Abstract
The lithium-ion batteries (LIBs) have been widely equipped in electric/hybrid electric vehicles (EVs/HEVs) and the portable electronics due to their excellent electrochemical performances. However, a large number of retired LIBs that consist of toxic substances (e.g., heavy metals, electrolytes) and valuable metals (e.g., Li, Co) will inevitably flow into the waste stream, and their incineration or landfill treatment will cause severe risks to ecosystem and human beings. The sustainable and efficient treatment or recycling of valuable resources from spent LIBs should be fully recognized for environmental and resource security. As one of the most important processes for spent LIBs recycling, the pretreatment is an indispensable step, which is directly related to the subsequent metal extraction and separation processes. Although considerable progresses have been made regarding the pretreatment technologies, there are few summarized reports concerning critical processes of spent LIBs recycling, especially combination of currently available recycling technologies with industrialized applications during pretreatments. Therefore, comprehensive review of the current prevailing pretreatment technologies in laboratory to existing scale-up applications is quite necessary to reveal cutting-edge development in the field of pretreatment. In this review, the current pretreatment technologies are systematically categorized and introduced, along with critical discussions. This review focused on the various options for pretreatment processes itself, instead of general spent LIBs recycling technologies without the focused topics that have been sophisticatedly reviewed by previous studies. Here, the deactivation, discharge, dismantling, separation, liberation of active material and electrolyte treatment have been summarized with the in-depth discussion of the technology development and current status of each category. Finally, current states of industrial development are also reviewed and discussed for the development of efficient and environmentally friendly recycling technologies for future applications. This review tends to present a focused topic concerning the pretreatment of spent LIBs to potential readers with a comprehensive illustration of the development on both cutting-edge technologies and scale-up applications.
Graphical abstract

摘要
锂离子电池(LIBs)由于其出色的电化学性能,已经被广泛地装备在电动/混合电动汽车(EVs/HEVs)和便携式电子产品中。然而,退役LIBs中含有大量的有毒物质(如重金属、电解质)和有价金属(如锂、钴),焚烧或填埋处理将对生态系统和人类造成严重风险。为了环境和资源的安全,可持续和有效地处理或回收废旧LIBs中的宝贵资源是十分重要的。预处理作为废LIBs回收的最重要、不可或缺的过程之一,其好坏直接关系到后续的金属提取和分离过程。尽管在预处理技术方面已经取得了相当大的进展,但有关废旧LIBs回收预处理过程的关键过程的总结却很少,特别是在预处理过程中把目前可用的回收技术与工业化应用相结合。因此,本文全面回顾目前在实验室中流行的预处理技术和现有的放大应用,以揭示预处理领域的前沿发展。在本综述中,对当前的预处理技术进行了系统的分类和介绍,并进行了重要的讨论。本综述侧重于预处理过程的各种方法,而不是介绍一般的废LIBs的回收技术,这在之前的综述中很少见。在这里,总结了失活、放电、拆解、分离、活性材料与集流体的分离和电解质处理等处理技术,并对每一类的技术发展和现状进行了深入讨论。最后,还回顾和讨论了工业发展的现状,以便为未来的应用开发出高效和环保的回收技术。本文倾向于向潜在的读者介绍有关废旧LIBs预处理部分,全面说明前沿技术和放大应用的发展。
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the further development of industrialization in the modern society, it has been well recognized that the alternative renewable energy resources should be one of the critical strategies to solve current energy and environmental issues [1, 2]. As one of the typical emerging energy storage devices, lithium-ion batteries (LIBs) are increasingly used as substitutes of the conventional fuels due to their superiorities regarding natural resource conservation, carbon emission reduction and environmental footprint [3,4,5]. Nowadays, an increasing number of LIBs have been applied as energy storage devices owing to their excellent electrochemical performances, like high energy efficiency, high power density, light weight, and long cycle life, since their commercialization by Sony in the 1990s [6, 7]. As shown in Fig. 1, they are widely used in consumer electronics products, electric/hybrid electric vehicles, aerospace industry, power transmission, and renewable energy industry [8,9,10]. It was reported that global electric vehicle reached 136 GWh in 2020 and was expected to 1163 GWh in 2025 [11]. As illustrated in Fig. 2, driven by the continuous electrification of auto industry, LIBs have reached millions of trading volumes as the main driver of electric vehicles will continue to grow in the future [4, 12].
a Global LIBs Market in the next decade (2021–2030). Reproduced with permission from Ref. [4]. Copyright 2021, Elsevier. b Automotive power LIBs shipments, growth rate and forecast in China in recent decade (2015–2025, E manifest forecast data); c Percentage of global distribution of critical materials for LIBs production. Reproduced with permission from Ref. [3, 13]. Copyright 2022, Elsevier
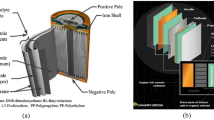
Generally, the LIBs are mainly composed of cathode, anode, separator, electrolyte and other appurtenances (Fig. 3) [13]. Usually, cathode materials that are coated on the current collectors (Al foils) consist of cathode materials, conductive reagents and polyvinylidene difluoride (PVDF) binders [14]. Recently, cathode materials of LiCoO2 (LCO), LiMn2O4 (LMO), LiFePO4 (LFP) and LiNixCoyMn1-x-yO2 (NCM) are the most widely used materials [15]. The anode is made of anode materials, conductive reagent and PVDF binders in uniform format, and then they are coated on the surface of current collectors (Cu foils) [16]. The PVDF is widely used as a binder and is a kind of polymer compound with high viscosity, good chemical stability and good mechanical processing properties, which interacts with cathode/anode materials to current collectors via weak van der Waals forces [17]. The electrolyte is a mixture of organic solvents and lithium salts with the most used lithium salts of LiPF6, LiBF4, LiClO4 and Li(SO2CF3)2 for LIBs [18]. Typical organic solvents include ethyl carbonate (EC), dimethyl carbonate (DMC), propylene carbonate (PC) and dimethyl sulfoxide (DMSO) due to their high solubility for lithium salts and suitable viscosity [19]. Currently, the most widely used separators are the microporous polyolefin membranes (e.g. polyethylene (PE), polypropylene (PP)) for their preferable porosity, liquid absorption capacity, and temperature tolerance [20].
With an explosive growth of rechargeable LIBs, large quantities of spent LIBs will be generated in next 3–10 years and approximately 500,000 metric tons of spent LIBs were generated in China in 2020, with an estimated amount of 11 million tons of spent LIBs by 2030 around the world based on the statistics [2, 21]. However, less than 6% of spent LIBs are recycled world widely, and most of them ended up in incineration or landfilling treatments [22, 23]. The inappropriate treatment of spent LIBs not only causes severely environmental issues, but also leads to scarcity of valuable resources (such as valuable metals). Besides, the flammable electrolytes may release toxic substances (e.g., ketone, aldehydes, HF and PF5) generated, causing serious contaminations to the environment (as shown in Eqs. (1–5)) [4, 14]. The heavy metals (e.g., Ni, Co, Mn) in cathodes are also harmful to the environment and human beings [8, 24]. According to London Metal Exchange, the official price for Ni and Co in the first quarter of 2022 was 27,915.50 $·t−1 and 76,107.20 $·t−1, respectively [25]. And the average export price for Li2CO3 reached around 42,500.00 $·t−1 in May of 2022 [26]. Spent LIBs have been regarded as “artificial minerals” for their high contents of valuable metals [14], especially the global limited reserves [3, 27].
Therefore, it is necessary for the recycling of spent LIBs to realize the sustainable and environmental strategy of “reduce, reuse and recycle” (3R), alleviating the depletion of mineral resources and reducing damages to human beings and environment [28, 29]. Currently, there are numerous methods towards recycling of valuable metals from spent LIBs [22, 30, 31]. Typically, main recycling methods include pyrometallurgy [32, 33], hydrometallurgy [34] (acid leaching [35,36,37,38], alkali leaching [39, 40], bioleaching [41, 42], solvent extraction [43,44,45] and electrochemical process [46]), mechanical method [47] and direct regeneration [48, 49]. Pyrometallurgy is used to decompose constituents of LIBs by thermal treatment and the products are metallic alloys, slags, and gases [50]. The metallic alloys (e.g. Co–Ni–Cu) or slags (e.g., Al, Mn, Li) can be recovered through hydrometallurgical process, and the gaseous products are comprised of volatile organics from binders and electrolytes [8]. The important process of hydrometallurgy is the leaching process, which usually uses acids [51, 52], alkalis [40, 53], ionic liquids [54, 55], etc. Then, the different valuable metals are recovered by a series of processes of precipitation [56], solvent extraction [57, 58] and electro-deposition [46]. The direct recycling can renovate active materials directly into battery grade active materials with minimized changes of crystal structures and morphologies [59]. However, impurities in waste cathode materials during the regeneration process prohibit its further large-scale applications, and therefore, the classification and sorting of these coarse spent LIBs into different compositions by pretreatment based on their physicochemical properties are necessary before the recycling of valuable metals enriched in spent cathode materials.
The pretreatment is an important step during spent LIBs recycling, which can greatly improve the overall recycling efficiency of spent LIBs. Detailed pretreatment procedure can include the deactivation, disintegration and separation, separation of cathode/anode materials and current collectors, and treatment of electrolytes (Fig. 4) [8]. Pretreatment for spent LIBs recycling will ensure the security for subsequent processes and facilitate the liberation of the active materials from current collectors [60, 61]. After pretreatment, the liberated active materials will be then sent to metallurgical processes for extraction of value-added metals. Therefore, pretreatment is critical in the recycling process of spent LIBs. A large number of reviews are mainly concentrated on the reports of metallurgical recycling processes, despite emerging of the pretreatment technologies being adopted [27]. There are few reviews that reported the highlights and technologies for some specific sections, while an overview of the pretreatment section is necessary for its importance in the overall recovery process [3, 8, 27]. Therefore, this paper tends to review the prevailing cutting-edge technologies in more comprehensive aspects, with a summary of the recent advances in pretreatment technologies for spent LIBs recycling, including their relevant advantages and disadvantages, and the brief extensions in scale-up applications at home and abroad.
2 Progress in pretreatment technology
The compositions of spent LIBs are complicated and some components are flammable and toxic, leading to incompatibility between the different components. Pretreatment that adopted discharging, dismantling and separation of cathode, anode, carbonaceous additives and electrolyte is used to liberate each component, which is critical to improving recycling efficiency of spent LIBs. The advantages and disadvantages of each process are listed in Table 1 [3, 27, 62,63,64,65,66]. The current progresses for the pretreatment of spent LIBs are demonstrated based on the above proposed recycling steps.
2.1 Theoretic analysis
The residual electricity of spent LIBs may cause a short-circuit current, which may lead to self-ignition and explosion [65]. Thus, deactivation is necessary to ensure the safety of the subsequent processing of spent LIBs.
Chemical solutions, such as salt, acid and alkali, with good electrical conductivity are good media for discharging. Spent LIBs are soaked in chemical solution to release the residual electricity with certain discharge efficiency. The advantages of this method are safe and stable discharge, due to the fact that the energy can be absorbed effectively by the solution. However, ambulatory electrolyte including toxic lithium salts and volatile organic compounds will leak out if the LIB is broken [62]. Li et al. [67] investigated the discharging rate in NaCl solutions with different concentrations. The 10 wt% NaCl solution was found to be more efficient in discharging and less harmful to environment. However, high discharging rate is always accompanied with high risk of environmental pollution as electrochemical corrosion can quickly destroy the metallic shells, as shown in Fig. 5a. The different solutions were used by Xiao et al. [62] to investigate the discharge performances, with relevant results shown in Fig. 5b. It can be found that the spent LIBs can be mildly discharged in MnSO4 solution and Mn2+ is converted into isolation layers during the discharging process, which can reduce oxygen contact with anode and effectively avoid galvanic corrosion and organic leakage. The NaCl solution, H2SO4 solution and NaOH solution were used by Wang et al. as conductive solutions, and concluded that salt solution was better than alkaline and acid solution [68, 69]. That is because the shell is usually made of Al, which is prone to react with acid and alkali, resulting in the losses of metals inside the battery and leakage of electrolytes.

Reproduced with permission from Ref. [67]. Copyright 2016, Elsevier. b Change curves and conductivity of different solutions with time and proposed reaction mechanism with conductive solution (Re: electricity percentage). Reproduced with permission from Ref. [62]. Copyright 2016, Elsevier
a Discharge and disassembly process of waste LIBs (supernate and concentrated liquor after discharging of LIBs).
Short-circuiting of the anodes or cathodes is commonly employed in the discharging process, which can facilitate complete discharging within relatively short time. In some cases, the high voltage inside the battery may lead to organic electrolyte leakage and local overheating [27]. Wang [68] and Zheng [69] explored discharging process of graphite powders and metal powders as discharging mediums. The temperature rises rapidly in metal powders due to the inability to dissipate heat quickly, while the discharge rate of graphite powder is mild. But this process holds its drawbacks, such as high heat production, which will limit its application [70].
Cryogenic temperatures (from − 175 to − 200 °C) (e.g., liquid N2) were adopted to freeze and crystallize the electrolyte in cryogenic deactivation [69]. The low temperature makes the lithium inert, eliminating the risk of fire caused by oxidation of the lithium element, which has the same effect as discharging. Zhang et al. [71] disassembled spent LIBs under low-temperature N2 conditions to limit the release of toxic gases and chemicals, then dried them in a vacuum oven at 60 °C for 24 h. Some companies have used this process in the industry, e.g., Umicore [64] and Toxco [72]. This method is suitable for a large handling capacity processing. While the high investment and rigorous requirement of equipment limit its application.
