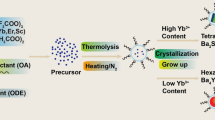
Abstract
Impurity doping not only provides a fundamental approach to impart unique electronic, magnetic and optical properties to target nanomaterials, but also has critical influence on nucleation and growth of many functional nanocrystals. In the current study, Y3+ and Sc3+ were adopted to tune the shape, size, and upconversion luminescence properties of Na3ScF6:Yb/Er and NaYF4:Yb/Er samples, respectively. When Y3+ doping concentration was lower than 10 mol%, the size and shape of Na3ScF6:Yb/Er (18/2) nanoparticles gradually changed from ~ 18 nm rhombus to 36 nm spheres. Subsequently, the co-existence of Na3ScF6:Yb/Er and NaYF4:Yb/Er nanoparticles was observed during 20 mol%–50 mol% Y3+ doping, and finally NaYF4:Yb/Er nanoparticles became the only product at > 60 mol% Y3+ doping where the role of Sc3+ turned into dopant and the size of nanocrystals decreased from ~ 30 to 20 nm gradually. Both Y3+ and Sc3+ ions doping could enhance the upconversion luminescence intensity of Na3ScF6:Yb/Er and NaYF4:Yb/Er samples, for which possible mechanism was proposed from the perspective of crystal structure. Finally, the upconversion luminescence details were disclosed.
Graphical abstract

摘要
掺杂不仅能赋予目标纳米材料独特的电学、磁性和光学特性, 还会影响许多功能纳米晶体的成核和生长。本文通过掺杂Y3+和Sc3+, 分别调控Na3ScF6:Yb/Er和NaYF4:Yb/Er试样的形貌、尺寸及上转换发光性能。当Y3+掺杂浓度低于10 mol%时, Na3ScF6:Yb/Er(18/2)纳米晶的大小和形貌由18 nm左右的菱形逐渐演变为36 nm左右的球形。随后, 在20 mol% ~ 50 mol% Y3+掺杂过程中, 发现单斜相Na3ScF6:Yb/Er和六方相NaYF4:Yb/Er纳米晶共存。最终在 > 60 mol% Y3+掺杂时, 只有六方相NaYF4:Yb/Er纳米晶存在。此时, Sc3+的角色转变为掺杂离子, 纳米晶的尺寸从 ~ 30 nm逐渐减小到20 nm。此外, Y3+和Sc3+掺杂均能增强Na3ScF6:Yb/Er和NaYF4:Yb/Er样品的上转换发光强度。我们从晶体结构的角度提出了可能的机制。最后, 通过对上转换发光过程及发光寿命的表征, 我们揭示了纳米晶上转换发光细节。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare-earth-based upconversion (UC) nanoparticles, enabling two or more low energy photons to convert into higher energy photons, have emerged as a unique kind of luminescent nanomaterials in the past decades [1,2,3,4,5,6,7,8]. The unique optical properties of UC nanoparticles, including high photostability, low background, fingerprint emission and tunable luminescent lifetime, make them promising candidates for sensing, anti-counterfeiting, and bioimaging [9,10,11,12,13,14]. Moreover, by integrating the luminescent features with other properties like magnetic properties, UC nanoparticles offer an opportunity to construct a multifunctional platform on a single particle for the rising theranostics [15,16,17].
Among various rare-earth-doped UC nanomaterials, Y-based fluorides are recognized as the most efficient ones for their wide band gap (> 10 eV), low phonon energy (350 cm−1), good thermal stability and environmental safety [18,19,20], which can greatly minimize the non-irradiative relaxation during the photon UC process, thus acquiring higher efficient luminescence emission and having been widely used as both UC and even down-shifting host matrixes for various applications [21,22,23,24,25,26]. However, there is almost no study on the impact of Y3+ ions acting as dopants on crystal structure and UC luminescence (UCL) properties of nanocrystals containing other rare-earth elements to the best of our knowledge.
Very recently, continuous efforts have been devoted to the scandium (Sc)-based luminescent nanomaterials, owing to the distinct electronic configuration of Sc3+ [27,28,29,30,31]. Notably, as the smallest rare-earth element, lanthanide-doped Sc-based compounds exhibit different optical behaviors compared with those of the rest, such as NaYF4 nanomaterials. For example, NaScF4:Yb/Er generates intensive red emission under 980 nm excitation, while NaYF4:Yb/Er nanoparticles emit strong green light under identical conditions, induced by the different crystal structures of host materials and small ionic radii of Sc3+ [28, 32]. Moreover, by changing solvent polarity, hetero-structured Na3ScF6@NaScF4 core-shell nanoparticles can be obtained, which indicates the potentiality for controlled synthesis of Sc-based UC nanoparticles [32]. Despite the advances in Sc-based nanomaterials, there are rare achievements on their size, morphology and crystal structure manipulation toward optical property tuning. In addition, it is still an urgent challenge to improve their luminescence efficiency. A variety of methods have been adopted to enhance the UC intensity, such as bringing in a co-dopant sensitizer [33, 34], constructing core-shell structures [35, 36], host lattice manipulation [37, 38], broadband sensitization [39], surface passivation and so on [40, 41]. Among these methods, introducing dopants into host lattice to change the lattice symmetry is useful to enhance UCL intensity. Moreover, due to the small ionic radius and the same valence, Sc3+ ions can also easily enter into the crystal lattices of other rare-earth-based nanomaterials as dopants for modulation of crystallographic parameters toward UCL tuning [42, 43].
