All-inorganic perovskites CsPbX3 (X = Cl, Br, I) have attracted worldwide interest due to their excellent luminescent performances. Meanwhile, Cs4PbBr6 have been studied because they can enhance the luminescent efficiency and stability of CsPbBr3. Herein, we introduced a microfluidic method based on room-temperature supersaturated recrystallization to synthesize blue-emitting CsPbBr3 nanocrystals (NCs) and green-emitting CsPbBr3/Cs4PbBr6 NCs. The ultrasmall CsPbBr3 NCs emitted at a deep blue wavelength of 461 nm with the full width at half maximum (FWHM) of 15 nm. transmission electron microscopy (TEM) results demonstrated that ultrasmall CsPbBr3 NCs with the average particle size of 3.8 nm were synthesized and the CsPbBr3 NCs were crystallized as monoclinic structure. X-ray photoelectron spectrometer (XPS) analysis suggested that ultrasmall CsPbBr3 suffered from VBr defect and the surface passivation of Cs4PbBr6 possessed a low level of VBr defect density. The lifetime of CsPbBr3/Cs4PbBr6 was much longer than that of CsPbBr3. The results showed that ultrasmall CsPbBr3 NCs can be regarded as a source of blue-emitting material and CsPbBr3/Cs4PbBr6 NCs had a better stability.
Graphic abstract

摘要
纯无机钙钛矿CsPbX3 (X=Cl, Br, I)因其优异的光学性能而引起了人们的广泛关注。同时, Cs4PbBr6常被用来提高CsPbBr3的发光效率和稳定性。因此, 基于室温过饱和再结晶法, 我们在微流控平台上同时合成蓝光发射的CsPbBr3和绿光发射的CsPbBr3/Cs4PbBr6。超小尺寸的CsPbBr3实现了在461 nm处的深蓝光发射, 其半高宽为15 nm。TEM结果表明合成的超小CsPbBr3属于单斜晶相, 其平均尺寸为3.8 nm。XPS表明超小尺寸的CsPbBr3存在更多的VBr缺陷, 在CsPbBr3/Cs4PbBr6中Cs4PbBr6的表面钝化降低了VBr缺陷密度, 并且与超小尺寸的CsPbBr3相比CsPbBr3/Cs4PbBr6具有更长的荧光寿命。结果表明, 超小尺寸CsPbBr3可以作为蓝色发光材料, 而CsPbBr3/Cs4PbBr6具有较好的稳定性。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
All inorganic perovskites CsPbX3 (X = Cl, Br, I) have attracted worldwide interest due to their excellent luminescent performances. Meanwhile, Cs4PbBr6 have been studied because they can enhance the luminescent efficiency and stability of CsPbBr3. Herein, we introduced a microfluidic method based on room-temperature supersaturated recrystallization to synthesize blue-emitting CsPbBr3 nanocrystals (NCs) and green-emitting CsPbBr3/Cs4PbBr6 NCs. The ultrasmall CsPbBr3 NCs emitted at a deep blue wavelength of 461 nm with the full width at half maximum (FWHM) of 15 nm. The transmission electron microscopy (TEM) results demonstrated that ultrasmall CsPbBr3 NCs with the average particle size of 3.8 nm were synthesized and the CsPbBr3 NCs were crystallized as monoclinic structure. X-ray photoelectron spectrometer (XPS) analysis suggested that ultrasmall CsPbBr3 suffered from VBr defect and the surface passivation of Cs4PbBr6 possessed a low level of VBr defect density. The lifetime of CsPbBr3/Cs4PbBr6 was much longer than that of CsPbBr3. The results showed that ultrasmall CsPbBr3 NCs can be regarded as a source of blue-emitting material and CsPbBr3/Cs4PbBr6 NCs had a better stability. Nowadays, all inorganic halide perovskite NCs and several classes of perovskite-related materials have attracted worldwide interest due to the unique luminescence properties [1, 2]. All inorganic perovskite NCs have been promising materials for applications in LEDs [3, 4], photodetectors [5, 6], solar cells [7] and lasers [8] due to their unique optical and electronic properties. CsPbX3 NCs have narrow emission line width, wide color tunability in the whole visible range, high photoluminescence quantum yields (PLQYs) and short radiative lifetimes [9,10,11,12,13]. Up to now, the hot injection (HI) method and room-temperature supersaturated recrystallization (RTSR) approach are two popular strategies to synthesize all inorganic CsPbX3 NCs. In the HI process, a Cs-oleate precursor is injected into lead halide solution at high temperature under nitrogen atmosphere, which increases cost and complexity of preparation. The RTSR method, a low-cost and facile approach, uses the difference in solubility of cesium, lead and bromide ions in dimethyformamide (DMF) and toluene. When the DMF solution is rapidly injected into toluene, the cesium lead bromide NCs will crystalize and disperse in toluene at room temperature.