Thermal deactivation is typically employed prior to mechanical process to avoid sudden thermal events. The organics of spent LIBs can be removed in a controlled manner [73]. This method can destroy the safety of the structures, but the deactivation process takes place under higher temperatures with high energy consumption. The ecoBatRec process is a patented thermal treatment process with the core purpose of lithium recovery, providing an alternative to the deactivation pathway. After disassembly, the module undergoes thermal deactivation. Flammable electrolytes, decomposing organics and products of the separators can be removed in vacuum or atmospheric environment [74].
2.2 Mechanical treatment
2.2.1 Disassembly and shredding
Disassembly and shredding are recognized as critical premises and a bottleneck process due to the complicated structure of spent LIBs, which are typically described as manual disassembly and automatic disassembly [75].
Manual disassembly is mainly operated in the laboratory. The disassembly procedure is normally hazardous due to the generation of harmful substances (e.g., LiPF6, HF and residual lithium), requiring well-protected operators and a fume hood. The deactivated LIBs are disassembled into the metal casing, plastic, separator, cathode, and anode [76]. The separated components obtained by manual disassembly have a high yield (> 80%) and satisfactory purity, which facilitates the subsequent separation process. Zhao et al. [77] manually disassembled the charged LIBs in water with no emissions and near-perfect recycling efficiencies. This method allows the complete separation of each component, avoiding uncontrollable reaction and combustion from anode or electrolytes (Fig. 6a). Manual disassembly of spent LIBs was adopted by LithoRec in Germany, followed by calcination and leaching processes to recover the metals. The final products were Li2CO3, Al-Cu alloy and plastic component, with only loss of electrolytes throughout the process [78]. However, its application was limited for the lower processing capacity, poor efficiency and severe safety hazards.

Reproduced with permission from Ref. [77]. Copyright 2022, Elsevier. b Sketch of wet impact crusher and particle size distribution of dry or wet crushed products. Reproduced with permission from Ref. [80]. Copyright 2013, Elsevier
a Schematic pyrometallurgy, hydrometallurgy, direct-recycling and manual depiction of disassembling spent LIBs and separated materials.
By contrast, automatic mechanical disassembly is widely used in large-scale industrial processes owing to higher processing capacity/efficiency [11, 65]. Shredding requires the mechanical disassembly of the spent LIBs using a series of techniques (e.g., tearing, shearing, crushing and grinding) [79]. It is clear that the principle for the mechanical disassembly is mainly due to the different physical properties of LIBs components. Dry and wet crushing were commonly used in the recycling process of the spent LIBs [11]. Dry crushing is the direct crushing of spent LIBs without the presence of water. It was employed to crush and separate different components in spent LIBs, resulting in the co-existence of separators, Al foils, Cu foils and plastics in the coarse particles and active materials in the fine particles [80]. Wet crushing requires crushing the LIBs components in the solution that converts the LIBs into unreactive state, which cannot reactive with electrolytes. Zhang et al. [80] explored dry and wet crushing of spent LIBs, as shown in Fig. 6b, and they found that selective crushing was presented in both wet and dry crushing processes. Separators, Al foils, Cu foils and plastics mainly existed in coarse particles, while the fine particles were mainly composed of active materials of graphite and LiCoO2. Dry crushing can make full use of selective crushing. In comparison, wet crushing offers a higher safety performance but more impurities are present in the fine fraction for the scouring action of the water flow [28]. Several researchers have directed their attention to the crushing of spent LIBs under an inert atmosphere (e.g., N2 or CO2) or cryogenic (e.g., liquid N2). The reactivity of Li and the explosion of flammable gas components can be suppressed during crushing [81,82,83]. In the process of Recupyl, LIBs undergo shearing in a rotary shearer in an inert atmosphere (Ar or CO2) and CO2 can react with Li to produce Li2CO3 with reduced reactivity, which can reduce the risk of fire and explosion [84].
2.2.2 Sieving and separation
After the disassembly and shredding of spent LIBs, coarse separation is employed based on the differences in the physical properties, such as the particle size, magnetism, eddy current, electrostatic, gravity separation and froth flotation [27, 85]. The schematic illustration of the typical sieving process in pretreatment is exhibited in Fig. 7.

Reproduced with permission from Ref. [11]. Copyright 2022, Elsevier
Schematic illustration of typical sieving and separation process in pretreatment.
The particle size separation is simplified sieving method that utilizes different particle sizes. The crushed separators, Al foils, Cu foils and plastics are mainly as the form of coarse particles, while the graphite and cathode materials are relatively fine in particle size [11]. Pindar et al. [85] crushed the disassembled sheet in the attritor and then sieved them at 53 μm to obtain active materials. The materials above 53 μm for the anode and cathode contained Cu and Al, respectively. Granata et al. [86] identified that the active materials were primarily concentrated in the particle size fraction < 0.25 mm, while the metals such as Fe, Al and Cu were mainly distributed in the size fraction > 0.20 mm. According to Zhang et al. [71], the crushed products can be divided into three fractions: Al-enriched fraction (+ 2 mm), Cu-Al-enriched fraction (− 2 + 0.25 mm) and Co-graphite-enriched fraction (− 0.25 mm). The result shows that fraction of − 0.25 mm size partially recycled active materials, keeping as the original crystal form and chemical state of the LIBs (eight size fractions can be seen in Fig. 8a).

Reproduced with permission from Ref. [71]. Copyright 2014, Elsevier. b Particles and aggregates existing in Magnetic-Waterflow separation. Reproduced with permission from Ref. [88]. Copyright 2021, Elsevier. c Sample of granule mixture and corona electrostatic separator (CES) in electrostatic separation. Reproduced with permission from Ref. [91]. Copyright 2020, Sage. d Schematic diagram for graphite and LCO separation using Fenton reagent-assisted flotation. Reproduced with permission from Ref. [100]. Copyright 2017, Elsevier. e Separation diagram of eddy current. Reproduced with permission from Ref. [104]. Copyright 2019, Elsevier. f Schematic of pneumatic separation of spent LIBs in industrial process. Reproduced with permission from Ref. [106]. Copyright 2020, Elsevier
a Eight size fractions of crushed products of spent LIBs in particle size separation.
Magnetic separation takes advantage of the fact that Fe and Co are strongly magnetic (ferromagnetic), while plastics and separators are not magnetic (diamagnetic), which can be separated in one step through magnetic force [28]. Herein, Costa et al. [87] investigated the influence of processing parameters on the metal distribution among the different fractions. They found that magnetic separation was efficient for particle sizes from 1 to 2 mm, and Co mainly existed in fine materials with particle sizes < 1 mm. The results reflected that abundant Co and Cu concentrates could be obtained after the magnetic separation and that the concentration of Al as the by-product was feasible by applying an additional mechanical process. Huang et al. [88] developed a technology of ultrasonic dispersion and water flow-magnetic separation to recover micro-magnetic particles and the maximum recovery rate of Co particles and purity of Co particles was higher than 95% and 90%, respectively, under optimized conditions (aggregates and particles positioned in Fig. 8b).
Electrostatic separation, which is a very promising process for their separation, has been widely used in the fields of separation processes of plastics, metals, coals and other ores on account of their difference in conductivity [89, 90]. Recently, electrostatic separation has also been proposed to separate LIBs components after mechanical crushing. Cao et al. [89] proposed a multi-stage electrostatic separation method to recover Al foils from fine granules of black dross. After three separation steps, the Al2O3 content of collection products upgraded to 63.94%. Bi et al. [91] discussed the separation of Al and crushed spent LFP from non-metal particles. They placed spent LIBs into a sieve with a particle size of − 1.25 + 0.40 mm for corona electrostatic separation after mechanical crushing. The metal particles (size: − 1.50 + 0.20 mm) were separated from the non-metal particles of crushed spent LFP batteries (the samples of granule mixture and corona electrostatic separators are shown in Fig. 8c). The electrostatic separation was adopted by Silveira et al. [90] for the recycling of the conductive and non-conductive portions of the LIBs after the particle size separation. The recovered conductive fraction contained 98.98% of metal fraction, along with non-conductive fraction containing 99.60% polymers under the conditions of roll rotation speed of 20 r·min−1, electrode voltage of 25 kV, electrostatic electrode distance of 6 cm and deflector inclination angle of 0° by electrostatic separator, suggesting that the electrostatic separation could be a promising and effective method for recovering high purity materials from spent LIBs.
Gravity separation has proven to be an effective method for the separation of spent LIBs of different sizes and densities [92]. There is obvious density difference among different components of spent LIBs, which is crucial to achieve effective gravity separation [92]. Different spent LIBs components will form different states in certain separation medium [8]. Low-density fractions are mainly composed of separators, plastics, and Al foils. The densities of Cu and polyethylene are 8.96 and 0.90 g·cm−3, with a large density difference. Gratz et al. [93] realized the perfect separation of current collectors and separators in heavy media, such as diiodomethane (3.30 g·cm−3).
Flotation technology is an efficient physicochemical process for the separation of fine particles based on their surface hydrophilicity difference. Hydrophobic particles can be selectively attached to air bubbles and separated from hydrophilic particles [94]. It has been well known that the cathode materials of spent LIBs are hydrophilic, while anode materials (C) are hydrophobic. Based on their differences, Saneie et al. [95] studied flotation separation for recycling of Cu, Al, and plastics from spent LIBs and explored their hydrophobicity by contact angle measurement. Firstly, the plastics were separated from Cu and Al mixture by flotation. Then, the added Na2S (as an activator of Cu), potassium amyloid xanthate (collector) and methyl isobutyl carbinol (MIBC) (frother) were utilized to separate Al and Cu, with the separation efficiency as high as 100%. Usually, active materials from spent LIBs are coated by some organics, which reduces hydrophilicity difference between cathode materials and anode materials. Some researchers have used different technologies of roasting [96, 97], pyrolysis [98], Fenton oxidation [99, 100] and grinding [94] to remove organics from surface of active materials and restore the surface hydrophilicity difference. As illustrated in Fig. 8d, He et al. [100] modified the surface of active materials with Fenton reagent and modified active materials were separated by flotation to recover cathode materials and anode materials, respectively. Most of organic outer layers covering the surface of the active materials were removed under the optimized conditions (Fe2+/H2O2 molar ratio = 1:120, liquid–solid ratio = 5:1). The wettability of LiCoO2 and graphite was restored after the modification by Fenton's reagent. Then the crushed products can be effectively separated by the flotation process.
Eddy current separation is an efficient and environmentally friendly technology for separation of magnetic metals and non-magnetic metals [101, 102]. With time-varying magnetic field will induce an electrical current to flow throughout the whole body of a conductive particle [103]. This method is effective for removing the residual non-liquid organic substances from LIBs, to solve the problem of incomplete removal of organic impurities in industry and laboratory [60]. Bi et al. [104] explored the force and kinematic model of the cathode and anode plates during the eddy current separation in crushed products of spent LIBs. As shown in Fig. 8e, test results agreed with the simulated results in a range of 2–20 mm. Cu and Al foil can be separated to a maximum particle size ratio of 1.72 due to their good electrical conductivity at magnetic roll speed of 800 r·min−1. Therefore, it is considered to be a green and effective technology for separation of nonferrous metals from crushed spent LFP batteries.
Pneumatic separation is a method that uses airflow to lift light materials and heavy materials based on the difference in density [105]. It is widely used in the recovery of waste printed circuit boards. Recently, it has been gradually applied to the separation of anodes and cathodes of spent LIBs [106, 107]. Bi et al. [108] achieved effective separation of the Cu and Al fragments without screening by using pneumatic separation, with the achieved recovery efficiency of 97% and 96% for Al and Cu, respectively. Zhong et al. [106] investigated the separation of current collectors and separators using a special Z-shaped pneumatic separator. The industrial experiments of pneumatic separation indicate that the recovery of current collectors and separators are approximately 99.23% and 98.64%, respectively. The turbulence and the changes in high-speed zones in the pneumatic separator favor the separation of current collectors and separators. It can be a promising technology to separate the current collectors and separators in crushed spent LIBs. The schematic illustration for pneumatic separation of spent LIBs in industrial process is shown in Fig. 8f.
2.3 Separation of active materials and current collectors
The active materials are generally connected to current collectors with adhesive organic binders [65]. PVDF is widely used as the binder between cathode materials and current collectors due to its strong adhesion and high stability [45, 109]. However, the strong adhesion makes it difficult for the separation of cathode materials and current collectors, which has a significant adverse impact on the subsequent metal extraction process. In contrast, the bond between anode materials and current collectors is much weak during repeated charging and discharging process, which can be separated by simple physical operations, such as sieving, pulverizing and striking. Therefore, this paper is currently focused on the separation of cathode materials [110]. A summary of different methods for the separation of cathode materials from Al foils is demonstrated in Table 2. There are three types of separation methods commonly used: dissolution of Al foils, removal of binders and supporting method.
2.3.1 Dissolution of Al foils
In many recycling processes, the dissolution of Al foils can be achieved by the addition of acid or alkali [93, 111]. However, the addition of acid will cause the structure of the cathode materials to be destroyed and dissolved. Alkali solution is commonly used to dissolve the Al foils to separate cathode materials due to the amphoteric behavior of Al [112]. The equations involved in the reaction are as follows:
Nan et al. [112] used 10 wt% NaOH solution with a solid–liquid ratio of 1:10 at room temperature to achieve the complete dissolution of the Al foils after 5 h. Then, it could be deposited as Al(OH)3 and recovered by changing pH of the lixivium from 4.80 to 5.20. This is a simple and efficient separation method. However, Al will be entered into the ionic form that is difficult to be recovered, and alkaline wastewater (i.e., NaOH solution) is also harmful to the ecosystem.
