Abstract
In this study, silicone-oil-based γ-Fe2O3 magnetic fluid was successfully prepared by thermal oxidizing of Fe3O4 magnetic nanoparticles, which were prepared by chemical co-precipitation with FeSO4·7H2O and FeCl3·6H2O, and their surface was modified by oleate ligands. Silicone oil was used as carrier liquid and oleic acid was as surfactant for preparing γ-Fe2O3 magnetic fluid. It is found that the Fe3O4 nanoparticles surrounded by oleate ligands are not damaged during the thermal oxidizing. The shape of γ-Fe2O3 magnetic nanoparticles prepared is similar to spherical, and their mean size is about 10–20 nm, which has nothing obvious difference compared with Fe3O4. The saturation magnetization of γ-Fe2O3 magnetic fluid prepared is 14.25 A·m2·kg−1 and that of γ-Fe2O3 nanoparticles is 57.56 A·m2·kg−1. The needle of γ-Fe2O3 magnetic fluid is much bigger than that of Fe3O4 magnetic fluid under the same magnetic field, which shows better magnetic properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Magnetic fluid is a colloidal solution or suspension with ferromagnetic and superparamagnetic fine particles that were dispersed in the solvent with the help of surfactant which is appropriate for the particles and solvent [1–3]. After decades of research and development, magnetic fluid has been used in a large variety of biomedical and engineering applications [4–8], such as aerospace, electronics, chemical industry, machinery, energy, metallurgy, environmental protection, and medical instrument. However, the application fields have gradually been enlarged for further study of the magnetic fluid.
The γ-Fe2O3 magnetic powder, one of the qualified magnetic materials, is widely applied in various storage medium and magnetic recordings for its lower price and great oxidation resistance. Compared with the usual structure, the γ-Fe2O3 gets more excellent magnetic properties while its grain comes into nanosize. And its coercive force improved so greatly that the magnetic recording materials made by the γ-Fe2O3 powder can achieve high-density information recording while getting better signal-to-noise ratio and image quality. The traditional method for preparing γ-Fe2O3 is heating Fe3O4 or γ-FeOOH in appropriate temperature, and the particle size is in micrometer. With the development of nanomaterials, preparation of γ-Fe2O3 has also been improved, and kinds of high-performance γ-Fe2O3 nanoparticles have been prepared. However, most of them are complicated and expensive. In order to realize industrial production, the most important thing is to cut the cost and make the large-scale preparation of uniform γ-Fe2O3 nanoparticles that have controllable size happen [9–12].
In this paper, the γ-Fe2O3 magnetic nanoparticles were prepared by thermal oxidation of Fe3O4 nanoparticles directly, and silicone oil was used as carrier liquid and oleic acid was as surfactant for preparing γ-Fe2O3 magnetic fluid. The prepared silicone-oil-based γ-Fe2O3 magnetic fluid could be widely used in sealing device.
2 Experimental
2.1 Preparation of γ-Fe2O3 magnetic fluid
The reaction process of chemical co-precipitation for Fe3O4 was: Fe2+ + Fe3+ + OH− → Fe3O4 + H2O. According to Fe3+ to Fe2+ mole ratio of 3:2, FeSO4·7H2O and FeCl3·6H2O were mixed into deionized water and placed into a 75 °C thermostatic beaker for 10–15 min [13, 14]. Then, enough 25 vol%–28 vol% NH3·H2O was fast poured into the mixed solution. After 10–15 min, the sodium oleate powder was put into the solution and isothermally stirred for 40 min to ensure that the Fe3O4 particles were fully covered by oleate ligands [15–17]. Under the external magnetic field, the supernate was removed and the deposition was washed by deionized water until the pH of supernate was about 7. Finally, the products were dried at low temperature. Then, the Fe3O4 magnetic nanoparticles were obtained, as shown in Fig. 1a. The γ-Fe2O3 was prepared by fully thermal oxidizing of Fe3O4 in a poor vacuum drying oven, as shown in Fig. 1b. The γ-Fe2O3 magnetic fluid was proceeded as follows: 3.5 ml organic silicone oil (VPO-705) was used as the solvent, 1.0 ml oleic acid and 0.5 ml emulsifier OP-7 as surfactant, and 5 g γ-Fe2O3 ultrasonic dispersion in it for 0.5 h. All reagents were analytically pure.
