005). The results of the present study showed that the Delta-SEL population of P. solenopsis (100-fold) developed no cross-resistance to profenofos (95% FL overlap), while significant cross-resistance to lambda-cyhalothrin and acetamiprid (95% FL did not overlap) compared to the UNSEL. Lack of cross-resistance between deltamethrin and profenofos suggests that this insecticide could be used as alternatives due to low resistance risk for delaying the development of resistance to deltamethrin in P. solenopsis.
The results of the present study showed that the costs of fitness are related with the resistance of deltamethrin in this pest. Insecticides can change the biology of resistant organisms. Studying the relative fitness of the resistant population is essential for understanding and managing the resistance problems (Abbas et al . 2014b; Georghiou and Taylor 1977). It is usually believed that the biological characteristics (e.g. declined fecundity and hatchability) change the relative fitness of the resistant population. The present results showed that under continuous selection pressure, the Delta-SEL population had significantly lower number of eggs laid by the females, lower hatching and lower biotic potential. Therefore, deltamethrin resistance in P. solenopsis population corresponded with significant decrease in most of the life history traits and these results indicate the occurrence of trade off in resource distribution between the fitness costs and resistance. Previously, fitness costs associated with deltamethrin resistance has been documented in H. virescens (Sayyed et al . 2008). This is the first report of fitness cost of deltamethrin resistance in P. solenopsis worldwide to the best of author’s knowledge.
Realized heritability (h 2) is the proportion of phenotypic variation (VP) accounted for by additive genetic variation (VA), which may decrease either due to the decrease in VA or to the increase in environmental variance (VE) (Falconer et al . 1996). In this study, the high h 2 after six generations of selection with deltamethrin indicated that P. solenopsis may have higher chances of resistance development to deltamethrin. This high h 2 reflects higher additive genetic and lower environmental variations for deltamethrin resistance. The present results are similar to those obtained with deltamethrin (h 2 = 0.49) in P. xylostella (Sayyed et al . 2005) and lambda-cyhalothrin (h 2 = 0.2) and beta-cypermethrin (h 2 = 0.3) in house fly, Musca domestica (Abbas et al . 2014a; Zhang et al . 2008). This is in agreement with the fact that the population was collected from a cotton field where a variety of insecticides, including deltamethrin, lambda-cyhalothrin, profenofos and acetamiprid were being used to control different pests (Afzal et al . 2015a; Saddiq et al . 2014). Moreover, the other likely explanation for high heritability is that h 2 is artificially inflated by the decrease in VE, as expected from the field to the laboratory (Abbas et al . 2014a; Klerks et al . 2011). The number of generations required for a ten-fold increase in LC50 value of the Delta-SEL strain was estimated to be 4 generations if a field population received prolonged exposure to deltamethrin and 72% of individuals survived the selection pressure of different concentration of deltamethrin. Although laboratory experiments do not always reflect field conditions, h 2 estimated in the laboratory provides useful information on the potential for resistance increase in P. solenopsis (Tabashnik 1992).
Management of insecticide resistance mainly depends on the costs of fitness, such as the number of resistance controlling factors which would be reduced when selection pressure is stopped (Ferré and Van Rie 2002). Analysis of models recommends that fitness costs may delay the development of resistance to insecticides within a pest population (Tabashnik et al . 2003). In the present study, selected population had disadvantage than the UNSEL which showed that the resistance development against deltamethrin would be delayed. All the tested insecticides are used by the farmers in Pakistan for the control of cotton pests including P. solenopsis. Based on the findings of the present research, it can also be concluded that for good management of P. solenopsis under field conditions, rotation of deltamethrin with profenofos which have no/weak cross-resistance should be implemented in resistance management strategies. Moreover, insecticides with novel modes of action like insect growth regulators should be rotated to delay the development of resistance.
References
Abbas, G., Arif, M. J., Ashfaq, M., Aslam, M., & Saeed, S. (2010). Host plants, distribution and overwintering of cotton mealybug (Phenacoccus solenopsis; Hemiptera: Pseudococcidae). International Journal of Agriculture and Biology, 12, 421–425.
Abbas, G., Arif, M. J., Saeed, S., & Karar, H. (2009). A new invasive species of genus Phenacoccus Cockerell attacking cotton in Pakistan. International Journal of Agriculture and Biology, 11, 54–58.
Abbas, N., Khan, H. A. A., & Shad, S. A. (2014a). Resistance of the house fly Musca domestica (Diptera: Muscidae) to lambda-cyhalothrin: mode of inheritance, realized heritability, and cross-resistance to other insecticides. Ecotoxicology, 23, 791–801.
Abbas, N., Shad, S. A., & Razaq, M. (2012). Fitness cost, cross resistance and realized heritability of resistance to imidacloprid in Spodoptera litura (Lepidoptera: Noctuidae). Pesticide Biochemistry and Physiology, 103, 181–188.
Abbas, N., Shad, S. A., Razaq, M., Waheed, A., & Aslam, M. (2014b). Resistance of Spodoptera litura (Lepidoptera: Noctuidae) to profenofos: relative fitness and cross resistance. Crop Protection, 58, 49–54.