2.3.2 Heat treatment of organic binders
Heat treatment involves heating cathodes to melt or decompose adhesive by reducing the bonding force between cathode materials and current collectors for the better separation in this process. Typically, the PVDF binders will be decomposed at a temperature of about ~ 350 °C, while other components (e.g. acetylene black and conductive carbon) can be decomposed only above 600 °C. During the heat treatment process, the organic binders undergo oxidation reactions, C–F bond breaking, etc. [65]. At the same time, metals (e.g., Li, Co, Ni) combine F to form M–F (M = Li, Co, Ni) bonds with lower binding energy, reducing the surface binding energy of cathode materials. Graphite can be removed by oxidation to form CO/CO2 [113]. The heat treatment method holds the advantages of simplified operation and high processing capacity, which is suitable for scale-up application. However, high energy consumption and pollution are also the bottlenecks for this technology [114].
High-temperature roasting, which is used to remove PVDF and acetylene black from waste cathodes in air or oxygen, is simple to operate [115]. The selection of the suitable temperature range for incineration can be beneficial for the subsequent metal extraction [114]. As reported by Fan et al. [116], the cathode was calcined at 600 °C for 5 h, and Al foils and cathode materials could be directly obtained after the removal of carbon and organics. However, the Al foils would become fragile at temperatures over 600 °C. Further increase in temperature would result in the melting of Al foils and the cover of the active materials, causing difficulties for the subsequent separation and recovery [117]. For this purpose, Hanisch et al. [118] proposed the adhesion neutralization via incineration and impact liberation (ANVIL) to enhance separation efficiency between current collectors and cathode materials (as shown in Fig. 9a). After heating at 500 °C to decompose the PVDF, an air-jet separator was used to separate the cathode materials from the current collectors. To eliminate the environmental impact of thermal decomposition of PVDF in recycling, calcium oxide (CaO) was adopted by Wang et al. [66] as a low-temperature reaction medium and PVDF in the cathode could be decomposed at a lower temperature of 300 °C when using CaO as the reaction medium. The peeling-off efficiency for the cathode materials exceeded 95% without the release of hydrogen fluoride detected during the decomposition process. It is indicated that the use of CaO can reduce the processing cost and avoid the release of hydrogen fluoride, which has economic and environmental benefits (Fig. 9b). High-temperature roasting mainly involves with some exothermic reactions, and the energy can be recovered as steam, which can be reused in generators. Therefore, this technology was widely used in the industrialized recycling. However, organics contained F will release fluorinated gases above 350 °C and conductive carbon produces CO at high temperature. The extra tail gas treatment devices will increase the equipment cost [119].

Reproduced with permission from Ref. [118]. Copyright 2015, Elsevier. b Flowchart for cathode separation by using CaO as heat treatment medium and possible reaction mechanism for PVDF. Reproduced with permission from Ref. [66]. Copyright 2019, ACS. c Separation of active materials from current collectors after pyrolysis. Reproduced with permission from Ref. [123]. Copyright 2016, Elsevier. d Digital pictures of cathode after different treatments under a CO2 atmosphere at 600 °C. Reproduced with permission from Ref. [115]. Copyright 2022, RSC. e Pyrolysis mechanism of cathode at different temperatures. Reproduced with permission from Ref. [125]. Copyright 2022, Elsevier. f Schematic illustration of separating cathode materials and Al foils by low-temperature molten salt (AlCl3-NaCl). Reproduced with permission from Ref. [128]. Copyright 2019, ACS
a Experimental set-up for ANVIL and diagram of separation efficiency with varied calcination time.
Pyrolysis is thermal treatment in inert atmosphere to facilitate thermal decomposition of organics (e.g., PVDF, electrolyte) into lower molecular weight products, which were used as fuel or chemical feedstock [120]. Pyrolysis can not only effectively separate the cathode materials, but also has the potential to recover the organics, which is considered to be an alternative method for the pretreatment of spent LIBs [98, 121]. Pyrolysis was also used to deactivate spent LIBs with residual electricity. This technology was carried out in an inert atmosphere or under vacuum conditions to avoid the reaction of Li [107, 122]. Simultaneously, the decomposition temperature of the byproducts could be reduced and the efficiency of this process might be improved [117]. The spent cathode was heated in high purity N2 at 600 °C for 15 min by Yang et al. [123] for pyrolysis. The cathode materials can be easily separated from current collectors for their significant differences in density after pyrolysis. The Al foils can be recovered by water washing, as shown in Fig. 9c. Meanwhile, the transition metals in the cathode materials are reduced to the low valence state, which makes their subsequent leaching easier and more efficient. Similarly, Deng et al. [124] put waste cathodes acquired by disassembly and thermally treated at CO2 atmosphere at 600 °C. After heat treatment, Al foils and cathode materials could be easily separated by manual shaking. Intact Al foils with obvious metallic luster were recovered and PVDF would be converted into graphite-like carbon under the high temperatures (Fig. 9d). Tao et al. [125] combined one-step pyrolysis and reductant-free acid leaching to realize the recycling of spent LIBs. The electrolytes volatilized and the high molecular weight polymer (binder and separator) were degraded to pyrolysis gases and oil during pyrolysis process. Under synergistic effect of pyrolysis gas and graphite, LiMn2O4 can be deconstructed and converted into MnO and Li2CO3, and F and P are absorbed by Ca(OH)2 solution, avoiding the production of toxic gases. The flowchart for the pyrolysis mechanism of spent LiMn2O4 is illustrated in Fig. 9e. More efforts should be paid to address complicated operating conditions for their scale-up application, due to enhanced processing costs by high requirement of equipment.
Low-temperature molten salt technology was developed to separate cathode materials from current collectors through one or several low melting point salts as reaction media [126, 127]. It is the robust thermal treatment process to destroy the organic-containing substances, retaining the inorganic and radioactive materials as their in-situ states [128]. Cathode materials and current collectors can be obtained after cooling and washing salt away [129]. Zhao et al. [130] studied the pyrolysis of spent printed circuit boards in molten salt (Na2CO3-K2CO3) and they found that both the gas yield and carbon conversion increased with increasing equivalence ratio. The main pyrolysis products were phenol, 2-methylphenol, 4-methylphenol, naphthalene, etc. More than 90% metals were retained in molten salt, which could be recovered by collecting and treating bottom layer of molten salt. However, only few studies focused on the molten salt technology. Wang et al. [128] proposed a similar process that adopted the low-temperature molten salt (AlCl3-NaCl) technology to achieve separation of cathode materials and Al foils from spent LIBs (Fig. 9f). The highest peeling-off efficiency was over 95% under optimized conditions: temperature of 160 °C, mass ratio of molten salt:cathode being 10:1, and retention time of 20 min. These results support that molten salt system is an efficient, low-cost and environmentally friendly technology to separate cathode materials from current collectors of Al foils.
2.3.3 Other approaches for binder removal
Removal of the binders is crucial for the separation of active materials from the current collectors. Crushed cathodes usually contain Al foils and cathode materials, but most of active materials are still attached to surface of Al foils [44, 45]. Organic binders (e.g., PVDF) in the LIBs effectively connect cathode materials with Al foils [11]. PVDF has excellent chemical stability and mechanical property that cannot be removed by strong acids and alkalis. Instead of the heat treatment decomposition, some technologies have also been reported regarding removing organic binders, e.g., organic solvent dissolution [131, 132], deep eutectic solvent dissolution [133, 134], advanced oxidation process [135], the application of some new solvents [129, 136].
Organic solvent dissolution utilizes principle of “Like Dissolves Like” theory. PVDF has strong polarities, which can be effectively dissolved using the polar organic solvents. And the N-methyl pyrrolidone (NMP) [137], dimethylacetamide (DMAC) [137], N, N-dimethylformamide (DMF) [138], dimethylsulfoxide (DMSO) [43], etc., are frequently employed to dissolve PVDF. Among them, NMP was widely utilized for its extremely high solubility to PVDF and high recovery efficiency. The peeling-off efficiency of the cathode materials could achieve as high as 99% using NMP under optimized conditions of dissolving temperature of 70 °C, ultrasonic power of 240 W and reaction time of 90 min, according to He et al. [44]. The low agglomeration of cathode materials separated from Al foils facilitates subsequent leaching process. DMF is another effective solution for the dissolution of PVDF with similar procedure to NMP. Xu et al. [138] placed the cathode in DMF and stirred it at room temperature to achieve complete dissolution of PVDF. Results show that solvent can be recycled until saturation is reached, reducing the cost of the reaction process. Song et al. [137] put cathode sheets (uniform size: 1 cm × 1 cm) into different organic solvents (NMP, DMF, DMAC and acetone) to dissolve them at different temperatures (30–70 °C). Then the cathode materials could automatically fall off after a certain time and part of residues could be wiped out manually (Fig. 10a). Organic solvent dissolution is widely accepted in this field and the Al/Cu foils can be directly recovered after simple treatment. Organic solvents can be recycled through distillation or other methods. The dissolution effect of binders can be enhanced by auxiliary means such as ultrasonication (introduced in a separate section of “Assisted methods for peeling off process”) [11]. Nevertheless, the dissolved cathode materials existed in fine powders show poor filtration performance [139, 140]. The relatively high viscosity, high price and toxicity of the organic solvents (e.g., NMP) restrict the practical application of this technology [11].

Reproduced with permission from Ref. [137]. Copyright 2015, RSC. b Diagram of peeling process and peeling off efficiency by heating ILs ([BMIm][BF4]). Reproduced with permission from Ref. [144]. Copyright 2014, Elsevier. c Schematic of recycling and diagram of DES (choline chloride-ethylene glycol). Reproduced with permission from Ref. [21]. Copyright 2019, Nature. d Schematic of methyl ester solvent synthesis and cathode materials/Al foils separation. Reproduced with permission from Ref. [136]. Copyright 2020, ACS. e Scheme and schematic representation of PVDF dissolution in SC CO2 process. Reproduced with permission from Ref. [154]. Copyright 2021, Elsevier. f Peeling off mechanism with ultrasound-assisted Fenton reaction system. Reproduced with permission from Ref. [135]. Copyright 2021, Elsevier
a Schematic diagram of separating cathode materials (a1) from Al foil by DMSO (a2), acetone (a3), DMF (a4), NMP (a5) and DMAC (a6).
Ionic liquids (ILs) are adopted as the green solvents with specific properties, such as extremely low vapor pressure, low combustibility, decent thermal stability and wide temperature range in the liquid states [54, 141, 142]. Some studies have proven that ILs are effective for the recycling of waste printed circuit boards [55] and the dissolution of organics [143]. Zeng and Li [144] reported separation of Al foils and cathode materials by heated ILs. 1-Butyl-3-methylimidazolium-tetrafluoroborate ([BMIm][BF4]) was used as the solvent owing to wide temperature range of its liquid state, suitable viscosity, excellent hydrophilicity and easy syncretization. The relationship between reaction temperature and retention time was investigated. More than 95% of cathode materials can be removed from Al foils under the optimized experimental conditions of heating temperature of 180 °C, stirrer speed of 300 r·min−1 and retention time of 25 min. In addition, ILs were also used in selective extraction of metals from spent LIBs [54, 55], schematic diagrams for different peeling processes and the peeling-off efficiency are shown in Fig. 10b. However, high cost, complex synthesis steps and non-reusability of ILs limited their further application, making it difficult for industrialized applications [142].
Deep eutectic solvents (DESs) are a new kind of green solvent with low synthesis cost, biodegradability, low toxicity, and low vapor pressure. DESs have been widely used in many fields, such as metal extraction, gas absorption, separation, catalysis and material synthesis, for their eco-friendly characteristics [133, 145, 146]. It usually consists of a hydrogen bond donor (HBD) (e.g., alcohols, carboxylic acids and amides) and hydrogen bond acceptor (HBA) (e.g., choline chloride) through the hydrogen bonds. Therefore, their melting points are lower than melting points of respective components [146,147,148,149]. Tran et al. [21] used choline chloride-ethylene glycol as solvent to separate the cathode first [44, 137, 138] and recovered metals in one step. The schematic of recycling and the diagram of DES is shown in Fig. 10c. In their study, the Al foils and PVDF could be recovered separately, with leaching efficiencies for Co and Li > 90%. Wang et al. [134] studied the peeling-off process of cathode through DESs specifically. Choline chloride-glycerol was synthesized and applied to solve the separation dilemma. The separation of Al foils and cathode materials could be achieved by heating temperature of 190 °C, choline chloride: glycerol molar ratio of 2.30:1, and heating time of 15 min. Due to the attack of the hydroxide of choline chloride on the acidic hydrogen atom in PVDF, the organic binder was deactivated. DES can be a low-cost, high-efficiency, and environmentally friendly solvent for the separation of cathode materials from current collectors.
Some other researchers have begun to explore the possibility of new solvents to replace the radiational organic solvents [44, 137, 138]. Pant and Dolker [150] reported that citrus fruit juice (CJ) could be an excellent candidate that combined the removal of the PVDF binders and facilitated the subsequent leaching. The CJ contains organic acids, such as citric acid, malic acid and ascorbic acid, which can avoid the use of traditional organic solvent to peel off active materials. The leaching of metals can be completed at the same time. It can make the process safe for the environment with an effective recovery. However, CJ is expensive and susceptible to the season, which will limit its industrialized application. According to the study by Zhang et al. [151], trifluoroacetic acid (TFA) was used for dissolving the polytetrafluoroethylene (PTFE) in nickel manganese cobalt oxide (NCM)-based LIBs, which was a relatively simple and environmentally friendly process. The cathode materials can be separated with 15 vol% TFA solution, liquid to solid (L/S) ratio of 8.00 ml·g−1, reaction temperature of 40 °C and reaction time of 180 min. The regenerated cathode materials have good electrochemical performance. Wang et al. [136] proposed a novel "waste + waste = resource" recycling method. They prepared fatty acid methyl esters (FAMEs) solvent from waste cooking oil to separate cathode materials and Al foils of spent LIBs due to cross-linking of the hydrogen bonds (Fig. 10d). The results show that over 99% of cathode materials in spent LIBs can be peeled off from Al foils after heating in FAMEs solvents at 190 °C for 20 min. This method holds features of low cost and wide availability, which is promising to separate cathode materials from Al foils. New solvent dissolution often holds the feature of environmentally friendliness, but the solvent source is limited and difficult to apply on large scale [43, 136, 150].