In general, the CsPbBr3 NCs synthesized by the preparation methods mentioned above are green emitting. The doping of rare earth ions into hosts has been applied in traditional luminescence materials [14,15,16]. For perovskite NCs, the luminescence properties can be adjusted by controlling the size and morphology. Yang et al. controlled the reaction temperature of HI method to obtain the blue-emitting CsPbBr3 nanoplatelets [17]. The two-dimensional (2D) perovskite nanoplatelets attract attention of researchers and are regarded as promising candidate of blue-emitting materials because the anion exchange can be avoided when the different halide perovskite NCs are coexistence in optical devices [18,19,20,21]. Blue-emitting CsPbBr3 nanoplatelets are always synthesized via the hot injection method in a narrow temperature range, which increases the controllability and difficulty. Another method is taking advantage of strong quantum confinement of CsPbBr3 NCs [22,23,24]. Nevertheless, it is a huge challenge to shrink the size of CsPbBr3 NCs to exciton Bohr radius (7 nm).
In this paper, we introduced a microfluidic method based on RTSR to synthesize CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs. In this method, a microfluidic platform was used to control the reaction rate by operating the feeding rate of precursors and poor solvent solution. PbBr2 and CsBr were chosen as the sources for lead, cesium and bromine. The ratio of PbBr2 and CsBr played an important role in the final products. In addition, there were different products and sizes of NCs in the precipitation and supernatant at the same mole ratio of PbBr2 and CsBr. Notably, the composites of CsPbBr3 and Cs4PbBr6 were detected in the precipitation (PbBr2:CsBr = 2 or 3). A large number of reports suggested that the presence of Cs4PbBr6 can enhance the luminescent properties of CsPbBr3 [25,26,27]. Therefore, blue-emitting NCs and green-emitting NCs can be simultaneously obtained in one pot and can be directly separated by facile centrifugation. The method provided a new pathway to synthesize blue band emission CsPbBr3 NCs.
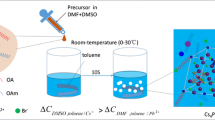
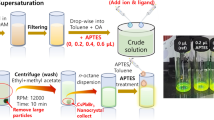
Figure 1a shows the schematic diagram of the synthesis of the nanocrystals on a microfluidic platform. CsBr and PbBr2 were put into a beaker with the stoichiometric ratio (PbBr2:CsBr = 1:1, 1:2, 1:3 and 1:4) and 10 ml dimethylformamide (DMF) was poured into the beaker to dissolve the precursor at room temperature, then 1 ml oleic acid (OA) and 0.5 ml oleylamine (OAm) were added to stabilize the solution. After the precursor became clear, the clear precursor and toluene were transferred into 2.5- and 20-ml syringes, respectively. The flow rates were 6 μl·min−1 for the precursor and 120 μl·min−1 for the toluene. At the junction, the precursor was recrystallized in toluene due to the difference in solubility. Under ultraviolet light, it was obvious that the supernatants emitted blue light and the precipitations emitted green light. Finally, the product was collected in the beaker and separated by centrifugation.