Abbott, W. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265–267.
Afzal, M. B. S., Abbas, N., & Shad, S. A. (2015a). Inheritance, realized heritability and biochemical mechanism of acetamiprid resistance in the cotton mealybug, Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae). Pesticide Biochemistry and Physiology
Afzal, M. B. S., Shad, S. A., Abbas, N., Ayyaz, M., & Walker, W. B. (2015b). Cross-resistance, the stability of acetamiprid resistance and its effect on the biological parameters of cotton mealybug, Phenacoccus solenopsis (Homoptera: Pseudococcidae), in Pakistan. Pest Management Science, 71, 151–158.
Ahmad, M., Iqbal Arif, M., & Ahmad, M. (2007a). Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Protection, 26, 809–817.
Ahmad, M., Sayyed, A. H., Crickmore, N., & Saleem, M. A. (2007b). Genetics and mechanism of resistance to deltamethrin in a field population of Spodoptera litura (Lepidoptera: Noctuidae). Pest Management Science, 63, 1002–1010.
Anonymous (2005). Statistix for windows. Tallahassee:Analytical software.
Cao, G., & Han, Z. (2006). Tebufenozide resistance selected in Plutella xylostella and its cross-resistance and fitness cost. Pest Management Science, 62, 746–751.
Culik, M. P., & Gullan, P. J. (2005). A new pest of tomato and other records of mealybugs (Hemiptera: Pseudococcidae) from Espírito Santo, Brazil. Zootaxa, 964, 1–8.
Falconer, D. S., Mackay, T. F., & Frankham, R. (1996). Introduction to quantitative genetics (4th edn). Trends in Genetics, 12, 280.
Ferré, J., & Van Rie, J. (2002). Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annual Review of Entomology, 47, 501–533.
Finney, D. (1971). A statistical treatment of the sigmoid response curve. Probit analysis (3rd ed., p. 333). London: Cambridge University Press.
Georghiou, G. P., & Taylor, C. E. (1977). Genetic and biological influences in the evolution of insecticide resistance. Journal of Economic Entomology, 70, 319–323.
Groeters, F. R., Tabashnik, B. E., Finson, N., & Johnson, M. W. (1994). Fitness costs of resistance to Bacillus thuringiensis in the diamondback moth (Plutella xylostella). Evolution, 197-201.
Hodgson, C., Abbas, G., Arif, M. J., Saeed, S., & Karar, H. (2008). Phenacoccus solenopsis Tinsley (Sternorrhyncha: Coccoidea: Pseudococcidae), an invasive mealybug damaging cotton in Pakistan and India, with a discussion on seasonal morphological variation. Zootaxa, 1913, 1–35.
Ishtiaq, M., Razaq, M., Saleem, M. A., Anjum, F., Noor ul Ane, M., Raza, A. M., et al. (2014). Stability, cross-resistance and fitness costs of resistance to emamectin benzoate in a re-selected field population of the beet armyworm, Spodoptera exigua (Lepidoptera: Noctuidae). Crop Protection, 65, 227–231.
Jan, M. T., Abbas, N., Shad, S. A., & Saleem, M. A. (2015). Resistance to organophosphate, pyrethroid and biorational insecticides in populations of spotted bollworm, Earias vittella (Fabricius) (Lepidoptera: Noctuidae), in Pakistan. Crop Protection, 78, 247–252.
Jia, B., Liu, Y., Zhu, Y. C., Liu, X., Gao, C., & Shen, J. (2009). Inheritance, fitness cost and mechanism of resistance to tebufenozide in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Pest Management Science, 65, 996–1002.
Klerks, P. L., Xie, L., & Levinton, J. S. (2011). Quantitative genetics approaches to study evolutionary processes in ecotoxicology; a perspective from research on the evolution of resistance. Ecotoxicology, 20, 513–523.
Kliot, A., & Ghanim, M. (2012). Fitness costs associated with insecticide resistance. Pest Management Science, 68, 1431–1437.
Kranthi, K., Jadhav, D., Kranthi, S., Wanjari, R., Ali, S., & Russell, D. (2002). Insecticide resistance in five major insect pests of cotton in India. Crop Protection, 21, 449–460.
Kumashiro, B. R., Heu, R. A., Nishida, G. M., & Beardsley, J. W. (2001). New state records of immigrant insects in the Hawaiian Islands for the year 1999. Proceedings of the Hawaiian Entomological Society, 35, 170–184.
Liu, Z., & Han, Z. (2006). Fitness costs of laboratory-selected imidacloprid resistance in the brown planthopper, Nilaparvata lugens Stål. Pest Management Science, 62, 279–282.
Mansoor, M. M., Abbas, N., Shad, S. A., Pathan, A. K., & Razaq, M. (2013). Increased fitness and realized heritability in emamectin benzoate-resistant Chrysoperla carnea (Neuroptera: Chrysopidae). Ecotoxicology, 22, 1232–1240.