Several studies utilize the supercritical (SC) fluids for the recycling of valuable metals from spent LIBs and separate its components by removing the polymers [152, 153]. Fu et al. [154] utilized SC-CO2 in combination with the cosolvent DMSO to extract organic binders (e.g., PVDF) from spent LIBs, facilitating the liberation of the cathode materials from Al foils. The mechanism of SC–CO2 dissolution was explored by using pure PVDF. More than 95 wt% PVDF was dissolved in the SC–CO2–DMSO system under optimal conditions: reaction temperature of 70 °C, reaction pressure of 8 MPa and reaction time of 13 min. The surficial chemical group and content of treated PVDF remained the same after treatment. The scheme and schematic representation of PVDF dissolution in SC-CO2 system are illustrated in Fig. 10e. The SC–CO2–DMSO system can effectively release waste cathode materials from Al foils after removal of binders. It is an effective and sustainable approach to liberate cathode materials. Although some studies focused on the implementation of SC fluid technology for recovery of spent LIBs, there is still a lack of systematic investigation to eliminate the hazardous wastes, and the high levels of equipment requirement limit its further application [155].
Advanced oxidation processes (AOPs), based on generation of diverse reactive oxygen species (e.g., standard reduction potentials of hydroxyl radical, E0 = 2.70 V vs. normal hydrogen electrode (NHE) and sulfate radical, E0 = 3.10 V vs. NHE), are an effective method during the peeling off process [156, 157]. It has been widely used to decompose numerous pollutants (e.g., polyethylene [158] and polyvinyl chloride [159]) in various environmental applications since its high efficiency, compactness and cost-effectiveness. AOPs were firstly introduced in our previous study for the peeling-off process of cathode in the pretreatment of spent LIBs [160]. A new process for in-situ separation and recovery of cathode materials and Al foils from spent LIBs with ultrasonic-assisted was established and only mild acid was used in this process with 99% of cathode materials detached from Al foils. The Al foils can be obtained in the in-situ form with the purity of over 98%. The ˙OH radicals with intense oxidation formed and enhanced in the acidic ultrasonic solution can result in the degradation of the PVDF. In our subsequent studies, an innovative approach of the application of ultrasound-assisted Fenton reaction for the selective removal of PVDF binders by ˙OH radicals generated by Fenton's reagent and adequate degradation of PVDF binder with enhancement under ultrasonic environment was also proposed [135]. About 97% of cathode materials can be peeled off from Al foils under the optimized experimental conditions of ultrasonic power of 100 W, Fe2+ concentration of 1 mmol·L−1, reaction temperature of 50 °C and retention time of 20 min, with minor loss of Li (Fig. 10f). In this process, recovered LiFePO4 can be directly refabricated through the lithium compensation with little degradation in electrochemical performances. ˙OH radicals generated by Fenton agents with ultrasonic enhancement can effectively degrade PVDF binders. AOPs can be green and effective candidates for in-situ recycling of cathode materials from spent LIBs.
2.3.4 Assisted methods for peeling off process
Some enhancement methods (e.g., ultrasonic treatment, low-temperature grinding and microwave treatment) of the improvement of separation efficiency of cathode materials from Al foils have been widely utilized.
Ultrasonic treatment is a conventional technology in industry and laboratory. He et al. [44] combined organic solvents and ultrasonic treatment to separate the cathode based on “Like Dissolves Like” theory. The cavitation effect is caused by ultrasound, and the waste cathode materials with low agglomeration are obtained. Mechanism of ultrasonic cleaning is shown in Fig. 11a. Ultrasound-assisted AOPs were used to separate cathodes in our previous research [135, 160]. Kayakool et al. [161] first peeled off the graphite from Cu foils by ultrasonic treatment. They investigated electrochemical performance of recovered graphite at different temperatures. Ultrasonic treatment is widely used as an auxiliary method with the feature of simplicity and low cost. Cryogenic grinding for recovery of cathode materials from spent LIBs has been studied in recent years, which is an environmentally friendly technology. This assisted method has been mentioned by Wang et al. [162] to separate different components. In their study, grinding temperature declined to 77 K which was lower than the glass transition temperature of PVDF (235 K), resulting in the change of PVDF from a highly elastic state to a glassy state (Fig. 11b). They observed that the strength of the current collectors increased significantly at low temperatures, causing the fracture of the organic binders. The peeling-off efficiency of cathode materials was improved to 87.29% after cryogenic grinding. Concurrently, Liu et al. [163] combined cryogenic grinding with froth flotation for the separation of LCO and graphite. Cryogenic grinding with the liquid N2 facilitated the removal of organic binders on the surface of active materials. Hydrophilicity of LCO and hydrophobicity of graphite were improved by cryogenic grinding, which contributed to the excellent flotation separation. But its application was limited due to the extensive consumption of liquid N2 in the cryogenic grinding system. In addition, it is not convenient to use such a large amount of liquid N2 on a commercial scale [164]. Microwave treatment used the microwaves in frequency range of 300–300 GHz with electromagnetic waves in wavelength range of about 1 m to 1 mm, absorbing and converting microwave energy into thermal energy. It has been widely used in metal recycling [165], effluent treatment [166] and material synthesis [167, 168]. Fu et al. [169] proposed microwave-assisted carbon thermal reduction to recycle spent LIBs and the leaching experimental results showed that the optimal efficiencies of 97% transition metals (Co, Ni and Mn) and 99% Li were obtained with microwave-assisted. Chao et al. [167] utilized the excellent microwave absorption ability of anode to make binder and electrolytes in spent graphite volatilize rapidly in the microwave field. In addition, microwave radiation also causes the remaining Li in graphite to form Li2CO3, which can be extracted by water leaching. The lithium-containing wastewater can be recycled for the leaching of Li. Microwave treatment has also been used in the enhancement of solubility and AOPs, promoting the production of free radicals and thus accelerating their reactions [167].
2.4 Disposal of electrolyte
The disposal of electrolytes is a major challenge regarding their adverse impacts on the environment. The organic solvents, usually consisting of ethylene carbonate (EC) and dimethyl carbonate (DMC), are highly combustible [65, 170, 171]. The electrolyte can be easily volatilized or decomposed to generate toxic gases (even HF under humidity conditions) and some of them are considered nerve agents. However, there are only few studies that paid attention to the recycling of electrolytes due to the recycling of electrolytes to their complicated composition and issues in terms of separation and secondary contamination. A brief summary of the advantages and disadvantages for different technologies is listed in Table 3.
2.4.1 Organic solvent extraction
Organic solvent extraction is one of the most efficient separation methods for recovery of electrolytes from spent LIBs. Solvent extraction has excellent adjustability, with the controllable components of the targeted products and the relatively pure materials [172,173,174]. Electrolytes are dispersed between the active materials and separators and can be transferred into the organic solvents through immersion. Distillation is then used to separate the organic solvent and electrolyte based on the differences in boiling points [77, 171].
Several organic solvents used as extracting agents were examined. Tong et al. [175] compared the recovery efficiency of electrolytes with different solvents, such as PC, diethyl carbonate (DEC) and dimethyl ether (DME), the electrolytes could be completely removed after 2 h using PC as solvent. He et al. [171] prepared the exfoliation and extraction solution (AEES) as the peeling-off agent to recover both active materials and hazardous electrolytes, which could avoid the infiltration of impurities into the active materials. Nearly 100% electrolyte was dissolved within 20 min. The EC and PC could be extracted via distillation. LiPF6 could be precipitated from EC and PC and recovered via filtration. The recovery efficiency of electrolytes reached 95.60% in the form of organic mixture (Fig. 12a). The organic solvent extraction is effective to recover electrolytes, which can avoid the generation of wastewater, waste gases and residues, and reduce environment footprints, with recycled extractants through distillation. However, the impurity and low extraction yield restrict the application [174].

Reproduced with permission from Ref. [171]. Copyright 2019, Elsevier. b Schematic illustration of reclaimed electrolytes from spent LIBs. Reproduced with permission from Ref. [178]. Copyright 2017, ACS. c Schematic of low-temperature volatilization. Reproduced with permission from Ref. [23]. Copyright 2020, Elsevier. d Schematic illustration of lab-scale pyrolysis system. Reproduced with permission from Ref. [173]. Copyright 2011, Elsevier
a Experimental set-up for active materials separation.
2.4.2 Supercritical fluid extraction (SFE) system
SFE is a method of extracting organics using a fluid to reach above its critical point (at high temperature and high pressure) and transform into a supercritical fluid state, which gained increasing interest. SC is a state where gas and liquid states are indistinguishable, with liquid-like density and gas-like viscosity [176]. In SFE, CO2 (critical temperature Tc = 31.10 °C, critical pressure Pc = 7.38 MPa) is the most commonly used for extraction due to its good solvating property, high removal efficiency, minimized impurities and environment safety [153]. It can diffuse through solid particles and dissolve chemical compounds, especially non-polar ones [177].
Grützke et al. [172] investigated the extraction of electrolytes with supercritical helium head pressure carbon dioxide (SC-HHPCO2), and the extractants were consisted of DMC, EMC, EC and a trace amount of LiPF6. The aging products of electrolyte degradation were identified as DEC, dimethyl,2,5-dioxahexane dicarboxylate (DMDOHC), ethylmethyl-2,5-dioxahexane dicarboxylate (EMDOHC) and diethyl-2,5-dioxahexane dicarboxylate (DEDOHC). It is a suitable method to recover organics and electrolytes. While further purification (e.g., distillation) of extractants is necessary to separate the organic carbonates from the aging compounds, to improve the extraction of conducting salts. Grützke et al. [76] developed an SFE system through addition of different solvents to achieve quantitative extraction of the electrolyte. Nearly 90% of electrolytes can be recovered after extracting for 30 min with SC-CO2 (25 °C, 6 MPa) and 0.50 ml·min1 acetonitrile (ACN)/PC in a mixture (molecular ratio = 3:1). Usually, the recovered electrolytes with SC fluids cannot be directly reused. Liu et al. [178] proposed a recycling approach that consists of SC-CO2 extraction, weakly basic anion exchange resin, molecular sieve purification and components supplements. The recovered electrolytes exhibited a high ionic conductivity of 0.19 mS·cm−1 at 20 °C, which was close to commercial electrolytes with the same composition (see Fig. 12b). In the subsequent study, Rothermel et al. [179] found that subcritical CO2 was also an effective method to recycle electrolytes of LIBs. SFE system can simplify purification process of extracted product without impurities introduced [23, 180]. The operation conditions are relatively moderate to the heat-sensitive substances (e.g. LiPF6). Compared with the conventional extraction methods, the SFE system has a short processing time and a low impact on environment. The high cost and low processing capacity are the key issues that limit its further application of SC solvent.
2.4.3 Pyrolysis treatment
The organic materials can be broken down as low molecular weight liquid or gas by the pyrolysis process in an inert atmosphere. This process is harmless to the electrolyte and separator [173].
Zhong et al. [181] presented low-temperature volatilization to recover the electrolytes, more than 95% of electrolytes could be recycled, and LiPF6 was disposed of by pyrolysis process. The active materials can be effectively separated from current collectors under N2 at 550 °C for 2 h in this process. LiPF6 was decomposed to LiF and PF5, which could react with water by pyrolysis at above 180 °C. The final residues contained few harmful elements (e.g., F) (Fig. 12c). Recently, some novel pyrolysis methods have been proposed. Sun and Qiu [173] proposed a novel process to treat electrolytes by vacuum pyrolysis (Fig. 12d), and electrolytes and binders were evaporated or decomposed through pyrolysis. Jung et al. [180] performed a catalytic thermolysis process to convert the electrolyte (EC/DMC/LiPF6) into value-added chemicals and the cathode materials could be used as the catalyst. They have confirmed the different volatile compounds (e.g., carbonates, cyclic hydrocarbons, and aliphatic hydrocarbons) and gaseous products (e.g., H2, CH4, CO2) from pyrolysis. The mixtures were further converted into syngas, which was catalyzed by NCM and CO2-assisted at 500 °C. Rothermel et al. [179] adopted thermal evaporation of volatile electrolyte components without recovery. This process can be directly recovered to obtain graphite without the subsequent separation process, but decomposition of toxic gases (e.g., HF) has proceeded. The non-recycling treatment of electrolytes often simplifies the recycling process, while these toxic gases generated by fluorinated compounds will pose a risk to environment and equipment, causing serious environmental pollution and high energy consumption [182].
3 Industrialized applications of pretreatment process
Not all pretreatment methods reported in the literature in the current study are likely to be adopted by LIBs recycling companies due to the different types of LIBs [3, 8]. Based on this reason, the current representative pretreatment processes in the major spent LIBs recycling companies around the world were present to bridge the current technological status with the scale-up industrialized applications.
3.1 Umicore, Belgium
The recycling process explored by Umicore is mainly composed of smelting (in shaft furnaces) and hydrometallurgy steps. And the pretreatment mainly utilized the ultra-high temperature (UHT) smelting technology without discharging treatment [114, 183], which could treat all types of spent LIBs. This reaction process takes place in a vertical shaft furnace, which can be divided into three parts [83, 184] (Fig. 13a). (1) the upper zone (< 300 °C): electrolytes are evaporated without the risk of explosions; (2) the middle zone (about 700 °C): the organic components are decomposed, which can be used as fuel and (3) the bottom zone (1200–1450 °C): the feed materials are smelt and reduced to metal alloys (e.g., Co–Ni-Cu) and slags (e.g., Li oxide). The output streams are scheduled for the subsequent hydrometallurgical process [170, 183].

Reproduced with permission from Ref. [83]. Copyright 2019, Springer. b Schematic diagram of Retriev process. Reproduced with permission from Ref. [11]. Copyright 2021, Elsevier. c Schematic diagram of Accurec process. Reproduced with permission from Ref. [83]. Copyright 2019, Springer
a Schematic diagram of Umicore process.