a Synthesis of nanocrystals using microfluidic platform; b XRD patterns of corresponding samples at different ratios of CsBr to PbBr2; schematic crystal structure of c CsPbBr3, d Cs4PbBr6 and e CsPb2Br5; PL emission spectra of f supernatants and g precipitations at different ratios of CsBr and PbBr2; h UV–Vis absorption spectra
As shown in Fig. 1b–e, the different products were synthesized via changing the amount of CsBr and PbBr2. Notably, the products collected from supernatants and precipitates were different with the same amount of CsBr and PbBr2. Through adjusting the consumption of CsBr and PbBr2, the CsPbBr3 perovskite and perovskite-related nanocrystals (Cs4PbBr6 and CsPb2Br5) can be obtained from supernatants or precipitates. Cs4PbBr6 and CsPb2Br5, as the families of the CsPbBr3, are always used to modify the properties of CsPbBr3 [28,29,30,31]. It is reported that the mole ratio of Cs and Br results in different phases of the final product. In a Cs-rich system, there is a tendency to form Cs4PbBr6. On the other hand, Br-rich conditions lead to CsPb2Br5 formation. Although Cs4PbBr6, CsPb2Br5 and CsPbBr3 contain the same elements, they possess quite different structures and properties. Cs4PbBr6 NCs, with the lowest symmetry, are considered to be a zero-dimensional structure, in which the PbBr64− octahedrons are completely separated from each other. CsPb2Br5 NCs have a 2D structure, in which the Pb2Br5− layer is separated by Cs+. The different structures result in different band gaps. CsPbBr3 has the smallest band gap (about 2.48 eV) while the band gaps of Cs4PbBr6 (> 3.2 eV) and CsPb2Br5 (2.98 eV) are larger. The products collected from supernatants and precipitates (CsBr:PbBr2 = 1:2) were denoted as 1–2-s and 1–2-p, respectively, and the same simplification was applied for other samples.
The solubility of CsBr in DMF is less than that of PbBr2 and the introduction of PbBr2 can accelerate the solubility of CsBr [32]. At the junction, a blend of the trace of precursor and toluene which created a local Cs-rich environment (inducing the formation of Cs4PbBr6 and the products) were transported away by the flow. The 1–1-s and 1–1-p samples were obtained by centrifugation, and the supernatant was transparent in the daylight, suggesting that there were trace of production with small size. X-ray diffractometer (XRD) pattern showed that the characteristic peaks assigned to Cs4PbBr6 were found in the pattern of 1–1-p and 1–1-s, especially for the (110), (113), (223) and (324). When the amount of PbBr2 increased into CsBr: PbBr2 = 1:2 or 1:3, XRD patterns of 1–2-s and 1–3-s matched well with the standard PDF card of monoclinic CsPbBr3 (PDF No. 18–0364). However, the patterns of both precipitations indicated the products consisted of two phases that were well coincident with monoclinic CsPbBr3 and rhombohedral Cs4PbBr6 (PDF 3 No. 73–2478). The increase of amount of PbBr2 resulted in a much faster precipitation rate of PbBr2 than that of CsBr at the beginning, which caused the formation of Pb-Br abundant Cs2PbBr5. Therefore, the new phases CsPb2Br5 were detected in the precipitations when CsBr:PbBr2 = 1:4.
The optical properties of the prepared nanocrystals at different ratios of CsBr and PbBr2 were studied. As shown in Fig. 1f–h, the supernatants showed bright emission with a photoluminescence (PL) peak at ~ 461 nm arising from ultrasmall CsPbBr3 NCs, and the emission peaks had a slightly red-shift with the increasing PbBr2. The absorption had the shoulder absorptions at ~ 456 nm, and the peaks shifted to long wavelength coincided with the emission spectra. However, when CsBr:PbBr2 = 1:1, almost no emission and absorption peaks were observed in the prepared supernatants. The emission peak of 1–1-s located at ~ 510 nm, which was similar to that of the 1–1-p. Chen’s group [33] reported that the luminescence of Cs4PbBr6 did not depend on the wide size distribution. The results suggested that products of 1–1-s contained a trace of small size Cs4PbBr6 particles. Under 365-nm excitation, all precipitations had the bright green emission at 516 nm. The absorption spectrum of 1–1-p showed an obvious absorption peak at 317 nm, stemming from the 1S0 → 3P1 transition of Pb2+ centers [34, 35]. With the increase of mole ratio of PbBr2 and CsBr, the absorption peak at 317 nm decreased, while the shoulder absorption peak at 510 nm appeared arising from CsPbBr3. Correspondingly, the PL excitation spectrum of the precipitate (Figure S1) had a steep drop at 315 nm, which is consistent with the sharp absorption peak in the PL spectrum at the same wavelength, and the sharp drop gradually disappeared with the increase of PbBr2. As shown in Fig. S2, the amount of PbBr2 had different effects on the emission wavelength of supernatants and precipitations. The band gap of bulk CsPbBr3 crystal was 2.30 eV and the emission wavelength located at 539 nm [36, 37]. With the decrease of CsPbBr3 NCs size, the PL peaks of NCs will shift to blue because of quantum confinement effect. Hence, the PL emission wavelength of blue CsPbBr3 NCs was tuned from 461 to 476 nm as well as the band gaps decreased from 2.67 to 2.66 eV with the increase of PbBr2.
