Abstract
Single-phase SrM ultrafine powders with single-domain particle ranging from 200 to 400 nm in size were successfully synthesized by a cetyltrimethylammonium bromide (CTAB)-assisted co-precipitation method, and excellent magnetic properties with saturation magnetization of 68.6 A·m2·kg−1 and coercive field of 0.5250 T are obtained. As CTAB content exceeds 5.0 wt%, the impurities emerge due to the deterioration of simultaneous precipitations of the starting materials and the deviation of Sr/Fe molar ratio from stoichiometry ratio in the local microdomains by employing CTAB in co-precipitation process. Also, the use of CTAB can effectively improve the homogeneities of Fe-precursor particles with Sr-precursor particles, exert surface barriers to inhibit interdiffusion, and constrain the crystal growth in the local microdomains during sintering, resulting in smaller particle sizes and more uniform particle size distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As one of the typical M-type hexagonal ferrites, strontium hexa-ferrite or SrM ferrite attracts more and more attention due to their excellent magnetic properties and low cost [1, 2]. Nowadays, with the rapid development of electromagnetic technique, the application fields of SrM ferrite have extended to high-performance anisotropic magnetisms, magnetic fillers for elastomeric composites, perpendicular recording materials, electromagnetic wave absorbing materials, microwave gyromagnetic materials, etc. [1, 3–6]. It is believed that the high-quality M-type hexagonal ferrite ultrafine powders with precisely controlled chemical compositions and uniform size distribution are often required as the starting materials to develop SrM ferrite-related functional devices with higher performance [7–9]. Several chemical techniques were used to synthesize high-quality SrM ultrafine powders including sol–gel method [10], hydrothermal process [11], sol–gel auto-combustion [12, 13] as well as chemical co-precipitation method [14–17].
Generally speaking, chemical co-precipitation method is supposed to be a relatively technologically feasible and cost-effective technique, which is quite suitable for synthesizing complex compounds with mass production. However, there are several obstacles constraining its application in fabricating high-quality complex oxide ultrafine powders such as ferrites: (1) the simultaneous co-precipitation of the starting metal ions is very difficult but important for the formation of the targeted phase; (2) the required secondary heating treatment at a relatively high temperature to accomplish the solid-state reaction inevitably leads to coarsened particle and agglomeration. In order to effectively improve the chemical homogeneities of precursors, decrease the secondary heating temperature, and alleviate the sintering aggregation between the particles, it was believed to be an effective way to employ proper surfactants in co-precipitating process [16–20]. Referring to SrM ferrite ultrafine powders synthesized by the surfactant-assisted co-precipitation method, there were still rare reports to our knowledge [16, 17]. Lu et al. [16] synthesized SrM ferrite powders by surfactant-assisted co-precipitation route. The results indicated that SrM powders prepared using cetyltrimethylammonium bromide (CTAB) as the surfactant in co-precipitation route exhibited the best magnetic properties compared with using sodium dodecyl sulfate (SDS) and polyethylene glycol 6000 (PEG-6000). However, the pre-treatment before heating treatment similar to the molten salt method made the problem more complex [16]. It cannot be simply deduced that the improvement in the phase formation temperature and the particle dispersibility as well as the properties are just ascribed to the employed surfactants rather than the pre-treatment of planetary milling with KCl–NaCl salts. Chen et al. [17] synthesized SrM ferrite nanopowders with particle size ranging from 50 to 150 nm via co-precipitation method using CTAB as the surfactant, followed by heat treatment at 900 °C for 2 h. The formation mechanism of platelet-like or rod-like particle was primarily investigated, and CTAB was supposed to act as a crystallization master, controlling the nucleation and growth of the crystals. The starting solution was directly dropped into the KOH solution, which would lead to a rapid nucleation of precipitates and the alteration in the particle shapes.
Therefore, focusing on synthesizing single-phase SrM ultrafine powders, CTAB-assisted co-precipitation method was used. The influences of CTAB content on phase formation and particle sizes as well as the magnetic properties were studied. Based on these results, the formation mechanism was also clarified.