3.2 Retriev, USA
In the Retriev pretreatment process, formerly the Toxco process, large number of LIBs were manually disassembly to separate assemblies and circuits [185, 186]. Separated LIBs were put into an automatic crusher, under the aqueous environment to prevent the generation of hazardous gases and to reduce the reactivity of these spent LIBs. Physical sorting (e.g., magnetic separation, sieving) yielded metal solids, metal-enriched liquids and plastic fractions. They were treated separately in subsequent technology [11, 185, 187], as illustrated in Fig. 13b.
3.3 Accurec, Germany
Accurec is a dedicated battery recycling company for all kinds of rechargeable batteries. The treatment capacity has reached 3000 t·year−1 of LIBs in 2019 [188]. Accurec proposed a vacuum thermal recycling (VTR) technology to recycle spent LIBs. The pretreatment process is divided into thermal pretreatment and mechanical separation. A schematic illustration of Accurec recycling process is shown in Fig. 13c. Firstly, spent LIBs are sorted and opened to remove the organic components in a rotary kiln by thermal treatment. The electrolyte can be collected and recovered by vacuum heat treatment below 250 °C. And then temperature is maintained below 600 °C to remove organic components, without the changes for the states of metals. The excess energy generated by the incineration is utilized for high-pressure steam [114]. Then, these pyrolyzed batteries are milled and separated by magnetic separation, air separation, zig-zag classier and vibrating screen. Finally, the obtained products (e.g., steel fraction, Cu/Al fraction and Co- and Ni-rich active powder) can be delivered to corresponding technologies for their final recycling processes [107, 113].
3.4 Recupyl, France
Recupyl proposed the combination of the recycling spent batteries and precious metals through mechanical and chemical methods, which are recognized as "Urban Mines" [189]. The spent LIBs are mechanically dismantled in an inert atmosphere mixed with CO2 and H2 with low lithium activity. Milling can reduce the particle size to 3 mm, and then it can be sorted by vibrating screening and magnetic separation. Different fractions are obtained after the following separation step: fine fraction rich in metal oxides and carbon, magnetic fraction composed of casings, current collectors and plastics. Then, a hydrometallurgical process is adopted to recover the rest of feed materials [4].
3.5 BatRec, Sweden
BatRec is a specialist in recycling of hazardous industrial wastes. BatRec's processing units are capable to recycle any type of batteries and accumulators [190]. This process is a combination of mechanical and thermal pretreatment to enable the recovery of all battery components. Firstly, the spent LIBs are sorted and the packaged materials are removed, followed by disassembling to the module level [191]. Then the modules are subjected to pyrolysis, which can cause thermal deactivation and evaporation of the organic components. The harmful gases generated during pyrolysis can be treated by a condenser unit and gas treatment mechanism. The target products (e.g., Al, Cu and steel) can be separated by comminution, and mechanical classification [81].
3.6 AkkuSer Oy’s battery recycling process, Finland
AkkuSer recycles spent batteries through its own “Dry- technology”, a mechanical process based on two-stage crushing, followed by a magnetic and mechanical separation unit [192]. The electrolytes and reaction gases are removed by a cyclonic air separator and then cleaned by filters in the first crushing process. In the following crushing process, the battery is crushed to 6 mm. All the dust is removed in this process. This process is efficient and safe to recycle these fire and explosion-sensitive batteries. Efficient dust and gas handling minimizes the environmental impact and maximizes the recovery of valuable metals [184].
3.7 Green eco-manufacturer (G.E.M), China
G.E.M is a famous recycling company that is capable of the treatment of electronic wastes, battery wastes, discarded cars and metal scraps [193]. And it is one of the largest recycling companies of recycling spent LIBs in China. The process chosen by G.E.M is “mechanical sorting + hydrometallurgy” model, with the pretreatment part mainly using mechanical separation technology. The waste materials are firstly crushed and sorted to remove metal fragments. Then, metal salt solutions (e.g., Cu2+, Al3+, Fe2+, Co2+ and Ni2+) are obtained through acid leaching and extraction separation. The ternary precursor products are prepared through co-precipitation and cobalt carbonate is prepared from cobalt chloride. The lithium-containing extract is used to prepare lithium hydroxide products [22, 193].
3.8 Brunp, China
Brunp is the second largest recycling company in China, and its main business segments include the recycling of spent batteries and cars. The annual waste batteries (including LIBs and other batteries) treatment capacity exceeded 30,000 t in 2018 [194]. And its pretreatment step mainly includes discharging, thermal pretreatment and mechanical treatment. The dried batteries after discharging, de-electrolyzing and drying are crushed, shaken and sorted to obtain two mixtures. The first mixture contains steel slags, Cu foils and graphite, which can be separated into steel slags and the third mixture (which contains Cu foils and graphite) by magnetically sorted. The second mixture composed of Al foils and black powders is sieved by roasting under an inert atmosphere, which is also used to sieve the Cu foils and graphite in the third mixture [3, 65]. The whole process can be completed by thermal and mechanical treatment without adding reagents, which is a green and efficient separation process.
4 Conclusion and perspective
This paper has focused on the prevailing cutting-edge technologies of different pretreatment processes for spent LIBs, with a summary of recent advances in deactivation, disintegration and separation, separation of active materials and current collectors and the treatment of electrolytes. An in-depth discussion of the technology development and current status in both laboratory and industry have been reviewed for future possible guidelines. Future development in the area of pretreatment still has great potential, although there is much research on pretreatment. An effective and environmentally friendly treatment method has not yet been established. To achieve the high processing efficiency and selectivity of spent LIBs, the combination of multiple technologies is critical to learn from other's strong points to offset one's weaknesses. The high-efficiency discharge, high-release efficiency of active materials and electrolyte treatment/recycling are highly required to pay due attention. Furthermore, the industrialization of technology is difficult to realize. The efficient, simple and eco-friendly pretreatment process may be improved and realized through the deconstruction and a combination of different treatment processes in the future.
References
Fan ES, Li L, Wang ZP, Lin J, Huang YX, Yao Y, Chen RJ, Wu F. Sustainable recycling technology for Li-ion batteries and beyond: challenges and future prospects. Chem Rev. 2020;120(14):7020. https://doi.org/10.1021/acs.chemrev.9b00535.
Natarajan S, Aravindan V. Burgeoning prospects of spent lithium-ion batteries in multifarious applications. Adv Energy Mater. 2018;8(33):1802303. https://doi.org/10.1002/aenm.201802303.
Ma LX, Chen TD, Hai CX, Dong SD, He X, Xu Q, Feng H, Xin A, Chen JT, Zhou Y. Surface engineering of Li- and Mn-rich layered oxides for superior Li-ion battery. Tungsten. 2022. https://doi.org/10.1007/s42864-022-00187-w.
Zhao Y, Fang LZ, Kang YQ, Wang L, Zhou YN, Liu XY, Li T, Li YX, Liang Z, Zhang ZX, Li BH. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Met. 2021;40(6):1431. https://doi.org/10.1007/s12598-020-01587-y.
Zhang ZQ, Li L, Li X, Hu YC, Huang K, Xue BY, Wang YQ, Yu YJ. State-of-health estimation for the lithium-ion battery based on gradient boosting decision tree with autonomous selection of excellent features. Int J Energy Res. 2022;46(2):1756. https://doi.org/10.1002/er.7292.
Lv WG, Wang ZH, Cao HB, Sun Y, Zhang Y, Sun Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain Chem Eng. 2018;6(2):1504. https://doi.org/10.1021/acssuschemeng.7b03811.
Fan MC, Wozny J, Gong J, Kang YQ, Wang XS, Zhang ZX, Zhou GM, Zhao Y, Li BH, Kang FY. Lithium metal recycling from spent lithium-ion batteries by cathode overcharging process. Rare Met. 2022;41(6):1843. https://doi.org/10.1007/s12598-021-01918-7.
Li WB, Wu K, Feng H, Wang N, Zhang JH, Wang JJ, Li XF. Atomic layer deposition of ultrafine Pd nanoparticles for enhancing the rate capability of LiNi0.8Co0.1Mn0.1O2 cathode. Tungsten. 2022;4(4):346. https://doi.org/10.1007/s42864-022-00178-x.
Yu DW, Huang Z, Makuza B, Guo XY, Tian QH. Pretreatment options for the recycling of spent lithium-ion batteries: a comprehensive review. Miner Eng. 2021;173:107218. https://doi.org/10.1016/j.mineng.2021.107218.
Assefi M, Maroufi S, Yamauchi Y, Sahajwalla V. Pyrometallurgical recycling of Li-ion, Ni-Cd and Ni-MH batteries: a mini-review, current opinion in green and sustainable. Chemistry. 2020;24:26. https://doi.org/10.1016/j.cogsc.2020.01.005.
SNE research, 2022. https://www.sneresearch.com/kr/home/ (Accessed Aug 1, 2022).
Ggii. Power battery shipments of China, 2022. https://www.gg-lb.com/art-42171.html (Accessed Aug 1, 2022).
Zhang JR, Lan ZW, Xi RH, Li YY, Wang JT, Zhang Caihong. Review on deficiency and modification of high nickel ternary materials for lithium-ion batteries. Chin J of Rare Met. 2022,46(3):367. https://doi.org/10.13373/j.cnki.cjrm.XY20090004.
Li M, Cheng LL, Yang YM, Niu F, Zhang XL, Liu DH. Development of technology for spent lithium-ion batteries recycling: a review. Chin J of Rare Met. 2022;46(3):349. https://doi.org/10.13373/j.cnki.cjrm.XY20020020.
Su MM, Huang G, Wang SQ, Wang YJ, Wang HH. High safety separators for rechargeable lithium batteries. Sci China-Chem. 2021;64(7):1131. https://doi.org/10.1007/s11426-021-1011-9.
Reddy MV, Mauger A, Julien CM, Paolella A, Zaghib K. Brief history of early lithium-battery development. Materials. 2020;13(8):1884. https://doi.org/10.3390/ma13081884.
Li JT, Wu ZY, Lu YQ, Zhou Y, Huang QS, Huang L, Sun SG. Water soluble binder, an electrochemical performance booster for electrode materials with high energy density. Adv Energy Mater. 2017;7(24):1701185. https://doi.org/10.1002/aenm.201701185.
He YQ, Yuan X, Zhang GW, Wang HF, Zhang T, Xie WN, Li LP. A critical review of current technologies for the liberation of electrode materials from foils in the recycling process of spent lithium-ion batteries. Sci Total Environ. 2020;766:142382. https://doi.org/10.1016/j.scitotenv.2020.142382.
Zhu T. Mechanics of high-capacity electrodes in lithium-ion batteries. Chin Phys B. 2016;25(1):014601. https://doi.org/10.1088/1674-1056/25/1/014601.
Liu ZF, Jiang YJ, Hu QM, Guo ST, Yu L, Li Q, Liu Q, Hu XL. Safer lithium-ion batteries from the separator aspect: development and future perspectives. Energy Environ Mater. 2021;4(3):336. https://doi.org/10.1002/eem2.12129.
Tran MK, Rodrigues MTF, Kato K, Babu G, Ajayan PM. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy. 2019;4(4):339. https://doi.org/10.1038/s41560-019-0368-4.
Roy JJ, Rarotra S, Krikstolaityte V, Zhuoran KW, Cindy YDI, Tan XY, Carboni M, Meyer D, Yan Q, Srinivasan M. Green recycling methods to treat lithium-ion batteries E-waste: a circular approach to sustainability. Adv Mater. 2021. https://doi.org/10.1002/adma.202103346.
Zhong XH, Liu W, Han JW, Jiao F, Qin WQ, Liu T. Pretreatment for the recovery of spent lithium ion batteries: theoretical and practical aspects. J Clean Product. 2020;263:121439. https://doi.org/10.1016/j.jclepro.2020.121439.
Chagnes A, Pospiech B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J Chem Technol Biotechnol. 2013;88(7):1191. https://doi.org/10.1002/jctb.4053.
London metal exchange home page, 2022. https://www.lme.com/ (Accessed Aug 1, 2022).
Shanghai metals market new energy division home page. 2022. https://price.metal.com/ (Accessed Aug 1, 2022).
Zhang GW, Yuan X, He YQ, Wang HF, Zhang T, Xie WN. Recent advances in pretreating technology for recycling valuable metals from spent lithium-ion batteries. J Hazard Mater. 2021;406:124332. https://doi.org/10.1016/j.jhazmat.2020.124332.
Zhang XX, Li L, Fan ES, Xue Q, Bian YF, Wu F, Chen RJ. Toward sustainable and systematic recycling of spent rechargeable batteries. Chem Soc Rev. 2018;47(19):7239. https://doi.org/10.1039/C8CS00297E.
Ordonez J, Gago EJ, Girard A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew Sustain Energy Rev. 2016;60:195. https://doi.org/10.1016/j.rser.2015.12.363.
Chaudhary V, Lakhera P, Kim KH, Deep A, Kumar P. Insights into the eco-friendly recovery process for valuable metals from waste lithium-ion batteries: organic acids leaching. Sep Purif Rev. 2022. https://doi.org/10.1080/15422119.2022.2164650.
Jiang YZ, Chen XP, Yan SX, Ou YD, Zhou T. Mechanochemistry-induced recycling of spent lithium-ion batteries for synergistic treatment of mixed cathode powders. Green Chem. 2022;24(15):5987. https://doi.org/10.1039/d2gc01929a.
Mousa E, Hu XF, Ye GZ. Effect of graphite on the recovery of valuable metals from spent Li-ion batteries in baths of hot metal and steel. Recycling. 2022;7(1):5. https://doi.org/10.3390/recycling7010005.