In order to characterize the CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs, TEM and high-resolution transmission electron microscopy (HRTEM) of 1–2-s and 1–2-p samples was performed to investigate the morphology and microstructure of CsPbBr3 NCs. As shown in Fig. 2a, b, the average size of blue NCs was 3.8 nm, which was smaller than the Bohr exciton radius (7 nm) of CsPbBr3. The ultrasmall perovskite NCs have strong quantum confinement effect, resulting in blue shift of emission wavelength. HRTEM image illustrated that the interplanar distances were 0.249 and 0.290 nm, and the intersection angle between the fringes was 90°, corresponding to the (002) and (\(\bar{2}00\)) of monoclinic CsPbBr3, respectively. The fast Fourier transformation (FFT) consisted of diffraction spots corresponding to the (002) and (\(\bar{2}00\)) planes of monoclinic CsPbBr3 with an intersection angle of 90°, which was consistent with the HRTEM results. The shape of CsPbBr3/Cs4PbBr6 NCs was irregular with sizes of 30–400 nm, and the Cs4PbBr6 particles had a hexagonal prisms shape (Fig. 2c). Moreover, energy-dispersive spectrometer (EDS) results in Fig. 2d presented that the ratio of Br:Pb was large than that of the stoichiometric ratio of Cs4PbBr6, suggesting that CsPbBr3 coexisted with Cs4PbBr6 in 1–2-p sample.
The luminescence properties of blue NCs and green NCs were investigated by PL, absorption and excitation spectra (Fig. 3a, b). The PL spectra were centered at 461 and 516 nm with the full width at half maximum (FWHM) of 15 and 20 nm of blue NCs and green NCs, respectively. The related Commission Internationale de I’Eclairage (CIE) chromaticity coordinates of 1–2-s and 1–2-p were plotted on the CIE 1931 diagram (Fig. 3c). The CIE color coordinates were (0.133, 0.063) and (0.091, 0.648) for 1–2-s and 1–2-p, respectively. The narrow FWHM of the blue NC indicated that the sample had good crystallinity and narrow size distribution, which was consistent with the XRD and TEM results. The first absorption onsets of the blue NCs and green NCs were about 456 and 514 nm, separately. The photoluminescence excitation (PLE) spectra demonstrated that both supernatants and precipitations NCs can be excited by the light of 345–430 and 288–450 nm. Notably, there was a depth at 315 nm in the PLE spectrum of green NCs and a strong absorption peak at the same wavelength. Hence, the PLE spectrum results of green NCs confirmed that Cs4PbBr6 NCs coexisted in CsPbBr3. Figure 3d, e shows the dependence of NCs PL intensity of CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs on the excitation wavelength. The CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs showed single emission peak at ~ 461 and 516 nm under different excitations, indicating that the intensity of the emission peaks rather than the position varies with the excitation wavelength.
XPS was performed to investigate the elemental compositions and bonding states of prepared colloidal NCs. The full-range survey-scan spectrum (Figs. 4a, S3) suggested that the products mostly consisted of Cs, Pb and Br, and their main peak positions, such as Cs 3d, Br 3d and Pb 4f (Fig. 4b–d), were in line with the results in Refs. [38,39,40]. Besides, C and O arose from the oleic acid and oleylamine surface ligands. Cs 3d spectrum showed two symmetric peaks at 738.2 and 724.5 eV, which were assigned to Cs 3d3/2 and Cs 3d5/2, respectively, indicating that the cesium element was present in the nanocrystals in the form of Cs+. The Br 3d spectrum showed two spectral peaks at 69.2 and 68.2 eV corresponding to Br 3d3/2 and Br 3d5/2, respectively. However, there were differences in the high-resolution Pb 4f spectra of CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs. In terms of high-resolution Pb 4f spectra for ultrasmall CsPbBr3 NCs, two peaks at 142.9 and 138.1 eV were contributed to Pb 4f5/2 and 4f7/2, respectively, which certified the existence of Pb2+ [41]. The peaks were fitted with two peaks: the peak at 142.9 eV consisted of two peaks at 143.1 and 142.7 eV; the peak at 138.1 eV consisted of 138.2 and 137.7 eV. The peaks at 143.1 and 138.2 eV were assigned to Pb-Br bonding, whereas the peaks at 142.7 and 137.7 eV were contributed to Pb-oleate [42], indicating that more VBr defects exist in the CsPbBr3 NCs. Compared with those of CsPbBr3 NCs, the spectral peaks of the CsPbBr3/Cs4PbBr6 NCs shifted to high binding energy of 143.1 and 138.2 eV, consistent with the Pb-Br bonding. The results suggested that the ultrasmall CsPbBr3 suffered from the VBr defect, which led to more non-radiation recombination. The stability of CsPbBr3 and CsPbBr3/Cs4PbBr6 was measured in air atmosphere (Fig. S4). As shown in Fig. S4a, the CsPbBr3 NCs showed significant PL quenching and a slight red shift after 24 h. On the contrary, the PL emission wavelength of CsPbBr3/Cs4PbBr6 NCs remained the same position and the PL intensity retained at ~ 80% of original intensity after 24 h.