2 Experimental
The typical procedure for synthesizing SrM ferrite ultrafine powders by CTAB-assisted co-precipitation route was described as follows: strontium nitrate [Sr(NO3)2] and iron nitrate nonahydrate [Fe(NO3)3·9H2O] with analytical reagent (AR) grade as the starting materials were weighed in the designed Sr/Fe molar ratios (1:10.0, 1:11.0, 1:11.5, 1:12.0) and dissolved together to form a clear aqueous solution. The surfactant, CTAB, was used and added into the above solution according to the designed contents of 0 wt%, 2.5 wt%, 5.0 wt%, or 10.0 wt% of the final product powders, respectively. Ammonia and ammonium carbonate mixed solution was employed as the composite precipitators with contents of 125 % or 150 % and added dropwise into the aqueous solution under the continuous stirring. After the complete reaction, the Sr–Fe co-precipitated precursors were collected by centrifugation and dried at 80 °C. Subsequently, the precursor powders were heat-treated at 400 °C for 2 h and calcined at 1000 °C for 4 h to form the targeted phase. The phase compositions were analyzed by X-ray diffraction (XRD, Brukers, D8 Advanced) with Cu Kα radiation, and the particle morphologies were observed by scanning electron microscope (SEM, JEOL, S-4800II). All the resulting powders were pressed into compacts with the same pressure of 25 MPa, and their magnetic properties were measured by a vibrating sample magnetometer (VSM, ADE, EV7) with a maximum applied field of 1.2 T.
3 Results and discussion
3.1 Optimizing co-precipitating conditions
Firstly, the experimental conditions including Sr/Fe molar ratios and the contents of the composite precipitators were optimized to promote the formation of SrM phase. Figure 1 gives the typical XRD patterns of the synthesized powders via the co-precipitation route with various Sr/Fe molar ratios and precipitator contents. It is obvious that most of the diffraction peaks are well indexed to SrM ferrite phase rather than any other. Meanwhile, the relative peak intensities are almost agreed with JCPDS No. 33-1340. All these facts demonstrate that SrM becomes the majority crystalline phase of as-synthesized powders. The weak peaks in 2θ range of 34°–35° are corresponding to α-Fe2O3 and Sr2Fe2O5, indicating that α-Fe2O3 and Sr2Fe2O5 exist as the impurity phases in synthesized powders.
Keeping the content of the composite precipitators of 25 % excess to the total content of metal ions, the content of impurity phase α-Fe2O3 decreases as the Sr/Fe molar ratio in the starting materials goes up from 1:12.0 to 1:11.0. A little excess of Sr2+ could compensate the incomplete precipitation of Sr2+ during the co-precipitating process for a small amount of strontium hydroxide could still dissolve into the solution. As the Sr/Fe molar ratio increases further to 1:10, the strontium-rich phase Sr2Fe2O5 companying with scarce α-Fe2O3 exists as the impurity phases, which indicates that the compensated Sr2+ is excess in the co-precipitates. As the content of the composite precipitators increases to 150 %, single-phase SrM powders without any other detective impurity phases were prepared with the Sr/Fe molar ratio in the starting materials ranging from 1:11.5 to 1:11.0. Therefore, the co-precipitating conditions could be optimized as the Sr/Fe molar ratio of 1:11 and the content of the composite precipitators of 150 % for further synthesis.
3.2 Influences of CTAB on formation of SrM phase
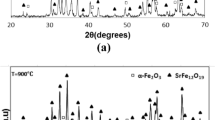
Figure 2 shows XRD patterns of synthesized powders with CTAB content ranging from 0 wt% to 10 wt%. It is clear to see that single-phase SrM powders without any other impurity phase were prepared with a little amount of CTAB surfactant. The synthesized powders could be maintained as the pure SrM phase with CTAB content increasing to 2.5 wt%. As CTAB content increases further, scarce α-Fe2O3 emerges as the impurity phase. The phase composition of the synthesized complex oxides manly relies on the reaction ratios of the solid-state reaction during the secondary heating treatment. With CTAB as the surfactant participating in the co-precipitation process, it does improve the homogeneities of the co-precipitates, but inhibit the interdiffusion of Sr2+ and Fe3+ to some extent as CTAB exceeds to a proper content. Such a changing trend is quite different from the presented results in Ref. [17], but appears to be more reasonable. Another point about the influences of CTAB content on the particle morphologies should be well considered.
3.3 Influences of CTAB on particle morphologies
Figure 3 demonstrates SEM images of the particle morphologies of the synthesized powders. Without any surfactant participating in co-precipitation process, the synthesized powders have tiny particles with irregular particle shape and broad particle size distribution ranging from 200 to 800 nm. The agglomeration between the particles appears to be very severe due to the magnetic attraction and the sintering adhesion. As CTAB increases to 2.5 wt%, the particle size decreases to 200–400 nm with the refined sintering adhesion. With more CTAB surfactant employed in co-precipitation process, the particle size of the synthesized powders decreases to about 150–300 nm, and the particle size distributions become relatively more uniform. The use of CTAB surfactant in co-precipitation process is effective in improving the particle agglomeration and controlling the particle sizes due to better dispersion of precursor particles and the inhibition to sintering adhesion during secondary heating treatment. Considering the fact that the critical size of the single magnetic domain of SrM ferromagnetic materials is about 0.5–1.0 μm, all the synthesized powders with CTAB as surfactant mainly consist of single-/quasi-single-domain particles.