Herein, we firstly introduced Y3+ as dopants into Na3ScF6:Yb/Er (18/2) system to tune the morphology, size, and UCL features. With the increasing of Y3+ concentration, hexagonal NaYF4:Yb/Er nanoparticle became the only product while the Sc3+ began to act as dopants. For NaYF4:Yb/Er/Sc (18/2/x mol%) systems, the variations of size, morphology and UCL intensity were also explored in detail. The crystal structure variation induced by doping from monoclinic Na3ScF6:Yb/Er to hexagonal NaYF4:Yb/Er was carefully illustrated in Scheme 1. Then we obtained nanomaterials with strong red emission and green emission, respectively. The possible inherent mechanism for enhanced luminescence intensity was proposed. Such tri-valent ions doping may give rise to interesting physical and chemical properties which are difficult to realize by mono- or bi-valent ions doping, such as Li+ [44], Na+ [45], or Zn2+/Ca2+ [46,47,48].
2 Experimental
2.1 Materials
Scandium (III) chloride hydrate (99.99 wt%), yttrium (III) chloride hydrate (99.99 wt%), erbium (III) chloride hydrate (99.99 wt%), ytterbium (III) chloride hydrate (99.99 wt%), sodium hydroxide (98 wt%), ammonium fluoride (98 wt%), methanol, cyclohexane, 1-octadecene (90 wt%) and oleic acid (90 wt%) were obtained from Sigma-Aldrich and used as received without further purification.
2.2 Characterization
Powder X-ray diffraction (XRD) analysis was carried out on a Bruker D8-advance diffractometer, operated at 40 kV and 40 mA with the monochromatized Cu Kα radiation (λ = 0.15406 nm) and the measurement was performed at ambient temperature in the range of 2θ = 10°–80° with 5 (°)·min−1. Transmission electron microscopy (TEM) measurements were conducted on a Hitachi 7700 transmission electron microscope at an acceleration voltage of 100 kV. High resolution TEM (HRTEM) images and energy-dispersive X-ray (EDX) spectra were acquired on a Tecnai G2 F20 microscope. Elemental analysis of rare-earth ions was conducted by inductively coupled optical emission spectrometer (ICP-OES) analysis on a PE Optima 5300DV spectrometer. The UCL properties of Na3ScF6:Yb/Er/Y (18/2/x mol%) and NaYF4:Yb/Er/Sc (18/2/x mol%) were characterized by Edinburgh F920 fluorescent spectrometer, which was equipped with a diode laser (980 nm). The pumping power of laser was fixed at a value of 20 mW for all samples.
2.3 Nanocrystals synthesis
In a typical experiment, 1 mmol RECl3·6H2O (x mol% Y, (80 − x) mol% Sc, 18 mol% Yb, 2 mol% Er) were added into a 50 ml three-necked flask containing oleic acid (12.5 ml) and 1-octadecene (12.5 ml) with magnetic stirring. The resulting mixture was heated to 150 °C and kept for 1 h under vigorous stirring before cooling down to 70 °C. Then, 10 ml methanol solution containing of 0.222 g NH4F and 0.12 g NaOH was put into the flask. Subsequently, the resulting mixture was stirred for 30 min to remove the methanol, the solution was then heated to 300 °C under an N2 environment for 1.5 h and cooled down to room temperature. Afterward, the reaction mixture was precipitated by addition of ethanol and collected by centrifugation for 5 min at 9000 r·min−1. The as-synthesized nanocrystals were washed with ethanol and water for four times and finally redispersed in cyclohexane for future use.
3 Results and discussion
TEM images shown in Fig. 1a–c indicate that the morphologies and sizes of as-synthesized Na3ScF6:Yb/Er/Y (18/2/x mol%) nanoparticles obviously change when Y3+ doping concentration is increased. Rhombus-like Na3ScF6:Yb/Er (18/2 mol%) nanocrystals were obtained at 0 mol% Y3+ doping, of which the average size is ~ 18 nm. Then the final products changed to cubes with the average size of ~ 30 nm at 5 mol% Y3+ doping. Nanospheres with average size of ~ 36 nm could be obtained if we kept increasing the doping concentration of Y3+ to 10 mol% (Fig. S1). XRD data in Fig. 1d indicate that the samples doping Y3+ ions at 0–10 mol% are all pure monoclinic phase Na3ScF6. The insets in Fig. 1a–c are HRTEM images of single Na3ScF6:Yb/Er/Y (18/2/x mol%) nanoparticles, indicating that the fringe distances are all ~ 0.47 nm and agree well with the lattice spacing of (011) planes of monoclinic Na3ScF6 crystals. Above results of XRD data and HRTEM analysis illustrate that introduction of Y3+ at 0–10 mol% did not alter the monoclinic single crystallinity of the final products and that Er3+, Yb3+ and Y3+ were successfully doped into Na3ScF6 crystal lattices, as confirmed by elemental mapping (Figs. S2 and S3) and ICP-OES analysis (Table S1), where the actual Y3+ concentrations doped in Na3ScF6:Yb/Er/Y (18/2/x mol%) samples increase with the theoretical contents, though it is always lower than the feed value.