a XPS survey scans of green CsPbBr3/Cs4PbBr6 NCs; XPS spectra of b Cs 3d and c Br 3d; d XPS spectra of Pb 4f of CsPbBr3/Cs4PbBr6 and CsPbBr3; e time-resolved PL decay (λex = 365 nm) of CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs; steady-state PL spectra excited with different excitation density of f blue CsPbBr3 NCs and g green CsPbBr3/Cs4PbBr6 NCs; logarithm plot of integrated PL intensity as a function of excitation density of h blue CsPbBr3 NCs and i green CsPbBr3/Cs4PbBr6 NCs
In order to understand the difference between the NCs, the time-solved decay spectra are shown in Fig. 4e. The decay curves can be well fitted by a second-order exponential formula [43]:
where A1 and A2 are the distribution, τ1 and τ2 are the excited-state fluorescence lifetime. After the excitation lights disappear, there are two ways for the electron transition from excited state to ground state. The fast τ1 is contributed to the lifetime of radiation recombination of intrinsic excitation, and the slow component τ2 is assigned to the non-radiation recombination from trap state [44]. It was clearly observed that the PL lifetime of CsPbBr3/Cs4PbBr6 NCs was far longer than that of CsPbBr3 NCs. The average lifetime (τavg) was calculated by the following formula:
The average lifetime was calculated to be 9.67 ns (τ1 = 2.68 ns, τ2 = 11.77 ns) and 22.08 ns (τ1 = 2.23 ns, τ2 = 27.22 ns) for the CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs, respectively. The radiation recombination state can be obtained by comparing the fast lifetime (τ1) and slow lifetime (τ2). As shown in Fig. 4e, the τ2 ratio of CsPbBr3/Cs4PbBr6 was lower than that of CsPbBr3. The results suggested that the Cs4PbBr6, with a nice lattice matching with CsPbBr3, passivated the surface of CsPbBr3 and possessed a low level of surface defects and traps.
The relationship of PL intensity and excitation power density can be used to deduce the origin of near-band-edge photoluminescence according to the following formula [45]:
where IPL and Iex represent the PL intensity and the excitation density, respectively; k can be calculated by the slope of the linear plot and 1 < k < 2 for recombination of excitons. Figure 4f, g shows the steady-state PL spectra of CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs under different excitation densities. The PL intensity increased with the increase of excitation density, but the positions of peaks and shapes of PL spectra were identical for all the excitation density. Figure 4h, i presents the logarithmic plot of the PL intensity vs. excitation density for CsPbBr3 and CsPbBr3/Cs4PbBr6 NCs and illustrates the PL intensity dependence on the excitation density with a slope of 1.02 and 1.06, respectively. The result implied that the PL originated from recombination of excitons [46, 47].
In summary, blue-emitting and green-emitting nanocrystals were synthesized in one pot by the room-temperature supersaturated recrystallization method on the microfluidic platform. The emission PL of ultrasmall CsPbBr3 NCs centered at 461 nm with the FWHM of 15 nm and corresponding CIE chromaticity coordinates of (0.133, 0.063). TEM results demonstrated that the average particle size was 3.8 nm. The emission peak of green-emitting CsPbBr3/Cs4PbBr6 NCs centered at 516 nm with the FWHM of 20 nm. The lifetime of CsPbBr3/Cs4PbBr6 was much longer than that of CsPbBr3, suggesting that the surface passivation of the Cs4PbBr6 possessed a low level of VBr defect density.