Murakami Y, Matsuzaki Y, Murakami K, Hiratani S, Shibayama A, Inoue R. Recovery rates of used rechargeable lithium-ion battery constituent elements in heat treatment. Metall Mater Trans B-Process Metall Mater Process Sci. 2020;51(4):1355. https://doi.org/10.1007/s11663-020-01834-8.
Pinna EG, Toro N, Gallegos S, Rodriguez MH. A novel recycling route for spent Li-ion batteries. Materials. 2022;15(1):44. https://doi.org/10.3390/ma15010044.
Zhu BW, Zhang YJ, Zou YL, Yang ZL, Zhang B, Zhao Y, Zhang MY, Meng Q, Dong P. Leaching kinetics and interface reaction of LiNi0.6Co0.2Mn0.2O2 materials from spent LIBs using GKB as reductant. J Environ Manag. 2021;300:113710. https://doi.org/10.1016/j.jenvman.2021.113710.
Zeng GS, Yao JX, Liu CL, Luo XB, Ji HY, Mi X, Deng CJ. Simultaneous recycling of critical metals and aluminum foil from waste LiNi1/3Co1/3Mn1/3O2 cathode via ethylene glycol-citric acid system. Acs Sustain Chem Eng. 2021;9(48):16133. https://doi.org/10.1021/acssuschemeng.1c04806.
Natarajan S, Boricha AB, Bajaj HC. Recovery of value-added products from cathode and anode material of spent lithium-ion batteries. Waste Manag. 2018;77:455. https://doi.org/10.1016/j.wasman.2018.04.032.
Jiang YZ, Chen XP, Yan SX, Li SZ, Zhou T. Pursuing green and efficient process towards recycling of different metals from spent lithium-ion batteries through ferro-chemistry. Chem Eng J. 2021;426:131637. https://doi.org/10.1016/j.cej.2021.131637.
Meng K, Cao Y, Zhang B, Ou X, Li DM, Zhang JF, Ji XB. Comparison of the ammoniacal leaching behavior of layered LiNixCoyMn1–x–yO2 (x = 1/3, 0.5, 0.8) cathode materials. ACS Sustain Chem Eng. 2019;7(8):7750. https://doi.org/10.1021/acssuschemeng.8b06675.
Qi YP, Meng FS, Yi XX, Shu JC, Chen MJ, Sun Z, Sun SH, Xiu FR. A novel and efficient ammonia leaching method for recycling waste lithium ion batteries. J Clean Product. 2020;251:119665. https://doi.org/10.1016/j.jclepro.2019.119665.
Roy JJ, Madhavi S, Cao B. Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process. J Clean Product. 2021;280:124242. https://doi.org/10.1016/j.jclepro.2020.124242.
Ma YY, Zhou XY, Tang JJ, Liu XJ, Gan HX, Yang J. Reaction mechanism of antibiotic bacteria residues as a green reductant for highly efficient recycling of spent lithium-ion batteries. J Hazard Mater. 2021;417:126032. https://doi.org/10.1016/j.jhazmat.2021.126032.
Kang SK, Park JW, Tikue ET, Zhang HX, Yang S, Lee PS. Self-cross-linking nanocomposite membranes for green recycling of the solvent during lithium-ion battery manufacturing. Acs Sustain Chem Eng. 2022. https://doi.org/10.1021/acssuschemeng.1c06715.
He LP, Sun SY, Song XF, Yu JG. Recovery of cathode materials and Al from spent lithium-ion batteries by ultrasonic cleaning. Waste Manag. 2015;46:523. https://doi.org/10.1016/j.wasman.2015.08.035.
Li JH, Shi PX, Wang ZF, Chen Y, Chang CC. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere. 2009;77(8):1132. https://doi.org/10.1016/j.chemosphere.2009.08.040.
Chan KH, Malik M, Azimi G. Separation of lithium, nickel, manganese, and cobalt from waste lithium-ion batteries using electrodialysis. Resour Conserv Recycl. 2022;178:106076. https://doi.org/10.1016/j.resconrec.2021.106076.
Widijatmoko SD, Gu F, Wang Z, Hall P. Selective liberation in dry milled spent lithium-ion batteries. Sustain Mater Technol. 2020;23:00134. https://doi.org/10.1016/j.susmat.2019.e00134.
Ma XT, Chen MY, Chen B, Meng ZF, Wang Y. High-performance graphite recovered from spent lithium-ion batteries. Acs Sustain Chem Eng. 2019;7(24):19732. https://doi.org/10.1021/acssuschemeng.9b05003.
Qin ZY, Wen ZX, Xu YF, Zheng ZC, Bai ML, Zhang N, Jia CK, Wu HB, Chen G. A ternary molten salt approach for direct regeneration of LiNi0.5Co0.2Mn0.3O2 cathode. Small. 2022;18(43):2106719. https://doi.org/10.1002/smll.202106719.
Xiao JF, Li J, Xu ZM. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J Hazard Mater. 2017;338(15):124. https://doi.org/10.1016/j.jhazmat.2017.05.024.
Choi JW, Cho CW, Yun YS. Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J Hazard Mater. 2022;423:127214. https://doi.org/10.1016/j.jhazmat.2021.127214.
Peng C, Liu FP, Aji AT, Wilson BP, Lundstrom M. Extraction of Li and Co from industrially produced Li-ion battery waste - using the reductive power of waste itself. Waste Manage. 2019;95:604. https://doi.org/10.1016/j.wasman.2019.06.048.
Wang HY, Li ZF, Meng Q, Duan JG, Xu ML, Lin Y, Zhang YJ. Ammonia leaching of valuable metals from spent lithium ion batteries in NH3-(NH4)2SO4-Na2SO3 system. Hydrometallurgy. 2022;208:105809. https://doi.org/10.1016/j.hydromet.2021.105809.
Zheng HS, Dong T, Sha YF, Jiang DF, Zhang HT, Zhang SJ. Selective extraction of lithium from spent lithium batteries by functional ionic liquid. ACS Sustain Chem Eng. 2021;9(20):7022. https://doi.org/10.1021/acssuschemeng.1c00718.
Zhu P, Chen Y, Wang LY, Zhou M. Treatment of waste printed circuit board by green solvent using ionic liquid. Waste Manag. 2012;32(10):1914. https://doi.org/10.1016/j.wasman.2012.05.025.
Wang BY, Liu F, Zhang F, Tan M, Jiang HQ, Liu Y, Zhang Y. Efficient separation and recovery of cobalt(II) and lithium(I) from spent lithium ion batteries (LIBs) by polymer inclusion membrane electrodialysis (PIMED). Chem Eng J. 2022;430:132924. https://doi.org/10.1016/j.cej.2021.132924.
Sio JEL, Escobar EC, Kim H, Chung WJ, Nisola GM. Hydroxypicolinic acid tethered on magnetite core-silica shell (HPCA@SiO2@Fe3O4) as an effective and reusable adsorbent for practical Co(II) recovery. Chemosphere. 2022;298:134301. https://doi.org/10.1016/j.chemosphere.2022.134301.
Tran TT, Moon HS, Lee MS. Recovery of cobalt, nickel and copper compounds from UHT processed spent lithium-ion batteries by hydrometallurgical process. Min Process Extract Metall Rev 2021; https://doi.org/10.1080/08827508.2021.1910508.
Li JL, Lu YQ, Yang TR, Ge DY, Wood DL, Li Z. Water-based electrode manufacturing and direct recycling of lithium-ion battery electrodes-a green and sustainable manufacturing system. iScience. 2020;23(5):101081. https://doi.org/10.1016/j.isci.2020.101081.
Zheng XH, Zhu ZW, Lin X, Zhang Y, He Y, Cao HB, Sun Z. A mini-review on metal recycling from spent lithium ion batteries. Engineering. 2018;4(3):361. https://doi.org/10.1016/j.eng.2018.05.018.
Li YK, Lv WG, Huang HL, Yan WY, Li XK, Ning PG, Cao HB, Sun Z. Recycling of spent lithium-ion batteries in view of green chemistry. Green Chem. 2021;23(17):6139. https://doi.org/10.1039/d1gc01639c.
Xiao JF, Guo J, Zhan L, Xu ZM. A cleaner approach to the discharge process of spent lithium ion batteries in different solutions. J Clean Product. 2020;255:120064. https://doi.org/10.1016/j.jclepro.2020.120064.
Yao LP, Zeng Q, Qi T, Li J. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries. J Clean Product. 2020;245:118820. https://doi.org/10.1016/j.jclepro.2019.118820.
Hagelüken C. Recycling of elctronic scrap at Umicore’s integrated metals smelter and refinery. Proc EMC. 2005;59(3):152.
Kim S, Bang J, Yoo J, Shin Y, Bae J, Jeong J, Kim K, Dong P, Kwon K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J Clean Product. 2021;294:126329. https://doi.org/10.1016/j.jclepro.2021.126329.
Wang MM, Tan QY, Liu LL, Li JH. A facile, environmentally friendly, and low-temperature approach for decomposition of polyvinylidene fluoride from the cathode electrode of spent lithium-ion batteries. ACS Sustain Chem Eng. 2019;7(15):12799. https://doi.org/10.1021/acssuschemeng.9b01546.
Li J, Wang GX, Xu ZM. Generation and detection of metal ions and volatile organic compounds (VOCs) emissions from the pretreatment processes for recycling spent lithium-ion batteries. Waste Manag. 2016;52:221. https://doi.org/10.1016/j.wasman.2016.03.011.
Wang HC. Study on recycling of spent lithium ion batteries containing cobalt and pilot scale experiment. Heilongjiang: Harbin Institute of Technology; 2013.
Zheng RJ. The resynthesis and reuse from materials of spent lithium ion batteries of phosphate and mixture. Heilongjiang: Harbin Institute of Technology; 2017.
Larsson F, Mellander BE. Abuse by external heating, overcharge and short circuiting of commercial lithium-ion battery cells. J Electrochem Soc. 2014;161(10):A1611. https://doi.org/10.1149/2.0311410jes.
Zhang T, He YQ, Wang FF, Ge LH, Zhu XN, Li H. Chemical and process mineralogical characterizations of spent lithium-ion batteries: an approach by multi-analytical techniques. Waste Manage. 2014;34(6):1051. https://doi.org/10.1016/j.wasman.2014.01.002.
Cardarelli. Method for recycling spent lithium metal polymer rechargeable batteries and related materials.US Patent; US 7192564 B2. 2007.
Bruckner L, Frank J, Elwert T. Industrial recycling of lithium-ion batteries-a critical review of metallurgical process routes. Metals. 2020;10(8):1107. https://doi.org/10.3390/met10081107.
Trager T, Friedrich B, Weyhe R. Recovery concept of value metals from automotive lithium-ion batteries. Chem Ing Tec. 2015;87(11):1550. https://doi.org/10.1002/cite.201500066.
Meng K, Xu GY, Peng XH, Youcef-Toumi K, Li J. Intelligent disassembly of electric-vehicle batteries: a forward-looking overview. Resour Conserv Recycl. 2022;182:106207. https://doi.org/10.1016/j.resconrec.2022.106207.
Grützke M, Monnighoff X, Horsthemke F, Kraft V, Winter M, Nowak S. Extraction of lithium-ion battery electrolytes with liquid and supercritical carbon dioxide and additional solvents. RSC Adv. 2015;5(54):43209. https://doi.org/10.1039/c5ra04451k.
Zhao Y, Kang YQ, Fan MC, Li T, Wozny J, Zhou Y, Wang X, Chueh YL, Liang Z, Zhou GM, Wang JX, Tavajohi N, Kang FY, Li BH. Precise separation of spent lithium-ion cells in water without discharging for recycling. Energy Storage Mater. 2022;45:1092. https://doi.org/10.1016/j.ensm.2021.11.005.
Velazquez-Martinez O, Valio J, Santasalo-Aarnio A, Reuter M, Serna-Guerrero R. A critical review of lithium-ion battery recycling processes from a circular economy perspective. Batteries-Basel. 2019;5(4):68. https://doi.org/10.3390/batteries5040068.
Diekmann J, Hanisch C, Frobose L, Schalicke G, Loellhoeffel T, Folster AS, Kwade A. Ecological recycling of lithium-ion batteries from electric vehicles with focus on mechanical processes. J Electrochem Soc. 2017;164(1):A6184. https://doi.org/10.1149/2.0271701jes.
Zhang T, He YQ, Ge LH, Fu RS, Zhang X, Huang YJ. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J Power Sources. 2013;240:766. https://doi.org/10.1016/j.jpowsour.2013.05.009.
Piatek J, Afyon S, Budnyak TM, Budnyk S, Sipponen M, Slabon A. Sustainable Li-ion batteries: chemistry and recycling. Adv Energy Mater. 2021;11(43):2003456. https://doi.org/10.1002/aenm.202003456.
Hossain R, Kumar U, Sahajwalla V. Selective thermal transformation of value added cobalt from spent lithium-ion batteries. J Clean Product. 2021;293:126140. https://doi.org/10.1016/j.jclepro.2021.126140.
Pinegar H, Smith YR. Recycling of end-of-life lithium ion batteries, part I: commercial processes. J Sustain Metall. 2019;5(3):402. https://doi.org/10.1007/s40831-019-00235-9.
Tedjar F, Foudraz JC. Method for the mixed recycling of lithium-based anode batteries and cells. US Patent; US 7820317 B2. 2010.
Pindar S, Dhawan N. Recycling of mixed discarded lithium-ion batteries via microwave processing route. Sustain Mater Technol. 2020;25:e00157. https://doi.org/10.1016/j.susmat.2020.e00157.
Granata G, Pagnanelli F, Moscardini E, Takacova Z, Havlik T, Toro L. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: accomplishment of European guidelines by optimizing mechanical pre-treatment and solvent extraction operations. J Power Sour. 2012;212:205. https://doi.org/10.1016/j.jpowsour.2012.04.016.
da Costa A, Matos J, Bernardes A, Muller I. Beneficiation of cobalt, copper and aluminum from wasted lithium-ion batteries by mechanical processing. Int J Miner Process. 2015;145:77. https://doi.org/10.1016/j.minpro.2015.06.015.