Under 980 nm laser excitation, the characteristic emission peaks of Er3+ centering at 545 and 654 nm (Fig. 1e) were observed for samples of Na3ScF6:Yb/Er/Y (18/2/x mol%, x = 0, 5, 10). The two emission bands could be ascribed to radiative energy transfer from 2H11/2, 4S3/2 and 4F9/2 levels of Er3+ to its 4I15/2 level, respectively. The emission focused on 654 nm was much stronger than that of 545 nm. Herein, stronger red UC emission were observed from all of Na3ScF6:Yb/Er/Y (18/2/x mol%, x = 0, 5, 10) samples, among which Na3ScF6:Yb/Er/Y (18/2/5 mol%) shows the strongest luminescence, Na3ScF6:Yb/Er/Y (18/2/10 mol%) ones are at the medium level, and Na3ScF6:Yb/Er (18/2 mol%) nanocrystals give the weakest. The intensity of Na3ScF6:Yb/Er/Y (18/2/5 mol%) sample is about 5 times as much as that of Na3ScF6:Yb/Er (18/2 mol%), which can be seen from Fig. S4a. Herein, Y3+ doping can effectively enhance the UCL intensity of Na3ScF6:Yb/Er (18/2 mol%) samples. It is worth pointing out that, though Y3+ introduction-induced particle size variation also gives rise to UCL enhancement because the surface defect density of corresponding sample reduces, it only takes up a very small portion because 10 mol% Y3+ doping nanocrystals own the largest size while the maximum enhancement is observed at 5 mol% Y3+ doping. Such comparison was carefully made by keeping other test conditions identical, which is helpful and straightforward to choose the most suitable one for further applications, such as biosensing, bioimaging and so on. The red to green ratio (R/G) also increases from 3.66 in Na3ScF6:Yb/Er (18/2 mol%) to 10.29 in Na3ScF6:Yb/Er/Y (18/2/5 mol%) and 4.88 in Na3ScF6:Yb/Er/Y (18/2/10 mol%) (Fig. S4b).
When Y3+ doping concentrations were set as 20 mol%–50 mol%, mixture was obtained. Corresponding TEM images, HRTEM images and XRD patterns are listed in Fig. 2. Small amount of nanospheres (Fig. 2a) which were recognized as hexagonal NaYF4:Yb/Er according to XRD patterns (Fig. 2g), started to show up when Y3+ doping concentration was higher than 20 mol% though monoclinic Na3ScF6:Yb/Er nanocrystals were dominant. Then, the amount of hexagonal NaYF4:Yb/Er samples kept increasing, which was accompanied with the decrease in monoclinic Na3ScF6:Yb/Er nanoparticles at increased Y3+ doping concentration until at 50 mol% Y3+ (Fig. 2g). Taking Na3ScF6:Yb/Er/Y (18/2/20 mol%) samples as an example, HRTEM images (Fig. 2e, f) corresponding to nanosphere and nanocubes selected from Fig. 2a show typical crystalline interplanar spacing of hexagonal NaYF4 and monoclinic Na3ScF6, which were identified as 0.52 and 0.47 nm and matched well with (100) facet of hexagonal NaYF4 crystals and (011) facet of monoclinic Na3ScF6, respectively. Herein, the two phased nanocrystals grew by themselves and existed as a physical mixture, rather than, for example, as core–shelled structures.
Typical TEM images of Na3ScF6:Yb/Er/Y (18/2/x mol%) nanocrystals with Y3+ at a 20 mol%, b 30 mol%, c 40 mol%, d 50 mol% doping concentrations, respectively; e, f HRTEM images of nanocrystals selected from a showing hexagonal NaYF4 and monoclinic Na3ScF6 crystal lattice constants, respectively; g XRD patterns of Na3ScF6:Yb/Er/Y (18/2/x mol%) nanocrystals
Further increasing Y3+ doping concentration to 60 mol%, monoclinic Na3ScF6 disappeared. TEM images, XRD patterns and UC luminescence spectra of pure hexagonal NaYF4 nanocrystals are presented in Fig. 3. Uniform nanospheres were gained at 60 mol% Y3+ doping concentration (Fig. 3a), of which the average size was ~ 30 nm and XRD data indicated that monoclinic Na3ScF6:Yb/Er nanocrystals completely transformed into hexagonal NaYF4:Yb/Er ones (Fig. 3e). Further increasing Y3+ concentration to 70 mol%, 75 mol% and even 80 mol% would not result in obvious morphology or phase structure variation of hexagonal NaYF4:Yb/Er ones (Fig. 3b–e) though the sizes of final products kept decreasing. Figure S5 shows that the sizes of NaYF4:Yb/Er/Sc (18/2/x mol%) samples with Y3+ concentrations of 70 mol%, 75 mol% and 80 mol% focus on 28, 24 and 22 nm, respectively. Compare the gradual variation of XRD patterns in Fig. 3e carefully, we found that diffraction peaks agree well with the standard hexagonal NaYF4 (JCPDS No. 16-0334) and no impure diffraction peaks are seen at 60 mol%–80 mol% Y3+ doping concentration. Actually, hexagonal NaYF4 became the host crystals while Sc3+ began to act as dopant and NaYF4:Yb/Er/Sc nanospheres were the final products during this stage, as confirmed by elemental mapping (Figs. S6 and S7) and the ICP results (Table S2). Under 980 nm laser excitation, NaYF4:Yb/Er/Sc (18/2/x mol%) nanocrystals all produce strong green UC emission (Fig. 3f), which is contrary to Na3ScF6:Yb/Er (18/2 mol%) nanoparticles generating strong red UC emission. The maximum UCL intensity enhancement of NaYF4:Yb/Er/Sc (18/2/x mol%) samples was obtained at 75 mol% Y3+ contents, namely, i.e., at 5 mol% Sc3+ doping where ~ 10 times enhancement was observed. When the concentration of Y3+ was 80 mol% and no Sc3+ doping, the sample shows the lowest luminescence intensity, suggesting that introduction of Sc3+ into NaYF4:Yb/Er (18/2) nanocrystals can also enhance UCL intensity. The integrated intensity of the samples can be directly observed from Fig. S8a. R/G ratio also varied with Y3+ contents increasing which is show in Fig. S8b.