References
Liu Y, Rong X, Li M, Molokeev MS, Zhao J, Xia Z. Incorporating rare-earth terbium(III) ions into Cs2AgInCl6: Bi nanocrystals toward tunable photoluminescence. Angew Chem Int Ed Engl. 2020;59(28):11634.
Gan Z, Zheng F, Mao W, Zhou C, Chen W, Bach U, Tapping P, Kee TW, Davis JA, Jia B, Wen X. The optical properties of Cs4PbBr6-CsPbBr3 perovskite composites. Nanoscale. 2019;11(31):14676.
Li J, Xu L, Wang T, Song J, Chen J, Xue J, Dong Y, Cai B, Shan Q, Han B, Zeng H. 50-Fold EQE improvement up to 6.27% of solution-processed all-inorganic perovskite CsPbBr3 QLEDs via surface ligand density control. Adv Mater. 2017;29(5):1603885.
Liu Y, Li F, Liu Q, Xia Z. Synergetic effect of postsynthetic water treatment on the enhanced photoluminescence and stability of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals. Chem Mater. 2018;30(19):6922.
Tan Q, Ye G, Zhang Y, Du X, Liu H, Xie L, Zhou Y, Liu N. Vacuum-filtration enabled large-area CsPbBr3 films on porous substrates for flexible photodetectors. J Mater Chem C. 2019;7(43):13402.
Liu R, Zhou H, Song Z, Yang X, Wu D, Song Z, Wang H, Yan Y. Low-reflection, (110)-orientation-preferred CsPbBr3 nanonet films for application in high-performance perovskite photodetectors. Nanoscale. 2019;11(19):9302.
Yao Y, Hang P, Wang P, Xu L, Cui C, Xie J, Xiao K, Li G, Lin P, Liu S, Xie D, Che S, Yang D, Yu X. CsPbBr3 quantum dots assisted crystallization of solution-processed perovskite films with preferential orientation for high performance perovskite solar cells. Nanotechnology. 2019;31(8):085401.
Wang Y, Li X, Nalla V, Zeng H, Sun H. Solution-processed low threshold vertical cavity surface emitting lasers from all-inorganic perovskite nanocrystals. Adv Func Mater. 2017;27(13):1605088.
Liu F, Zhang Y, Ding C, Kobayashi S, Izuishi T, Nakazawa N, Toyoda T, Ohta T, Hayase S, Minemoto T, Yoshino K, Dai S, Shen Q. Highly luminescent phase-stable CsPbI3 perovskite quantum dots achieving near 100% absolute photoluminescence quantum yield. ACS Nano. 2017;11(10):10373.
Li X, Cao F, Yu D, Chen J, Sun Z, Shen Y, Zhu Y, Wang L, Wei Y, Wu Y, Zeng H. All inorganic halide perovskites nanosystem: synthesis, structural features, optical properties optoelectronic applications. Small. 2017;13(9):1603996.
Nedelcu G, Protesescu L, Yakunin S, Bodnarchuk MI, Grotevent MJ, Kovalenko MV. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015;15(8):5635.
Koscher BA, Swabeck JK, Bronstein ND, Alivisatos AP. Essentially trap-free CsPbBr3 colloidal nanocrystals by postsynthetic thiocyanate surface treatment. J Am Chem Soc. 2017;139(19):6566.
Akkerman QA, D’Innocenzo V, Accornero S, Scarpellini A, Petrozza A, Prato M, Manna L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J Am Chem Soc. 2015;137(32):10276.
Qiao HN, Yang CL, Yin H, Hu YY, Ou MG. Luminescent properties of Gd2O3:Tb3+ nanoparticle by polyol method. Chin J Rare Met. 2019;43(1):81.
Fu R, Hu YY, Qiao HN, Yang CL, Yin H, Ou MG. Luminescence property and magnetic resonance imaging of Gd2O3:Tb3+ nanocrystals doped with Zn2+, Li+. Rare Met. 2021;40(8):2049.
Ding YJ, Han PD, Wang LX, Zhang QT. Preparation, morphology and luminescence of Gd2O3S: Tb with different Gd2O3 raw materials. Rare Met. 2019;38(3):221.
Bekenstein Y, Koscher BA, Eaton SW, Yang P, Alivisatos AP. Highly luminescent colloidal nanoplates of perovskite cesium lead halide and their oriented assemblies. J Am Chem Soc. 2015;137(51):16008.