3.4 Formation mechanism
Before clarifying the formation mechanism of the SrM ultrafine powders synthesized by surfactant-assisted co-precipitation method, the influences of CTAB surfactant on phase formation and particle morphologies should be summarized firstly as: (1) the participation of CTAB with higher contents leads to the emergence of the impurity phase; (2) the use of surfactant CTAB in co-precipitation process appears to be effective in improving the particle agglomeration and controlling the particle sizes. Generally, as to synthesizing complex oxides by co-precipitation method, the phase composition mainly depends on reaction ratios of the solid-state reaction which relies on the interdiffusion of the ions during the sintering process, and the phase morphologies may be determined by solid-state reaction and surface inhibition to the sintering adhesion or secondary crystal growth [17, 21].
A schematic illustration for CTAB participating in co-precipitating process is presented in Fig. 4 which helps for understanding the possible mechanism. As one of the familiar ionic surfactant, CTAB prefers to adhere on the particle surfaces of the precipitated precursors due to the surface electrostatic conjugation. However, the differences in Fe-precursor particles and Sr-precursor particles in surface electric potential will deteriorate the simultaneous precipitations of the starting materials to some extent, which alters Sr/Fe molar ratios in the local domains. If the Sr/Fe molar ratio deviates far from the stoichiometry ratio, a long domain interdiffusion during secondary heating treatment must be required to form the single phase. Unfortunately, that will be worse as CTAB content increases, leading to the emergences of the impurity phases. Referring to the particle morphologies, CTAB surfactant participating in the co-precipitation process does improve homogeneities of Fe-precursor particles with Sr-precursor particles during co-precipitating process, exert surface barriers to inhibit interdiffusion, and constrain the crystal growth in the local microdomains during sintering, which together depress particle size and improve size monodispersion.
3.5 Magnetic properties of synthesized SrM powders
The magnetic properties of the obtained powders prepared by CTAB-assisted co-precipitation method were measured by VSM with a maximum applied field of 1.2 T at room temperature, as shown in Fig. 5, demonstrating the typical ferromagnetic magnetization behavior. Without using any surfactant, the saturation magnetization at 1.2 T (M 1.2T) is about 66.7 A·m2·kg−1, and the coercive field (H c) is about 0.4125 T. With 2.5 wt% CTAB employed in co-precipitation process, M 1.2T and H c increase to 68.6 A·m2·kg−1 and 0.5250 T, respectively, which are near to the intrinsic magnetic properties of the SrM powders with single magnetic domain. The effective improvements in magnetic properties may be ascribed to the higher phase purity and more uniform particle size distribution. As the CTAB content increases to 5.0 wt%, M 1.2T deceases due to the existence of non-magnetic phase α-Fe2O3. It is interesting to find that M 1.2T of the powders synthesized with 10 wt% CTAB approaches to a higher value than that synthesized with 5.0 wt% CTAB since its relative impurity phase content is higher. Such a phenomenon may result from easy magnetization of particles with uniform particle size distribution.
4 Conclusion
Single-phase SrM ultrafine powders with particle sizes ranging from 200 to 400 nm were synthesized, and excellent magnetic properties with saturation magnetization of 68.6 A·m2·kg−1 and coercive field of 0.5250 T are obtained. The surfactant CTAB participating in the co-precipitation process deteriorates the simultaneous precipitations of starting materials to some extent and results in the deviation of Sr/Fe molar ratio from stoichiometry ratio in the local microdomains. As the CTAB content exceeded 5.0 wt%, the impurities emerge. The use of CTAB effectively improve homogeneities of Fe-precursor particles with Sr-precursor particles, exerts surface barriers to inhibit interdiffusion and constrains the crystal growth in the local microdomains during sintering, which together depress the particle sizes and improve size monodispersion. With CTAB content increasing, the particle size decreases from 200–800 to 150–300 nm, and the particle size distribution becomes more uniform.
References
Pullar RC. Hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrite ceramics. Prog Mater Sci. 2012;57(7):1191.
Went JL, Ratenau GW, Gorter EW, van Oosterhout GW. Ferroxdure, a class of new permanent magnet materials. Philips Tech Rev. 1952;13:194.
Harris VG, Geiler A, Chen YJ, Yoon SD, Wu MZ, Yang A, Chen ZH, He P, Parimi PV, Zuo X, Patton CE, Abe M, Acher O, Vittoria C. Recent advances in processing and applications of microwave ferrites. J Magn Magn Mater. 2009;321(14):2035.