2.2 Characterization method
The magnetic particles were identified by X-ray diffraction (XRD, DMAX-RB12 KW), Fourier transform infrared spectroscopy (FTIR, Vanquish Flex UHPLC), transmission electron microscopy (TEM, JEM-2010), and vibrating sample magnetometer (VSM, BKT-4500).
3 Results and discussion
3.1 XRD pattern of magnetic powder
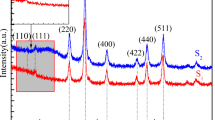
Figure 2 shows XRD pattern of γ-Fe2O3 nanoparticles prepared. The characteristic peaks at 2θ = 18.43°, 30.35°, 35.61°, 43.15°, 53.66°, 57.22°, 62.77°, 74.02°, 87.46°, 90.47° corresponding to (111), (220), (311), (400), (422), (511), (440), (532), (444), (800), respectively, which matches well with the standard JCPDS card of γ-Fe2O3, indicating that the products of thermal oxidizing of Fe3O4 are γ-Fe2O3 [18–23], and it is inverse cubic spinel phase. In addition, no characteristic peaks of impurities such as Na+, SO4 2−, and Cl− and other precursor compounds are found. It explains that the prepared product is pure phase. The particle size (D) is estimated by Scherrer formula.
where K = 0.89 is Scherrer constant, λ = 0.154 nm is the wavelength of X-ray, β is the diffraction peak width of (311) crystal surface, and θ is Prague diffraction angle. The grain size of both magnetic nanoparticles is about 10 nm.
3.2 FTIR analysis of magnetic particles
Figure 3 shows FTIR spectra of nanoparticles prepared. Figure 3(1) shows FTIR spectrum of Fe3O4 magnetic nanoparticles that are surrounded by oleate ligands. It can be seen that the possessed absorption band in 1634 and 1425 cm−1 is due to the symmetric and asymmetric carboxylate (COO–Fe) stretch. In Fig. 3(1), the peak at 575 cm−1 can be assigned as the characteristic absorption of Fe–O bond [24–26]. In Fig. 3(2), the thermal oxidation leads to the appearance of the bifurcated peak at 633 cm−1. In addition, the peak at 1042 cm−1 in Fig. 3(1) represents stretching vibration of (–C=C–) bond. However, the peak disappears in Fig. 3(2) after thermal oxidation. And the peaks in Fig. 3(2) have no obvious changes compared with those in Fig. 3(1). So the γ-Fe2O3 nanoparticles prepared are also covered with oleate ligands. It can be concluded that the thermal oxidation of Fe3O4 nanoparticles does not destroy the surface coating of oleic acid. The surface coating layer also prevents the growth and aggregation of the magnetic nanoparticles during the thermal oxidation.
3.3 Hysteresis loop of magnetic particles and magnetic fluid
Figure 4 shows the hysteresis loops of magnetic nanoparticles and magnetic fluid prepared. The hysteresis loop shows that the γ-Fe2O3 magnetic nanoparticles prepared have no remanence and coercivity, indicating that the obtained γ-Fe2O3 magnetic nanoparticles are superparamagnetic. After thermal oxidation, the saturation magnetization of magnetic nanoparticles decreases to 57.56 A·m2·kg−1 while that of Fe3O4 is 64.25 A·m2·kg−1. However, the saturation magnetization of γ-Fe2O3 magnetic fluid is 14.25 A·m2·kg−1, higher than that of Fe3O4 magnetic fluid (12.45 A·m2·kg−1). It is possible that the γ-Fe2O3 has higher solid content of 61 wt% (Table 1) than Fe3O4 of 50 wt% for the same carrier of magnetic fluid, while the amount of surfactant is also the same. It is the main factor that the saturation magnetization of γ-Fe2O3 magnetic fluid is higher than that of Fe3O4 magnetic fluid. Table 1 shows the component of different magnetic fluids that share the same viscosity.