According to the discussion above, we reason that the introduction of Y3+ or Sc3+ caused nanocrystal structures variation (expansion or contraction) takes responsibility for the UC emission enhancement. The introduction of dopants disturbed the local symmetry and resulted in changes in the crystal field. Consequently, the distance between sensitizer (Er3+) and activator (Yb3+) was affected, leading to energy transfer probabilities variation. Herein, UC emission intensity could be tuned [38]. To prove this viewpoint, we zoomed in the part of XRD patterns of Na3ScF6:Yb/Er/Y(18/2/x mol%) samples at 2θ of 20°–24°, finding that the positions of the diffraction peaks regularly shifted when Y3+ doping contents increased. As exhibited in Fig. 4a, the dominant diffraction peak at 22.038° which corresponds to (110) crystal planes of monoclinic phase Na3ScF6:Yb/Er/Y (18/2/x mol%) samples gradually moves to smaller angle with Y3+ contents increasing from 0 to 10 mol%. For the situation of NaYF4:Yb/Er/Sc (18/2/x mol%) samples, we pay attention to XRD patterns at 17.204° (Fig. 4b) which correspond to (100) facets of hexagonal phase NaYF4 nanoparticles. Obviously, the diffraction peaks move to small angle during the range of 60 mol%–80 mol% Y+ concentrations. That is to say, the diffraction peaks of NaYF4:Yb/Er/Sc (18/2/x mol%) nanocrystals shift gradually to larger angle with Sc3+ doping concentrations increasing. According to Bragg’s Law:
where n is the order of reflection, λ is the wavelength of the incident wave (Cu Kα, 0.154 nm), d is the interplanar distance, and θ is the angle between the incident wave and the scattering planes. Herein, the enlarged interplanar crystal spacing will induce shifting toward a small diffraction angle, and vice versa. The effective ionic radius of Y3+ is 0.106 nm, which is close to that of Sc3+ (0.083 nm) [32]. Considering the same valence, it is easy for Y3+ and Sc3+ to substitute each other as dopants. For the Na3ScF6:Yb/Er/Y (18/2/x mol%) system, Y3+ with larger ionic radius act as dopants, which results in larger interplanar crystal spacing, i.e., smaller diffraction angle. This is consistent with XRD results (Fig. 4a). As to NaYF4:Yb/Er/Sc (18/2/x mol%) nanoparticles, Sc3+ become dopants, the unit cell of NaYF4 would shrink, making XRD peak shift toward bigger angles according to the Bragg’s law, which agrees well with XRD patterns in Fig. 4b. Obviously, the introduction of dopants into host crystal structures will bring about changes in host lattice. Consequently, the local crystal field symmetry around emitters ions (Er3+) was affected, which was anticipated to regulate their radiation parameters and enhance corresponding UCL intensity.