Huang Z, Lin M, Qiu RJ, Zhu J, Ruan JJ, Qiu RL. A novel technology of recovering magnetic micro particles from spent lithium-ion batteries by ultrasonic dispersion and waterflow-magnetic separation. Resour Conserv Recycl. 2021;164:105172. https://doi.org/10.1016/j.resconrec.2020.105172.
Cao YX, Wang ZQ, Wang JJ, Li GF. Multi-stage electrostatic separation for recovering of aluminum from fine granules of black dross. J Wuhan Univ Technol-Mater Sci Ed. 2019;34(4):925. https://doi.org/10.1007/s11595-019-2139-2.
Silveira A, Santana M, Tanabe E, Bertuol D. Recovery of valuable materials from spent lithium ion batteries using electrostatic separation. Int J Miner Process. 2017;169:91. https://doi.org/10.1016/j.minpro.2017.11.003.
Bi HJ, Zhu HB, Zu L, Gao Y, Gao S, Bai YX. Environment-friendly technology for recovering cathode materials from spent lithium iron phosphate batteries. Waste Manage Res. 2020;38(8):911. https://doi.org/10.1177/0734242x20931933.
Fan MC, Zhao Y, Kang YQ, Wozny J, Liang Z, Wang JX, Zhou GM, Li BH, Tavajohi N, Kang FY. Room-temperature extraction of individual elements from charged spent LiFePO4 batteries. Rare Met. 2022;41(5):1595. https://doi.org/10.1007/s12598-021-01919-6.
Gratz E, Sa QN, Apelian D, Wang Y. A closed loop process for recycling spent lithium ion batteries. J Power Sour. 2014;262:255. https://doi.org/10.1016/j.jpowsour.2014.03.126.
Vanderbruggen A, Salces A, Ferreira A, Rudolph M, Serna-Guerrero R. Improving separation efficiency in end-of-life lithium-ion batteries flotation using attrition pre-treatment. Minerals. 2022;12(1):72. https://doi.org/10.3390/min12010072.
Saneie R, Abdollahi H, Ghassa S, Azizi D, Chehreh CS. Recovery of copper and aluminum from spent lithium-ion batteries by froth flotation: a sustainable approach. J Sustain Metall. 2022;8(1):386. https://doi.org/10.1007/s40831-022-00493-0.
Zhan RT, Yang ZZ, Bloom I, Pan L. Significance of a solid electrolyte interphase on separation of anode and cathode materials from spent Li-ion batteries by froth flotation. Acs Sustain Chem Eng. 2021;9(1):531. https://doi.org/10.1021/acssuschemeng.0c07965.
Wang FF, Zhang T, He YQ, Zhao YM, Wang S, Zhang GW, Zhang Y, Feng Y. Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J Clean Prod. 2018;185:646. https://doi.org/10.1016/j.jclepro.2018.03.069.
Zhang GW, He YQ, Feng Y, Wang HF, Zhu XN. Pyrolysis-ultrasonic-assisted flotation technology for recovering graphite and LiCoO2 from spent lithium-ion batteries. Acs Sustain Chem Eng. 2018;6(8):10896. https://doi.org/10.1021/acssuschemeng.8b02186.
Yu JD, He YQ, Li H, Xie WN, Zhang T. Effect of the secondary product of semi-solid phase Fenton on the flotability of electrode material from spent lithium-ion battery. Powder Technol. 2017;315:139. https://doi.org/10.1016/j.powtec.2017.03.050.
He YQ, Zhang T, Wang FF, Zhang GW, Zhang WG, Wang J. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation. J Clean Prod. 2017;143:319. https://doi.org/10.1016/j.jclepro.2016.12.106.
Huang Z, Zhu J, Wu XW, Qiu RJ, Xu ZM, Ruan JJ. Eddy current separation can be used in separation of non-ferrous particles from crushed waste printed circuit boards. J Clean Product. 2021;312:127755. https://doi.org/10.1016/j.jclepro.2021.127755.
Ruan JJ, Dong LP, Zheng J, Zhang T, Huang MZ, Xu ZM. Key factors of eddy current separation for recovering aluminum from crushed e-waste. Waste Manage. 2017;60:84. https://doi.org/10.1016/j.wasman.2016.08.018.
Smith YR, Nagel JR, Rajamani RK. Eddy current separation for recovery of non-ferrous metallic particles: a comprehensive review. Miner Eng. 2019;133:149. https://doi.org/10.1016/j.mineng.2018.12.025.
Bi HJ, Zhu HB, Zu L, Bai YX, Gao S, Gao Y. A new model of trajectory in eddy current separation for recovering spent lithium iron phosphate batteries. Waste Manage. 2019;100:1. https://doi.org/10.1016/j.wasman.2019.08.041.
Liang A. Recycling of spent lithium-ion batteries. Braunschweig: Springer Cham; 2019.
Zhong XH, Liu W, Han JW, Jiao F, Zhu HL, Qin WQ. Pneumatic separation for crushed spent lithium-ion batteries. Waste Manag. 2020;118:331. https://doi.org/10.1016/j.wasman.2020.08.053.
Zhang GW, Du ZX, He YQ, Wang HF, Xie WN, Zhang T. A sustainable process for the recovery of anode and cathode materials derived from spent lithium-ion batteries. Sustainability. 2019;11(8):2363. https://doi.org/10.3390/su11082363.
Bi HJ, Zhu HB, Zu L, He SH, Gao Y, Gao S. Pneumatic separation and recycling of anode and cathode materials from spent lithium iron phosphate batteries. Waste Manag Res. 2019;37(4):374. https://doi.org/10.1177/0734242x18823939.
Shi Y, Zhang J, Bruck AM, Zhang YM, Li J, Stach EA, Takeuchi KJ, Marschilok AC, Takeuchi ES, Yu G. A tunable 3D nanostructured conductive gel framework electrode for high-performance lithium ion batteries. Adv Mater. 2017;29(22):1603922. https://doi.org/10.1002/adma.201603922.
Zhong XH, Han JW, Chen LL, Liu W, Jiao F, Zhu HL, Qin WQ. Binding mechanisms of PVDF in lithium ion batteries. Appl Surf Sci. 2021. https://doi.org/10.1016/j.apsusc.2021.149564.
Nayaka GP, Zhang YJ, Dong P, Wang D, Zhou ZR, Duan JG, Li X, Lin Y, Meng Q, Pai KV, Manjanna J, Santhosh G. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J Environ Chem Eng. 2019;7(1):102854. https://doi.org/10.1016/j.jece.2018.102854.
Nan JM, Han DM, Yang MJ, Cui M, Hou XL. Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy. 2006;84(1–2):75. https://doi.org/10.1016/j.hydromet.2006.03.059.
Hu ZL, Zhu NW, Wei XR, Zhang SH, Li F, Wu PX, Chen YJ. Efficient separation of aluminum foil from mixed-type spent lithium-ion power batteries. J Environ Manag. 2021;298:113500. https://doi.org/10.1016/j.jenvman.2021.113500.
Makuza B, Tian QH, Guo XY, Chattopadhyay K, Yu DW. Pyrometallurgical options for recycling spent lithium-ion batteries: a comprehensive review. J Power Sour. 2021;491:229622. https://doi.org/10.1016/j.jpowsour.2021.229622.
Song DW, Wang XQ, Nie HH, Shi H, Wang DG, Guo FX, Shi XX, Zhang LQ. Heat treatment of LiCoO2 recovered from cathode scraps with solvent method. J Power Sour. 2014;249:137. https://doi.org/10.1016/j.jpowsour.2013.10.062.
Fan ES, Yang JB, Huang YX, Lin J, Arshad F, Wu F, Li L, Chen RJ. Leaching mechanisms of recycling valuable metals from spent lithium-ion batteries by a malonic acid-based leaching system. ACS Appl Energy Mater. 2020;3(9):8532. https://doi.org/10.1021/acsaem.0c01166.
Bridgwater AV. Waste incineration and pyrolysis. Resour Recov Conserv. 1980. https://doi.org/10.1016/0304-3967(80)90025-6.
Hanisch C, Loellhoeffel T, Diekmann J, Markley KJ, Haselrieder W, Kwade A. Recycling of lithium-ion batteries: a novel method to separate coating and foil of electrodes. J Clean Prod. 2015;108:301. https://doi.org/10.1016/j.jclepro.2015.08.026.
Haldar SK. Chapter 13 - Mineral processing, Edited by S. K. Haldar. Kolkata: Elsevier, Mineral Exploration (Second Edition). 2018:259.
Bernardes AM, Espinosa DCR, Tenório JAS. Recycling of batteries: a review of current processes and technologies. J Power Sour. 2004;130(1–2):291. https://doi.org/10.1016/j.jpowsour.2003.12.026.
Liu W, Zhong XH, Han JW, Qin WQ, Liu T, Zhao CX, Chang ZY. Kinetic study and pyrolysis behaviors of spent LiFePO4 batteries. Acs Sustain Chem Eng. 2019;7(1):1289. https://doi.org/10.1021/acssuschemeng.8b04939.
Tang YQ, Xie HW, Zhang BL, Chen X, Zhao ZQ, Qu JK, Xing PF, Yin HY. Recovery and regeneration of LiCoO2-based spent lithium-ion batteries by a carbothermic reduction vacuum pyrolysis approach: Controlling the recovery of CoO or Co. Waste Management. 2019; 97:140. https://doi.org/10.1016/j.wasman.2019.08.004.
Yang Y, Huang GY, Xu SM, He YH, Liu X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy. 2016;165:390. https://doi.org/10.1016/j.hydromet.2015.09.025.
Deng LP, Xu ZJ, Wang MM, Shentu HJ, Liu X, Xiong JW, Cheng YJ, Wang C, Zhou MJ, Gao J, Xia YG. CO2 treatment enables non-hazardous, reliable, and efficacious recovery of spent Li(Ni0.5Co0.2Mn0.3)O2 cathodes. Green Chem. 2022;24(2):779. https://doi.org/10.1039/d1gc02628c.
Tao R, Xing P, Li HQ, Cun ZG, Sun ZH, Wu YF. In situ reduction of cathode material by organics and anode graphite without additive to recycle spent electric vehicle LiMn2O4 batteries. J Power Sour. 2022;520:230827. https://doi.org/10.1016/j.jpowsour.2021.230827.
Xi XL, Feng M, Zhang LW, Nie ZR. Applications of molten salt and progress of molten salt electrolysis in secondary metal resource recovery. Int J Miner Metall Mater. 2020;27(12):1599. https://doi.org/10.1007/s12613-020-2175-0.
Uhlir J. Chemistry and technology of molten salt reactors - history and perspectives. J Nucl Mater. 2007;360(1):6. https://doi.org/10.1016/j.jnucmat.2006.08.008.
Wang MM, Tan QY, Liu LL, Li JH. Efficient separation of aluminum foil and cathode materials from spent lithium-ion batteries using a low-temperature molten salt. ACS Sustain Chem Eng. 2019;7(9):8287. https://doi.org/10.1021/acssuschemeng.8b06694.
Yao ZT, Li JH, Zhao XY. Molten salt oxidation: a versatile and promising technology for the destruction of organic-containing wastes. Chemosphere. 2011;84(9):1167. https://doi.org/10.1016/j.chemosphere.2011.05.061.
Zhao ZL, Li HB, Chen Y, Wu YM. Study on gasification characteristics of waste printed circuit boards (PCB) in molten salts. Acta Sci Circum. 2008;6:1161. https://doi.org/10.13671/j.hjkxxb.2008.06.020.
Xu Q, Wang Y, Shi XY, Zhong YJ, Wu ZG, Song Y, Wang GK, Liu YX, Zhong BH, Guo XD. The direct application of spent graphite as a functional interlayer with enhanced polysulfide trapping and catalytic performance for Li-S batteries. Green Chem. 2021;23(2):942. https://doi.org/10.1039/d0gc04033a.
Divya ML, Natarajan S, Lee YS, Aravindan V. Achieving high-energy dual carbon Li-ion capacitors with unique low- and high-temperature performance from spent Li-ion batteries. J Mater Chem A. 2020;8(9):4950. https://doi.org/10.1039/c9ta13913c.
Yuan MH, Wang HJ, Li HH, Yuan CL, Wang T, Yang HB. Deep eutectic solvent-a novel additive to induce gamma crystallization and alpha-to-gamma phase transition of PVDF. Macromol Chem Phys. 2022;223(4):2100416. https://doi.org/10.1002/macp.202100416.
Wang MM, Tan QY, Liu LL, Li JH. A low-toxicity and high-efficiency deep eutectic solvent for the separation of aluminum foil and cathode materials from spent lithium-ion batteries. J Hazar Mater. 2019;380:120846. https://doi.org/10.1016/j.jhazmat.2019.120846.
Chen XP, Li SZ, Wang Y, Jiang YZ, Tan X, Han WJ, Wang SB. Recycling of LiFePO4 cathode materials from spent lithium-ion batteries through ultrasound-assisted Fenton reaction and lithium compensation. Waste Manag. 2021;136:67. https://doi.org/10.1016/j.wasman.2021.09.026.
Wang MM, Tan QY, Liu LL, Li JH. Revealing the dissolution mechanism of polyvinylidene fluoride of spent lithium-ion batteries in waste oil-based methyl ester solvent. ACS Sustain Chem Eng. 2020;8(19):7489. https://doi.org/10.1021/acssuschemeng.0c02072.
Song X, Hu T, Liang C, Long HL, Zhou L, Song W, You L, Wu ZS, Liu JW. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017;7(8):4783. https://doi.org/10.1039/c6ra27210j.