Nagrare, V., Kranthi, S., Biradar, V., Zade, N., Sangode, V., Kakde, G., et al. (2009). Widespread infestation of the exotic mealybug species, Phenacoccus solenopsis (Tinsley) (Hemiptera: Pseudococcidae), on cotton in India. Bulletin of Entomological Research, 99, 537–541.
Robertson, J., & Preisler, H. (1992). Pesticide bioassays with arthropods. Boca Raton: CRC.
Roush, R. T., & Daly, J. C. (1990). The role of population genetics in resistance research and management. In R. T. Roush & B. E. Tabashnik (Eds.), Pesticide resistance in arthropods (pp. 97–152). New York: Chapman & Hall.
Roush, R. T., & McKenzie, J. A. (1987). Ecological genetics of insecticide and acaricide resistance. Annual Review of Entomology, 32, 361–380.
Saddiq, B., Shad, S. A., Khan, H. A. A., Aslam, M., Ejaz, M., & Afzal, M. B. S. (2014). Resistance in the mealybug Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae) in Pakistan to selected organophosphate and pyrethroid insecticides. Crop Protection, 66, 29–33.
Saeed, S., Ahmad, M., Ahmad, M., & Kwon, Y. J. (2007). Insecticidal control of the mealybug Phenacoccus gossypiphilous (Hemiptera: Pseudococcidae), a new pest of cotton in Pakistan. Entomological Research, 37, 76–80.
Sayyed, A. H., Ahmad, M., & Crickmore, N. (2008). Fitness costs limit the development of resistance to indoxacarb and deltamethrin in Heliothis virescens (Lepidoptera: Noctuidae). Journal of Economic Entomology, 101, 1927–1933.
Sayyed, A. H., Attique, M. N. R., Khaliq, A., & Wright, D. J. (2005). Inheritance of resistance and cross-resistance to deltamethrin in Plutella xylostella (Lepidoptera: Plutellidae) from Pakistan. Pest Management Science, 61, 636–642.
Sayyed, A. H., & Wright, D. J. (2001). Fitness costs and stability of resistance to Bacillus thuringiensis in a field population of the diamondback moth Plutella xylostella L. Ecological Entomology, 26, 502–508.
Software, L. (2005). POLO for windows. Petaluma: LeOra Software.
Sun, J., Liang, P., & Gao, X. (2012). Cross-resistance patterns and fitness in fufenozide-resistant diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Pest Management Science, 68, 285–289.
Tabashnik, B. E. (1992). Resistance risk assessment: realized heritability of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae), tobacco budworm (Lepidoptera: Noctuidae), and Colorado potato beetle (Coleoptera: Chrysomelidae). Journal of Economic Entomology, 85, 1551–1559.
Tabashnik, B. E., Carrière, Y., Dennehy, T. J., Morin, S., Sisterson, M. S., Roush, R. T., et al. (2003). Insect resistance to transgenic Bt crops: lessons from the laboratory and field. Journal of Economic Entomology, 96, 1031–1038.
Tabashnik, B. E., Cushing, N. L., & Johnson, M. W. (1987). Diamondback moth (Lepidoptera: Plutellidae) resistance to insecticides in Hawaii: intra-island variation and cross-resistance. Journal of Economic Entomology, 80, 1091–1099.
Tabashnik, B. E., & McGaughey, W. H. (1994). Resistance risk assessment for single and multiple insecticides: responses of Indianmeal moth (Lepidoptera: Pyralidae) to Bacillus thuringiensis. Journal of Economic Entomology, 87, 834–841.
Wang, Y., Watson, G. W., & Zhang, R. (2010). The potential distribution of an invasive mealybug Phenacoccus solenopsis and its threat to cotton in Asia. Agricultural and Forest Entomology, 12, 403–416.
YanPing, W., SanAn, W., & RunZhi, Z. (2009). Pest risk analysis of a new invasive pest, Phenacoccus solenopsis, to China. Chinese Bulletin of Entomology, 46, 101–106.
Yousuf, M., & Tayyib, M. (2007). Mealybug problem on cotton in Pakistan. Pakistan Entomologist, 29, 49–50.
Zaka, S. M., Abbas, N., Shad, S. A., & Shah, R. M. (2014). Effect of emamectin benzoate on life history traits and relative fitness of Spodoptera litura (Lepidoptera: Noctuidae). Phytoparasitica, 42, 493–501.
Zhang, L., Shi, J., & Gao, X. (2008). Inheritance of beta-cypermethrin resistance in the housefly Musca domestica (Diptera: Muscidae). Pest Management Science, 64, 185–190.
Acknowledgments
We are highly thankful to the national and international pesticide companies for the provision of chemicals for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saddiq, B., Abbas, N., Shad, S.A. et al. Deltamethrin resistance in the cotton mealybug, Phenacoccus solenopsis Tinsley: Cross-resistance to other insecticides, fitness cost analysis and realized heritability. Phytoparasitica 44, 83–90 (2016). https://doi.org/10.1007/s12600-015-0500-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-015-0500-3