To explore the details of Y3+ or Sc3+ as dopants induced UCL enhancement, the excitation power-dependent UC emissions were characterized (Fig. 5). In general, during the unsaturated UC process, the photon numbers consumed for giving rise to an UC emission band can be described as the following equation:
where Iup represents the UCL intensity, P represents the pumping laser power and n represents the number of required pumping photons. Herein, the value of n can be acquired from the slope of the linear plots between lnIup and lnP. For the case of Na3ScF6:Yb/Er/Y (18/2/x mol%), corresponding results are listed in Fig. 5a–c, which indicate that UC emissions fixing on 545 and 654 nm all abide by a two-photon process for the nanocrystals at Y3+ contents of 0 mol%, 5 mol% and 10 mol%, respectively. Figure S9a–d lists the photon numbers acquired for NaYF4:Yb/Er/Sc nanocrystals with Y3+ contents at 60 mol%, 70 mol%, 75 mol% and 80 mol%. Also, both the green (545 nm) and red (654 nm) emissions follow two-photon UC processes no matter doping without or with Sc+ at various contents. Herein, UC emission mechanism was not changed by Y3+ or Sc3+ doping. The outstanding variation in R/G ratios from Na3ScF6:Yb/Er/Y to NaYF4:Yb/Er/Sc samples was due to difference in the host crystal structures and the ionic radii of Y3+ (0.106 nm) and Sc3+ (0.083 nm). On the one hand, the hexagonal NaYF4 (JCPDS No. 16-0334) with the crystal lattice parameters of a = b = 0.596 nm and c = 0.353 nm tends to the energy transfer from 4S3/2 and 2H11/2 to 4I15/2, generating obvious green UC luminescence, which is exhibited in Fig. 3f [26]. On the other hand, Sc3+–Sc3+ distance is much shorter than that of Y3+–Y3+, which will create closer Er3+–Yb3+ cation pairs in Na3ScF6 system, which may result in much strong red emission [32].
Figure 5d depicts the energy level diagrams and UC processes of Er3+ and Yb3+ where Yb3+ was excited to the 2F5/2 level by absorbing a 980 nm photon. The corresponding energy was transferred to 4I11/2 level of nearby Er3+ and went back to its ground state. Population on the metastable 4I11/2 level, Er3+ can either relax to its lower state 4I13/2 through nonradiative transfer process or directly arrive at its higher energy level 4F7/2 by absorbing another photon at 980 nm. From 4I13/2 energy level, Er3+can reach to its higher energy level 4F9/2 by absorbing a 980 nm photon. Populating at 4F7/2 state, Er3+ will emit 525 nm, 545 nm photons via relaxing to 2H11/2 and 4S3/2 level and then return to the ground state 4I15/2. Meanwhile, the transitions from 4F9/2 level to the ground state of Er3+ produce 654 nm photons.
As for the great difference in R/G ratio of monoclinic Na3ScF6:Yb/Er and hexagonal NaYF4:Yb/Er, we speculate that the crystal structure of host material plays a crucial role. As the smallest rare-earth element whose ion radius was 0.083 nm (RSc = 0.083 nm), Sc-based materials may present shorter interatomic distances than other bigger rare-earth elements involved materials when present in the similar fluoride-bridged moieties inside the host lattice structure. Once Er3+ are introduced, they will occupy Sc sites. Consequently, red UC emission is realized for that relatively short Er3+–Er3+ distance strengthen the cross-relaxation between Er3+, which promote the photon population on 4F9/2 level of Er3+ (Fig. S10). Thus, monoclinic Na3ScF6:Yb/Er with relatively shorter Er3+−Er3+ distance shows red UC luminescence and hexagonal NaYF4:Yb/Er shows green UC luminescence [28, 32, 49, 50].
To further explore UC process and inherent mechanism, Er3+ decay curves for Na3ScF6:Yb/Er (18/2 mol%) and NaYF4:Yb/Er (18/2 mol%) samples were characterized. The UCL lifetimes of Er3+ ions were recorded at 545 and 654 nm under 980 nm excitation, as shown in Fig. S11. Corresponding lifetime fitting parameters are presented in Table S3 and Table S4. The decay behaviors of Er3+ all show second-order exponential decay, which can be described by:
where I is the emission intensity, t is the time, τ1 and τ2 are the shorter and longer lifetime constants, and A1, A2 are fitting parameters which represent the original contributions for the shorter and longer decay time. Using these parameters, the average decay time can be determined by the following formula [51]:
The lifetime values of 4F9/2–4I15/2 and 2H11/2/4S3/2–4I15/2 transitions for Er3+ were calculated in NaYF4:Yb/Er and Na3ScF6:Yb/Er nanocrystals, respectively. As shown in Tables S3 and S4, the effective lifetime values of 545/654 nm were determined to be 445/864 and 95/148 μs in Na3ScF6:Yb/Er (18/2 mol%) and NaYF4:Yb/Er (18/2 mol%), respectively. As is well known, the lifetime constant is the reverse of the sum of the radiative and nonradiative transition rates. Compared with NaYF4:Yb/Er nanocrystals, the shorter Yb3+–Er3+ distance in Na3ScF6:Yb/Er enhances energy transfer from Yb3+ to Er3+, which favors the phonon population of both 4S3/2/2H11/2 and 4F5/2 levels. Herein, it’s reasonable that both the luminescence lifetimes of 545 and 654 nm are longer than that in NaYF4:Yb/Er [49, 50]. By the way, for that the shorter Er3+-Er3+ distance in Na3ScF6:Yb/Er also favor the cross-relaxation process (4F7/2 + 4I11/2 − 4F9/2 + 4F9/2) of Er3+, much more enhanced luminescence lifetime of 654 nm (from 148 to 864 μs) is observed compared with 545 nm (from 95 to 445 μs).