Xu YA, Song DW, Li L, An CH, Wang YJ, Jiao LF, Yuan HT. A simple solvent method for the recovery of LixCoO2 and its applications in alkaline rechargeable batteries. J Power Sour. 2014;252:286. https://doi.org/10.1016/j.jpowsour.2013.11.052.
He K, Zhang ZY, Zhang FS. Synthesis of graphene and recovery of lithium from lithiated graphite of spent Li-ion battery. Waste Manag. 2021;124:283. https://doi.org/10.1016/j.wasman.2021.01.017.
Jiao CY, Sun L, Shao Q, Song JY, Hu Q, Naik N, Guo ZH. Advances in waterborne acrylic resins: synthesis principle, modification strategies, and their applications. ACS Omega. 2021;6(4):2443. https://doi.org/10.1021/acsomega.0c05593.
Nguyen VNH, Lee MS. Separation of Co(II), Ni(II), Mn(II) and Li(I) from synthetic sulfuric acid leaching solution of spent lithium ion batteries by solvent extraction. J Chem Technol Biotechnol. 2021;96(5):1205. https://doi.org/10.1002/jctb.6632.
Pham TP, Cho CW, Yun YS. Environmental fate and toxicity of ionic liquids: a review. Water Res. 2010;44(2):352. https://doi.org/10.1016/j.watres.2009.09.030.
Zheng HS, Huang JQ, Dong T, Sha YF, Zhang HT, Gao J, Zhang SJ. A novel strategy of lithium recycling from spent lithium-ion batteries using imidazolium ionic liquid. Chin J Chem Eng. 2022;41:246. https://doi.org/10.1016/j.cjche.2021.09.020.
Zeng XL, Li JH. Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries. J Hazard Mater. 2014;271:50. https://doi.org/10.1016/j.jhazmat.2014.02.001.
Tang SJ, Zhang M, Guo M. A novel deep-eutectic solvent with strong coordination ability and low viscosity for efficient extraction of valuable metals from spent lithium-ion batteries. Acs Sustain Chem Eng. 2022;10(2):975. https://doi.org/10.1021/acssuschemeng.1c06902.
Wang SB, Zhang ZT, Lu ZG, Xu ZH. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries. Green Chem. 2020;22(14):4473. https://doi.org/10.1039/d0gc00701c.
Wang K, Hu TY, Shi PH, Min YL, Wu JF, Xu QJ. Efficient recovery of value metals from spent lithium-ion batteries by combining deep eutectic solvents and coextraction. Acs Sustain Chem Eng. 2022;10(3):1149. https://doi.org/10.1021/acssuschemeng.1c06381.
Sekharan TR, Chandira RM, Tamilvanan S, Rajesh SC, Venkateswarlu BS. Deep eutectic solvents as an alternate to other harmful solvents. Biointerface Res Appl Chem. 2022;12(1):847. https://doi.org/10.33263/BRIAC121.847860.
Liu CY, Yan QB, Zhang XW, Lei LC, Xiao CL. Efficient recovery of end-of-life NdFeB permanent magnets by selective leaching with deep eutectic solvents. Environ Sci Technol. 2020;54(16):10370. https://doi.org/10.1021/acs.est.0c03278.
Pant D, Dolker T. Green and facile method for the recovery of spent lithium nickel manganese cobalt oxide (NMC) based lithium ion batteries. Waste Manag. 2017;60:689. https://doi.org/10.1016/j.wasman.2016.09.039.
Zhang XH, Xie YB, Cao HB, Nawaz F, Zhang Y. A novel process for recycling and resynthesizing LiNi1/3Co1/3Mn1/3O2 from the cathode scraps intended for lithium-ion batteries. Waste Manag. 2014;34(9):1715. https://doi.org/10.1016/j.wasman.2014.05.023.
Mu DY, Liu Z, Jin S, Liu YL, Tian S, Dai CS. The recovery and recycling of cathode materials and electrolyte from spent lithium ion batteries in full process. Prog Chem. 2020;32(7):950. https://doi.org/10.7536/PC191106.
Liu K, Zhang FS. Innovative leaching of cobalt and lithium from spent lithium-ion batteries and simultaneous dechlorination of polyvinyl chloride in subcritical water. J Hazard Mater. 2016;316:19. https://doi.org/10.1016/j.jhazmat.2016.04.080.
Fu YP, Schuster J, Petranikova M, Ebin B. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Resour Conserv Recycl. 2021;172:105666. https://doi.org/10.1016/j.resconrec.2021.105666.
Pavon S, Kaiser D, Mende R, Bertau M. The cool-process-a selective approach for recycling lithium batteries. Metals. 2021;11(2):259. https://doi.org/10.3390/met11020259.
Tufail A, Price WE, Hai FI. A critical review on advanced oxidation processes for the removal of trace organic contaminants: a voyage from individual to integrated processes. Chemosphere. 2020;260:127460. https://doi.org/10.1016/j.chemosphere.2020.127460.
Yan SX, Jiang YZ, Chen XP, Zhou T. Improved advanced oxidation process for in situ recycling of Al foils and cathode materials from spent lithium-ion batteries. Ind Eng Chem Res. 2022;61(34):12728. https://doi.org/10.1021/acs.iecr.2c01286.
Amelia D, Karamah EF, Mahardika M, Syafri E, Rangappa SM, Siengchin S, Asrofi M. Effect of advanced oxidation process for chemical structure changes of polyethylene microplastics. Mater Today-Proc. 2022;52:2501. https://doi.org/10.1016/j.matpr.2021.10.438.
Miao F, Liu YF, Gao MM, Yu X, Xiao PW, Wang M, Wang SG, Wang XH. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J Hazard Mater. 2020;399:123023. https://doi.org/10.1016/j.jhazmat.2020.123023.
Chen XP, Li SZ, Wu X, Zhou T, Ma HR. In-situ recycling of coating materials and Al foils from spent lithium ion batteries by ultrasonic-assisted acid scrubbing. J Clean Product. 2020;258:120943. https://doi.org/10.1016/j.jclepro.2020.120943.
Kayakool FA, Gangaja B, Nair S, Santhanagopalan D. Li-based all-carbon dual-ion batteries using graphite recycled from spent Li-ion batteries. Sustain Mater Technol. 2021;28:00262. https://doi.org/10.1016/j.susmat.2021.e00262.
Wang HF, Liu JS, Bai XJ, Wang S, Yang D, Fu YP, He YQ. Separation of the cathode materials from the Al foil in spent lithium-ion batteries by cryogenic grinding. Waste Manag. 2019;91:89. https://doi.org/10.1016/j.wasman.2019.04.058.
Liu JS, Wang HF, Hu TT, Bai XJ, Wang S, Xie WN, Hao J, He YQ. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Miner Eng. 2020;148:106223. https://doi.org/10.1016/j.mineng.2020.106223.
Liang SB, Hao YC. A novel cryogenic grinding system for recycling scrap tire peels. Adv Powder Technol. 2000;11(2):187. https://doi.org/10.1163/156855200750172303.
Liu Y, Yu HJ, Wang Y, Tang D, Qiu WX, Li WZ, Li J. Microwave hydrothermal renovating and reassembling spent lithium cobalt oxide for lithium-ion battery. Waste Manage. 2022;143:186. https://doi.org/10.1016/j.wasman.2022.02.024.
Usman M, Cheema SA, Farooq M. Heterogeneous Fenton and persulfate oxidation for treatment of landfill leachate: a review supplement. J Clean Product. 2020;256:120448. https://doi.org/10.1016/j.jclepro.2020.120448.
Chao YW, Liu BG, Zhang H, Tian SH, Zhang LB, Guo SH, Zhou BC. Efficient recovery and regeneration of waste graphite through microwave stripping from spent batteries anode for high-performance lithium-ion batteries. J Clean Product. 2022;333:130197. https://doi.org/10.1016/j.jclepro.2021.130197.
Fan WW, Zhang JL, Ma RX, Chen YQ, Wang CY. Regeneration of graphite anode from spent lithium-ion batteries via microwave calcination. J Electroanal Chem. 2022;908:116087. https://doi.org/10.1016/j.jelechem.2022.116087.
Fu YP, He YQ, Yang Y, Qu LL, Li JL, Zhou R. Microwave reduction enhanced leaching of valuable metals from spent lithium -ion batteries. J Alloy Compd. 2020;832:154920. https://doi.org/10.1016/j.jallcom.2020.154920.
Haccuria E, Crivits T, Hayes PC, Jak E. Selected phase equilibria studies in the Al2O3-CaO-SiO2 system. J Am Ceram Soc. 2016;99(2):691. https://doi.org/10.1111/jace.13991.
He K, Zhang ZY, Alai L, Zhang FS. A green process for exfoliating electrode materials and simultaneously extracting electrolyte from spent lithium-ion batteries. J Hazard Mater. 2019;375:43. https://doi.org/10.1016/j.jhazmat.2019.03.120.
Grützke M, Kraft V, Weber W, Wendt C, Friesen A, Klamor S, Winter M, Nowak S. Supercritical carbon dioxide extraction of lithium-ion battery electrolytes. J Supercrit Fluids. 2014;94:216. https://doi.org/10.1016/j.supflu.2014.07.014.
Sun L, Qiu KQ. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J Hazard Mater. 2011;194:378. https://doi.org/10.1016/j.jhazmat.2011.07.114.
Lei SY, Sun W, Yang Y. Solvent extraction for recycling of spent lithium-ion batteries. J Hazard Mater. 2022;424:127654. https://doi.org/10.1016/j.jhazmat.2021.127654.
Tong DG, Lai QY, Ji XY. Recycling of LiCoO2 cathode materials from spent lithium ion batteries. J Chem Ind Eng. 2005;56(10):1967.
Fomo G, Madzimbamuto TN, Ojumu TV. Applications of nonconventional green extraction technologies in process industries: challenges, limitations and perspectives. Sustainability. 2020;12(13):5244. https://doi.org/10.3390/su12135244.
Buszewski B, Wrona O, Mayya RP, Zakharenko AM, Kalenik TK, Golokhvast KS, Piekoszewski W, Rafińska K. The potential application of supercritical CO2 in microbial inactivation of food raw materials and products. Critic Rev Food Sci Nutr. 2021;62(24):6535. https://doi.org/10.1080/10408398.2021.1902939.
Liu YL, Mu DY, Li RH, Ma QX, Zheng RJ, Dai CS. Purification and characterization of reclaimed electrolytes from spent lithium-ion batteries. J Phys Chem C. 2017;121(8):4181. https://doi.org/10.1021/acs.jpcc.6b12970.
Rothermel S, Evertz M, Kasnatscheew J, Qi X, Gruetzke M, Winter M, Nowak S. Graphite recycling from spent lithium-ion batteries. Chemsuschem. 2016;9(24):3473. https://doi.org/10.1002/cssc.201601062.
Jung S, Kwon D, Park S, Kwon K, Tsang YF, Kwon EE. Valorization of a spent lithium-ion battery electrolyte through syngas formation using CO2-assisted catalytic thermolysis over a battery cathode material. J CO2 Utilization. 2021;50: 101591. https://doi.org/10.1016/j.jcou.2021.101591.
Zhong XH, Liu W, Han JW, Jiao F, Qin WQ, Liu T, Zhao CX. Pyrolysis and physical separation for the recovery of spent LiFePO4 batteries. Waste Manag. 2019;89:83. https://doi.org/10.1016/j.wasman.2019.03.068.
Yi XX, Qi YP, Li FF, Shu JC, Sun Z, Sun SH, Chen MJ, Pu SY. Effect of electrolyte reuse on metal recovery from waste CPU slots by slurry electrolysis. Waste Manag. 2019;95:370. https://doi.org/10.1016/j.wasman.2019.06.034.
Umicore recycling division home page. 2022. https://www.umicore.cn/ (Accessed Aug 1, 2022).
Mayyas A, Steward D, Mann M. The case for recycling: overview and challenges in the material supply chain for automotive li-ion batteries. Sustain Mater Technol. 2019;19:e00087. https://doi.org/10.1016/j.susmat.2018.e00087.
Retriev technologies home page. 2022. https://www.retrievtech.com/ (Accessed Aug 1, 2022).
Jin S, Mu DY, Lu Z, Li RH, Liu Z, Wang Y, Tian S, Dai CS. A comprehensive review on the recycling of spent lithium-ion batteries: urgent status and technology advances. J Clean Product. 2022;340:130535. https://doi.org/10.1016/j.jclepro.2022.130535.
Sonoc A, Jeswiet J, Soo VK. Opportunities to improve recycling of automotive lithium ion batteries. 22nd cirp conference on life cycle engineering. 2015;29:752. https://doi.org/10.1016/j.procir.2015.02.039.
Accurec battery recycling division home page. 2022. https://accurec.de/geschichte (2022.8.1).
Recupyl battery recycling division home page. 2022. www.recupyl.com (2022.8.1).
Batrec home page. 2022. https://batrec.ch/en/ (2022.8.1).
Hanisch C, Diekmann J, Stieger A, Haselrieder W, Kwade A. Recycling of lithium-ion batteries. In handbook of clean energy systems. 2015:1. https://doi.org/10.1002/9781118991978.hces221.
AkkuSer Oy Home page. 2022. https://www.akkuser.fi/en/home/ (2022.8.1).
Green eco-manufacturer home page. 2022. http://www.gemchina.com/ (2022.8.1).
Brunp home page. 2022. https://www.brunp.com.cn/ (2022.8.1).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 52074177 and 52174391) and Hunan Provincial Science and Technology Plan, China (No. 2017TP1001). The authors also appreciate the editor(s) and anonymous reviewer(s) with gratitude for their professional comments and constructive suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g., a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, SX., Jiang, YZ., Chen, XP. et al. Engineering classification recycling of spent lithium-ion batteries through pretreatment: a comprehensive review from laboratory to scale-up application. Rare Met. 43, 915–941 (2024). https://doi.org/10.1007/s12598-023-02377-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-023-02377-y