Xiao X, Li Y, Xie RJ. Blue-emitting and self-assembled thinner perovskite CsPbBr3 nanoplates: synthesis and formation mechanism. Nanoscale. 2020;12(16):9231.
Bohn BJ, Tong Y, Gramlich M, Lai ML, Doblinger M, Wang K, Hoye RLZ, Muller-Buschbaum P, Stranks SD, Urban AS, Polavarapu L, Feldmann J. Boosting tunable blue luminescence of halide perovskite nanoplatelets through postsynthetic surface trap repair. Nano Lett. 2018;18(8):5231.
Leng J, Wang T, Zhao X, Ong EWY, Zhu B, Ng JA, Wong YC, Khoo KH, Tamada K, Tan ZK. Thermodynamic control in the synthesis of quantum-confined blue-emitting CsPbBr3 perovskite nanostrips. J Phys Chem Lett. 2020;11(6):2036.
Ravi VK, Swarnkar A, Chakraborty R, Nag A. Excellent green but less impressive blue luminescence from CsPbBr3 perovskite nanocubes and nanoplatelets. Nanotechnology. 2016;27(32):325708.
Shao H, Bai X, Pan G, Cui H, Zhu J, Zhai Y, Liu J, Dong B, Xu L, Song H. Highly efficient and stable blue-emitting CsPbBr3@SiO3 nanospheres through low temperature synthesis for nanoprinting and WLED. Nanotechnology. 2018;29(28):285706.
Malgras V, Tominaka S, Ryan JW, Henzie J, Takei T, Ohara K, Yamauchi Y. Observation of quantum confinement in monodisperse methylammonium lead halide perovskite nanocrystals embedded in mesoporous silica. J Am Chem Soc. 2016;138(42):13874.
Takhellambam D, Meena TR, Jana D. Room temperature synthesis of blue and green emitting CsPbBr3 perovskite nanocrystals confined in mesoporous alumina film. Chem Commun (Camb). 2019;55(33):4785.
Quan LN, Quintero-Bermudez R, Voznyy O, Walters G, Jain A, Fan JZ, Zheng X, Yang Z, Sargent EH. Highly emissive green perovskite nanocrystals in a solid state crystalline matrix. Adv Mater. 2017;29(21):1605945.
Palazon F, Urso C, De Trizio L, Akkerman Q, Marras S, Locardi F, Nelli I, Ferretti M, Prato M, Manna L. Postsynthesis transformation of insulating Cs4PbBr6 nanocrystals into bright perovskite CsPbBr3 through physical and chemical extraction of CsBr. ACS Energy Lett. 2017;2(10):2445.
Ling Y, Tan L, Wang X, Zhou Y, Xin Y, Ma B, Hanson K, Gao H. Composite perovskites of cesium lead bromide for optimized photoluminescence. J Phys Chem Lett. 2017;8(14):3266.
Wang W, Wang D, Fang F, Wang S, Xu G, Zhang T. CsPbBr3/Cs4PbBr6 nanocomposites: formation mechanism, large-scale and green synthesis, and application in white light-emitting diodes. Cryst Growth Des. 2018;18(10):6133.
Kaur G, Justice BK, Ghorai N, Goswami T, Maiti S, Ghosh HN. Polaron-mediated slow carrier cooling in a Type-1 3D/0D CsPbBr3@Cs4PbBr6 core-shell perovskite system. J Phys Chem Lett. 2019;10(18):5302.
Jiang G, Guhrenz C, Kirch A, Sonntag L, Bauer C, Fan X, Wang J, Reineke S, Gaponik N, Eychmuller A. Highly luminescent and water-resistant CsPbBr3-CsPb2Br5 perovskite nanocrystals coordinated with partially hydrolyzed poly(methyl methacrylate) and polyethylenimine. ACS Nano. 2019;13(9):10386.
Zhu BS, Li HZ, Ge J, Li HD, Yin YC, Wang KH, Chen C, Yao JS, Zhang Q, Yao HB. Room temperature precipitated dual phase CsPbBr3-CsPb2Br5 nanocrystals for stable perovskite light emitting diodes. Nanoscale. 2018;10(41):19262.
Sun Q, Ni C, Yu Y, Attique S, Wei S, Ci Z, Wang J, Yang S. Design principle of all-inorganic halide perovskite-related nanocrystals. J Mater Chem C. 2018;6(46):12484.