4 Conclusion
Precise-tuning of the crystal structure, size, morphology, and UCL of Na3ScF6:Yb/Er/Y and NaYF4:Yb/Er/Sc has been realized through controlling Y3+ concentration, where ~ 5 and 10 times UCL enhancement was obtained in Na3ScF6:Yb/Er and NaYF4:Yb/Er samples, respectively. Based on sufficient experimental details, the possible mechanism for enhanced luminescence intensity was proposed from the point view of nanocrystal structure. The current work has further disclosed the key function of Y3+ and Sc3+ in the controlled synthesis and property modulation of rare-earth-based UC nanocrystals and may pave a new way for us to select new materials with unique UCL property.
References
Ge H, Wang D, Pan Y, Guo Y, Li H, Zhang F, Zhu X, Li Y, Zhang C, Huang L. Sequence-dependent DNA functionalization of upconversion nanoparticles and their programmable assemblies. Angew Chem Int Ed. 2020;59(21):8133. https://doi.org/10.1002/anie.202000831.
Chen HW, Weng XY, Ma ZJ, Guan ZH. Microwave absorption properties of Mn-Zn ferrite with different Pr3+ content. Chin J Rare Met. 2020;44(12):1339. https://doi.org/10.13373/j.cnki.cjrm.XY19050029.
Wen S, Zhou J, Schuck PJ, Suh YD, Schmidt TW, Jin D. Future and challenges for hybrid upconversion nanosystems. Nat Photonics. 2019;13(12):828. https://doi.org/10.1038/s41566-019-0528-x.
Wu Y, Xu J, Poh ET, Liang L, Liu H, Yang JKW, Qiu C, Vallee RAL, Liu X. Upconversion superburst with sub-2 mus lifetime. Nat Nanotechnol. 2019;14(12):1110. https://doi.org/10.1038/s41565-019-0560-5.
Zhong Y, Rostami I, Wang Z, Dai H, Hu Z. Energy migration engineering of bright rare-earth upconversion nanoparticles for excitation by light-emitting diodes. Adv Mater. 2015;27(41):6418. https://doi.org/10.1002/adma.201502272.
Zhuo Z, Liu Y, Liu D, Huang P, Jiang F, Chen X, Hong M. Manipulating energy transfer in lanthanide-doped single nanoparticles for highly enhanced upconverting luminescence. Chem Sci. 2017;8(7):5050. https://doi.org/10.1039/C7SC01393K.
Xie JH, Wang J, Qiu GH, Li XB, Huang WT, Zhang RR, Lin T, Wang LX, Zhang QT. A strategy to achieve efficient green-emission dual-mode luminescence of Yb3+, Er3+ doped NaBiF4. Rare Met. 2021;40(8):2040. https://doi.org/10.1007/s12598-020-01570-7.
Li X, Ma H, Wang XD, Chen FF, Zeng XH. Structural and luminescent properties of Eu, Mg co-doped GaN. Chin J Rare Met. 2020;44(11):1170. https://doi.org/10.13373/j.cnki.cjrm.XY19040031.
Li H, Tan M, Wang X, Li F, Zhang Y, Zhao L, Yang C, Chen G. Temporal multiplexed in vivo upconversion imaging. J Am Chem Soc. 2020;142(4):2023. https://doi.org/10.1021/jacs.9b11641.
Kong MY, Gu YY, Liu YL, Shi YB, Wu N, Feng W, Li FY. Luminescence lifetime–based in vivo detection with responsive rare earth–dye nanocomposite. Small. 2019;15(46):1904487. https://doi.org/10.1002/smll.201904487.
Wang J, Wei T, Li X, Zhang B, Wang J, Huang C, Yuan Q. Near-infrared-light-mediated imaging of latent fingerprints based on molecular recognition. Angew Chem Int Ed. 2014;53(6):1616. https://doi.org/10.1002/ange.201308843.
Yi Z, Luo Z, Qin X, Chen Q, Liu X. Lanthanide-activated nanoparticles: a toolbox for bioimaging, therapeutics, and neuromodulation. Acc Chem Res. 2020;53(11):2692. https://doi.org/10.1021/acs.accounts.0c00513.
Zhou L, Fan Y, Wang R, Li X, Fan L, Zhang F. High-capacity upconversion wavelength and lifetime binary encoding for multiplexed biodetection. Angew Chem Int Ed. 2018;57(39):12824. https://doi.org/10.1002/anie.201808209.
Mahata MK, Koppe T, Kumar K, Hofsäss H, Vetter U. Upconversion photoluminescence of Ho3+-Yb3+ doped barium titanate nanocrystallites: optical tools for structural phase detection and temperature probing. Sci Rep. 2020;10(1):8775. https://doi.org/10.1007/s12274-018-2159-9.
Cong TD, Wang ZM, Hu M, Han QY, Xing BG. Extra-specific manifestation of nanoheater’s position effect on distinctive cellular photothermal responses. ACS Nano. 2020;14(5):5836. https://doi.org/10.1021/acsnano.0c00951.
Liu Y, Wang D, Shi J, Peng Q, Li Y. Magnetic tuning of upconversion luminescence in lanthanide-doped bifunctional nanocrystals. Angew Chem Int Ed. 2013;52(16):4366. https://doi.org/10.1002/ange.201209884.
Lucky SS, Muhammad IN, Li Z, Huang K, Soo KC, Zhang Y. Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy. ACS Nano. 2015;9(1):191. https://doi.org/10.1021/nn503450t.