Li LC, Chen KY, Liu H, Tong GX, Qian HS, Hao B. Attractive microwave-absorbing properties of M–BaFe12O19 ferrite. J Alloys Compd. 2013;557:11.
Chen N, Yang K, Gu MY. Microwave absorption properties of La-substituted M-type strontium ferrites. J Alloys Compd. 2010;490(1–2):609.
Tian Y, Liu YQ, He MH, Zhao GZ, Sun YY. High damping properties of magnetic particles doped rubber composites at wide frequency. Mater Res Bull. 2013;48(5):2002.
Liu JL, Zhang W, Guo CJ, Zeng YW. Synthesis and magnetic properties of quasi single domain M-type barium hexaferrite powders via sol–gel auto-combustion: effects of pH and the ratio of citric acid to metal ions (CA/M). J Alloys Compd. 2009;479(1–2):863.
Chen YJ, Geiler AL, Chen TY, Sakai T, Vittoria C, Harris VG. Low-loss barium ferrite quasi-single-crystals for microwave application. J Appl Phys. 2007;101(9):09M501.
Liu JL, Zeng YW, Sheng XD, Guo CJ, Zhang W. Ba-hexaferrite quasi-single crystals prepared with single-domain crystallites: fabrication, structural characterization and magnetic properties. J Cryst Growth. 2009;311(8):2363.
Nga TTV, Duong NP, Loan TT, Hien TD. Key step in the synthesis of ultrafine strontium ferrite powders (SrFe12O19) by sol–gel method. J Alloys Compd. 2014;610:630.
Mocuta H, Lechevallier L, Le Breton JM, Wang JF, Harris IR. Structural and magnetic properties of hydrothermally synthesized Sr1−x Nd x Fe12O19 hexagonal ferrites. J Alloys Compd. 2004;364(1–2):48.
Luo H, Rai BK, Mishra SR, Nguyen VV, Liu JP. Highly aluminum doped strontium ferrite nanoparticles prepared by auto-combustion. J Magn Magn Mater. 2012;324(17):2602.
Fu YP, Lin CH. Fe/Sr ratio effect on magnetic properties of strontium ferrite powders synthesized by microwave-induced combustion process. J Alloys Compd. 2005;386(1–2):222.
Anis-ur-Rehman M, Asghar G. Variation in structural and dielectric properties of co-precipitated nanoparticles strontium ferrites due to value of pH. J Alloys Compd. 2011;509(2):435.
Hessien MM, Rashad MM, El-Barawy K. Controlling the composition and magnetic properties of strontium hexaferrite synthesized by co-precipitation method. J Magn Magn Mater. 2008;320(3–4):336.
Lu HF, Hong RY, Li HZ. Influence of surfactants on co-precipitation synthesis of strontium ferrite. J Alloys Compd. 2011;509(41):10127.
Chen DY, Meng YY, Zeng DC, Liu ZW, Yu HY, Zhong XC. CTAB-assisted low-temperature synthesis of SrFe12O19 ultrathin hexagonal platelets and its formation mechanism. Mater Lett. 2012;76:84.
Hasab MG, Ebrahimi SAS, Badiei A. Comparison of the effects of cationic, anionic and nonionic surfactants on the properties of Sr-hexaferrite nano powder synthesized by a sol–gel auto-combustion method. J Magn Magn Mater. 2007;316(2):e13.
Rashad MM, Radwan M, Hessien MM. Effect of Fe/Ba mole ratios and surface-active agents on the formation and magnetic properties of co-precipitated barium hexaferrite. J Alloys Compd. 2008;453(1–2):304.
Jadhav AP, Kim CW, Cha HG, Pawar AU, Jadhav NA, Pal U, Kang YS. Effect of different surfactants on the size control and optical properties of Y2O3:Eu3+ nanoparticles prepared by coprecipitation method. J Phys Chem C. 2009;113(31):13600.
Vadivel M, RameshBabu R, Arivanandhan M, Ramamurthic K, Hayakawa Y. Role of SDS surfactant concentrations on the structural, morphological, dielectric and magnetic properties of CoFe2O4 nanoparticles. RSC Adv. 2015;3(34):27060.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51103125), the China Postdoctoral Science Foundation (No. 2015M570483), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Natural Science Foundation of Yangzhou (No. YZ2014043), and the Science Innovative Foundation of Yangzhou University (No. 2014CXJ017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, JL., Chen, XL., Wang, SY. et al. Synthesis and properties of strontium hexa-ferrite ultrafine powders via a CTAB-assisted co-precipitation method. Rare Met. 36, 666–670 (2017). https://doi.org/10.1007/s12598-015-0604-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0604-4