3.4 TEM results of magnetic particles
Figure 5 shows TEM images of γ-Fe2O3 and Fe3O4 nanoparticles prepared. It can be seen that the nanoparticles show similar spherical. The diameter of the nanoparticle is within a range of 10–20 nm that matches well with XRD results. From the analysis above, it can be concluded that the γ-Fe2O3 magnetic nanoparticles have no obvious changes during the thermal oxidation. It is also found that the magnetic nanoparticles could be uniformly dispersed in the carrier liquid–silicone oil, and there is no obvious aggregation.
3.5 Appearance of magnetic fluid
Figure 6 shows the appearances of prepared magnetic fluid in different strength magnetic fields. The field strength was adjusted for these photographs by changing the distance between the magnet and glass dish. The magnetic nanoparticles are uniformly distributed along the magnetic field lines, and the magnetic needle appears. The conventional looking like puddle (Fig. 6a1, b1) becomes significantly distorted upon application of a weak field (Fig. 6a2, b2). The size of the needle (Fig. 6a3, b3) increases with the field increasing. Both of them have significant and uniform distribution magnetic needle, which shows excellent anisotropy. The needle of Fig. 6b is much bigger than that of Fe3O4 magnetic fluid, and it gets better magnetic properties.
4 Conclusion
The γ-Fe2O3 magnetic particles were successfully prepared by thermal oxidizing of Fe3O4 nanoparticles. The structure of Fe3O4 surrounded by oleic acid is not damaged during the thermal oxidizing. The coated oleic acid layer prevents the aggregation of magnetic nanoparticles, and the prepared γ-Fe2O3 nanoparticles have also been coated with oleate ligands. The γ-Fe2O3 magnetic nanoparticles prepared have lower saturation magnetization than that of Fe3O4, while the saturation magnetization of γ-Fe2O3 magnetic fluid prepared is improved because of its high solid content of 61 wt%, and the saturation magnetization of γ-Fe2O3 magnetic fluid is 14.25 A·m2·kg−1. Furthermore, it gets more obvious magnetic phenomenon in the same magnetic field. The needle of γ-Fe2O3 magnetic fluid is more clear and obvious than that of Fe3O4 magnetic fluid under magnetic field, and it shows better magnetic properties. The γ-Fe2O3 magnetic nanoparticles prepared remain similar spherical, and their mean size is about 10 nm.
References
Ladislau V, Doina B, Mikhail VA. Magnetic nanoparticles and concentrated magnetic nanofluids: synthesis, properties and some applications. China Particuol. 2007;5(1):43.
Kúdelčík Jozef, Bury Peter, Kopčanský Peter, Timko Milan. Structure of nanoparticles in transformer oil-based magnetic fluids, anisotropy of acoustic attenuation. J Magn Magn Mater. 2015;388(15):28.
Xie J, Li DC, Xing YS. The theoretical and experimental investigation on the vertical magnetic fluid pressure sensor. Sens Actuators, A. 2015;229(15):42.
Lan Q, Liu C, Yang F, Liu SY, Xu J, Sun DJ. Synthesis of bilayer oleic acid-coated Fe3O4 nanoparticles and their application in pH-responsive Pickering emulsions. J Colloid Interface Sci. 2007;310(1):260.
Sala F. Magnetic fluids effect upon growth processes in plants. J Magn Magn Mater. 1999;201(1–3):440.
Gu H, Tang X, Hong RY, Feng WG, Xie HD, Chen DX, Badami D. Ubbelohde viscometer measurement of water-based Fe3O4 magnetic fluid prepared by coprecipitation. J Magn Magn Mater. 2013;348(5):88.
Tadmor R, Rosenwsweig RE, Frey J, Klein J. Resolving the puzzle of ferrofluid dispersants. Langmuir. 2000;16(24):9117.