Weber MJ. Probabilities for radiative and nonradiative decay of Er3+ in LaF3. Phy Rev. 1967;157(2):262. https://doi.org/10.1103/physrev.157.262.
Xie J, Gao Z, Zhou E, Cheng X, Wang Y, Xie X, Huang L, Huang W. Insights into the growth mechanism of REF3 (RE = La-Lu, Y) nanocrystals: hexagonal and/or orthorhombic. Nanoscale. 2017;9(41):15974. https://doi.org/10.1039/C7NR06210A.
Xie J, Hu W, Tian D, Wei Y, Zheng G, Huang L, Liang E. Selective growth and upconversion photoluminescence of Y-based fluorides: from NaYF4:Yb/Er to YF3:Yb/Er crystals. Nanotechnol. 2020;31(50):505605. https://doi.org/10.1088/1361-6528/abb627.
Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev. 2014;114(10):5161. https://doi.org/10.1021/cr400425h.
Gai S, Li C, Yang P, Lin J. Recent progress in rare earth micro/nanocrystals: soft chemical synthesis, luminescent properties, and biomedical applications. Chem Rev. 2014;114(4):2343. https://doi.org/10.1021/cr4001594.
Gnach A, Lipinski T, Bednarkiewicz A, Rybka J, Capobianco JA. Upconverting nanoparticles: assessing the toxicity. Chem Soc Rev. 2015;44(6):1561. https://doi.org/10.1039/C4CS00177J.
Fu R, Hu YY, Qiao HN, Yang CL, Yin H, Ou MG. Luminescence property and magnetic resonance imaging of Gd2O3:Tb3+ nanocrystals doped with Zn2+, Li+. Rare Met. 2021;40(8):2049. https://doi.org/10.1007/s12598-020-01591-2.
Sun L, Wei R, Feng J, Zhang H. Tailored lanthanide-doped upconversion nanoparticles and their promising bioapplication prospects. Coord Chem Rev. 2018;364:10. https://doi.org/10.1016/j.ccr.2018.03.007.
Zuo SL, Chen P, Pan CF. Mechanism of magnetic field-modulated luminescence from lanthanide ions in inorganic crystal: a review. Rare Met. 2020;39(10):1113. https://doi.org/10.1007/s12598-020-01450-0.
Xie J, Zheng GC, Hu YM, Nosheen F, Zhang ZC, Liang EJ. Insight into crystal growth and upconversion luminescence property of tetragonal Ba3Sc2F12 nanocrystals. Rare Met. 2021;40(1):113. https://doi.org/10.1007/s12598-020-01631-x.
Ding Y, Teng X, Zhu H, Wang L, Pei W, Zhu JJ, Huang L, Huang W. Orthorhombic KSc2F7:Yb/Er nanorods:controlled synthesis and strong red upconversion emission. Nanoscale. 2013;5(23):11928. https://doi.org/10.1039/C3NR01840G.
Pan Y, Xie X, Huang Q, Gao C, Wang Y, Wang L, Yang B, Su H, Huang L, Huang W. Inherently Eu2+/Eu3+ codoped Sc2O3 nanoparticles as high-performance nanothermometers. Adv Mater. 2018;30(14):1705256. https://doi.org/10.1002/adma.201705256.
Wang Y, Wen T, Zhang H, Sun J, Zhang M, Guo Y, Luo WJ, Xia MJ, Wang YX, Yang BC. Low-temperature fluorination route to lanthanide-doped monoclinic ScOF host material for tunable and nearly single band up-conversion luminescence. J Phys Chem C. 2014;118(19):10314. https://doi.org/10.1021/jp5020274.
Xie J, Xie X, Mi C, Gao Z, Pan Y, Fan Q, Su HQ, Jin DY, Huang L, Huang W. Controlled synthesis, evolution mechanisms, and luminescent properties of ScFx: Ln (x = 2.76, 3) nanocrystals. Chem Mater. 2017;29(22):9758. https://doi.org/10.1021/acs.chemmater.7b03561.
Teng X, Zhu Y, Wei W, Wang S, Huang J, Naccache R, Hu W, Tok AIY, Han Y, Zhang QC, Fan QL, Huang W, Capobianco JA, Huang L. Lanthanide-doped NaxScF3+x nanocrystals: crystal structure evolution and multicolor tuning. J Am Chem Soc. 2012;134(20):8340. https://doi.org/10.1021/ja3016236.
Peng D, Ju Q, Chen X, Ma R, Chen B, Bai G, Hao J, Qiao X, Fan X, Wang F. Lanthanide-doped energy cascade nanoparticles: full spectrum emission by single wavelength excitation. Chem Mater. 2015;27(8):3115. https://doi.org/10.1021/acs.chemmater.5b00775.
Wang F, Liu X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem Soc Rev. 2009;38(4):976. https://doi.org/10.1039/B809132N.
Xie X, Gao N, Deng R, Sun Q, Xu QH, Liu X. Mechanistic investigation of photon upconversion in Nd3+-sensitized core-shell nanoparticles. J Am Chem Soc. 2013;135(34):12608. https://doi.org/10.1021/ja4075002.