Chen X, Chen D, Li J, Fang G, Sheng H, Zhong J. Tunable CsPbBr3/Cs4PbBr6 phase transformation and their optical spectroscopic properties. Dalton Trans. 2018;47(16):5670.
Zhang Y, Saidaminov MI, Dursun I, Yang H, Murali B, Alarousu E, Yengel E, Alshankiti BA, Bakr OM, Mohammed OF. Zero-dimensional Cs4PbBr6 perovskite nanocrystals. J Phys Chem Lett. 2017;8(5):961.
Zarur AJ, Ying JY. Reverse microemulsion synthesis of nanostructured complex oxides for catalytic combustion. Nature. 2000;403(6765):65.
Zhu J, Di Q, Zhao X, Wu X, Fan X, Li Q, Song W, Quan Z. Facile method for the controllable synthesis of CsxPbyBrz-based perovskites. Inorg Chem. 2018;57(11):6206.
Cottingham P, Brutchey R. On the crystal structure of colloidally prepared CsPbBr3 quantum dots. Chem Commun. 2016;52(30):5246.
Li Y, Lv Y, Guo Z, Dong L, Zheng J, Chai C, Chen N, Lu Y, Chen C. One-step preparation of long-term stable and flexible CsPbBr3 perovskite quantum dots/ethylene vinyl acetate copolymer composite films for white light-emitting diodes. ACS Appl Mater Interfaces. 2018;10(18):15888.
Di X, Hu Z, Jiang J, He M, Zhou L, Xiang W, Liang X. Use of long-term stable CsPbBr3 perovskite quantum dots in phospho-silicate glass for highly efficient white LEDs. Chem Commun (Camb). 2017;53(80):11068.
Li Y, Shi ZF, Li S, Lei LZ, Ji HF, Wu D, Xu TT, Tian YT, Li XJ. High-performance perovskite photodetectors based on solution-processed all-inorganic CsPbBr3 thin films. J Mater Chem C. 2017;5(33):8355.
Zhang J, Fan L, Li J, Liu X, Wang R, Wang L, Tu G. Growth mechanism of CsPbBr3 perovskite nanocrystals by a co-precipitation method in a CSTR system. Nano Res. 2018;12(1):121.
Wu Y, Wei C, Li X, Li Y, Qiu S, Shen W, Cai B, Sun Z, Yang D, Deng Z, Zeng H. In situ passivation of PbBr64– octahedra toward blue luminescent CsPbBr3 nanoplatelets with near 100% absolute quantum yield. ACS Energy Lett. 2018;3(9):2030.
Pan G, Bai X, Yang D, Chen X, Jing P, Qu S, Zhang L, Zhou D, Zhu J, Xu W, Dong B, Song H. Doping lanthanide into perovskite nanocrystals: highly improved and expanded optical properties. Nano Lett. 2017;17(12):8005.
Xie M, Liu H, Chun F, Deng W, Luo C, Zhu Z, Yang M, Li Y, Li W, Yan W, Yang W. Aqueous phase exfoliating quasi-2D CsPbBr3 nanosheets with ultrahigh intrinsic water stability. Small. 2019;5(34):1901994.
Shibata H, Sakai M, Yamada A, Matsubara K, Sakurai K, Tampo H, Ishizuka S, Kim K-K, Niki S. Excitation-power dependence of free exciton photoluminescence of semiconductors. Jpn J Appl Phys. 2005;44(8):6113.
Li J, Gan L, Fang Z, He H, Ye Z. Bright tail states in blue-emitting ultrasmall perovskite quantum dots. J Phys Chem Lett. 2017;8(24):6002.
He H, Yu Q, Li H, Li J, Si J, Jin Y, Wang N, Wang J, He J, Wang X, Zhang Y, Ye Z. Exciton localization in solution-processed organolead trihalide perovskites. Nat Commun. 2016;7(1):10896.
Acknowledgements
This study was financially supported by the National Frontier Technology Key R&D Project (Nos. XXX-125 and JXXX-2019-058), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Collaborative Innovation Center of Advanced Inorganic Functional Composites of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, XB., Huang, WT., Zhang, RR. et al. Synthesis and luminescence of ultrasmall CsPbBr3 nanocrystals and CsPbBr3/Cs4PbBr6 composites by one-pot method. Rare Met. 41, 1230–1238 (2022). https://doi.org/10.1007/s12598-021-01856-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01856-4