Muller S. Magnetic fluid hyperthermia therapy for malignant brain tumors: an ethical discussion. Nanomed Nanotechnol Biol Med. 2009;5(4):387.
Stefanescu O, Stefanescu M. New Fe(III) malonate type complex combination for development of magnetic nano-sized γ-Fe2O3. J Organ Metall Chem. 2013;740(3):50.
Lin JF, Tsai CC, Huang CH, Lee MZ, Lin YQ. Magneto-optical properties and magnetic inductive heating of sodium oleate coated Fe3O4 magnetic fluid. Optik-International Journal for Light and Electron Optics. 2015;126(9–10):907.
Chitra SR, Sendhilnathan S. Investigation on thermal studies of nanofluids related to their applications. Heat Transfer-Asian Res. 2015;44(5):420.
Chi YH, Zhuang J, Yu J, Tu MJ. γ-Fe2O3 nanoparticle prepared by solid-state reaction at low heating and its electromagnetic characters. Chin J Inorg Chem. 2004;20(4):479.
Tan YM, Shao HP, Ji Y, Lin T, Guo ZM. Preparation and research of stable Fe3O4 magnetic nanafluids in kerosene. Powder Metall Technol. 2012;30(3):163.
Zhao ZF, Shao HP, Sun S. The influence of pH on the properties of kerosene-based magnetic fluid. J Funct Mater. 2014;6(45):107.
Khollam YB, Dhage SR, Potdar HS, Deshpande SB, Bakare PP, Kulkarni SD, Date SK. Microwave hydrothermal preparation of submicron-sized spherical magnetite (Fe3O4) powders. Mater Lett. 2002;56(10):571.
Yang CC, Bian XF, Qin JY, Guo TX, Zhao SC. Fabrication and hyperthermia effect of magnetic functional fluids based on amorphous particles. Appl Surf Sci. 2015;330(1):216.
Zaharescu T, Setnescu R, Borbath I. Thermal oxidation of irradiated magnetic fluids and their component surfactants and dispersing oils. Cent Eur J Chem. 2014;12(7):782.
Ajay K, Mona G. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995.
Rosengart AJ, Kaminski MD, Chen HT, Caviness PL, Ebner AD, Ritter JA. Magnetizable implants and functionalized magnetic carriers: a novel approach for noninvasive yet targeted drug delivery. J Magn Magn Mater. 2005;293(1):633.
Lin KYA, Yang HT, Lee WD, Tsao KY. A magnetic fluid based on covalent-bonded nanoparticle organic hybrid materials (NOHMs) and its decolorization application in water. J Mol Liq. 2015;204(1):50.
Pei B, Thomas JM. Polymerization of monomer-based ferrofluids. Langmuir. 2010;26(9):6145.
Burenin V, Nikolaev A, Sova A, Katarzhin A, Eruslankin S. New magnetofluid seals for shafts of machines and mechanisms. Chem Pet Eng. 2014;49(11):754.
Junqiao Wang, Jizhou Chen. X-ray diffraction study of the microstructure of the acicular γ-Fe2O3. Chin J Chem Phys. 1994;7(1):44.
Kholmetskii AL, Vorobyova SA, Lesnikovich AI, Mushinskii VV, Sobal NS. A novel route for the preparation of magnetic fluids. Mater Lett. 2005;59(16):1993.
Wang LS, Ren ZQ, Gong CH, Hong RY. Preparation and application of methylbenzene-based magnetic fluid. Spec Petrochem. 2007;24(6):29.
Yoshida T, Bai S, Hirokawa A, Tanabe K, Enpuku K. Effect of viscosity on harmonic signals from magnetic fluid. J Magn Magn Mater. 2015;380(15):105.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 51274039).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, H., Shao, HP., Lin, T. et al. Preparation and characterization of silicone-oil-based γ-Fe2O3 magnetic fluid. Rare Met. 37, 803–807 (2018). https://doi.org/10.1007/s12598-016-0731-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0731-6