Zuo J, Sun D, Tu L, Wu Y, Cao Y, Xue B, Zhang Y, Chang Y, Liu X, Kong X, Buma W, Meijer E, Zhang H. Precisely tailoring upconversion dynamics via energy migration in core-shell nanostructures. Angew Chem Int Ed. 2018;57(12):3054. https://doi.org/10.1002/anie.201711606.
Hao D, Sun LD, Wang YF, Ke J, Si R, Xiao JW, Lyu G, Shi S, Yan CH. Efficient tailoring of upconversion selectivity by engineering local structure of lanthanides in NaxREF3+x nanocrystals. J Am Chem Soc. 2015;137(20):6569. https://doi.org/10.1021/jacs.5b01718.
Han S, Deng R, Xie X, Liu X. Enhancing luminescence in lanthanide-doped upconversion nanoparticles. Angew Chem Int Ed. 2014;53(44):11702. https://doi.org/10.1002/anie.201403408.
Zou W, Visser C, Maduro JA, Pshenichnikov MS, Hummelen JC. Broadband dye-sensitized upconversion of near-infrared light. Nat Photonics. 2012;6(8):560. https://doi.org/10.1038/nphoton.2012.158.
Schäfer H, Ptacek P, Zerzouf O, Haase M. Synthesis and optical properties of KYF4/Yb, Er nanocrystals, and their surface modification with undoped KYF4. Adv Funct Mater. 2008;18(19):2913. https://doi.org/10.1002/adfm.200800368.
Yi GS, Chow GW. Water-soluble NaYF4:Yb, Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence. Chem Mater. 2007;19(3):341. https://doi.org/10.1021/cm062447y.
He EJ, Chen SF, Zhang ML. Simultaneous morphology evolution and upconversion emission tuning of single Y-based fluoride microcrystal induced by Sc3+ co-doping. Mater Res Bull. 2017;87(Mar):61. https://doi.org/10.1016/j.materresbull.2016.11.025.
Huang Q, Yu J, Ma E, Lin K. Synthesis and characterization of highly efficient near-infrared upconversion Sc3+/Er3+/Yb3+ tridoped NaYF4. J Phys Chem C. 2010;114(10):4719. https://doi.org/10.1021/jp908645h.
Wang Y, Wei T, Cheng X, Ma H, Pan Y, Xie J, Su H, Xie X, Huang L, Huang W. Insights into Li+-induced morphology evolution and upconversion luminescence enhancement of KSc2F7:Yb/Er nanocrystals. J Mater Chem C. 2017;5(14):3503. https://doi.org/10.1039/C7TC00649G.
Ding Y, Gu J, Ke J, Zhang YW, Yan CH. Sodium doping controlled synthesis of monodisperse lanthanide oxysulfide ultrathin nanoplates guided by density functional calculations. Angew Chem Int Ed. 2011;50(51):12330. https://doi.org/10.1002/ange.201105025.
Lei L, Chen D, Huang P, Xu J, Zhang R, Wang Y. Modifying the size and uniformity of upconversion Yb/Er:NaGdF4 nanocrystals through alkaline-earth doping. Nanoscale. 2013;5(22):11298. https://doi.org/10.1039/C3NR03497F.
Liu X, Yi Z, Qin X, Liu H, Huang W, Liu X. Tuning long-lived Mn(II) upconversion luminescence through alkaline-earth metal doping and energy-level tailoring. Adv Opt Mater. 2019;7(15):1900519. https://doi.org/10.1002/adom.201900519.
Mahata MK, Koppea T, Mondalb T, Brüsewitza C, Kumarb K, Raib V, Hofsässa H, Vettera U. Incorporation of Zn2+ ions into BaTiO3:Er3+/Yb3+ nanophosphor: an effective way to enhance upconversion, defect luminescence and temperature sensing. Phys Chem Chem Phys. 2015;17(32):20741. https://doi.org/10.1039/C5CP01874A.
Cheng X, Ge H, Wei Y, Zhang K, Su W, Zhou J, Yin L, Zhan Q, Jing S, Huang L. Design for brighter photon upconversion emissions via energy level overlap of lanthanide ions. ACS Nano. 2018;12(11):10992. https://doi.org/10.1021/acsnano.8b04988.
Huang F, Yang T, Wang S, Lin L, Tao Hud T, Chen D. Temperature sensitive cross relaxation between Er3+ ions in laminated hosts: a novel mechanism for thermochromic upconversion and high performance thermometry. J Mater Chem C. 2018;6(45):12364. https://doi.org/10.1039/C8TC04733B.
Lakowicz JR. Principles of Fluorescence Spectroscopy. New York:Springer;2010.97. https://doi.org/10.1007/978-0-387-46312-4.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 11904323, 21871137, 21902148 and 21971113).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, MM., Zheng, GC., Wei, Y. et al. Doping induced morphology, crystal structure, and upconversion luminescence evolution: from Na3ScF6:Yb/Er/Y to NaYF4:Yb/Er/Sc nanocrystals. Rare Met. 42, 1018–1027 (2023). https://doi.org/10.1007/s12598-022-02159-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02159-y