Abstract
In this study, Al–Mg–Al trilaminated thin plates were fabricated by one-pass hot rolling to improve the processing capacity and bonding strength of magnesium alloy plates. The effects of processing parameters were investigated. The results show that the optimal bonding strength is up to 20 MPa with a reduction ratio of 40 % and rolling temperature of 400 °C, superior than that in other one-pass rolling studies with regard to thin laminated plates. In addition, a favorable property is achieved with the annealing temperature of 250–300 °C or annealing time of 1.5 h. When the annealing temperature exceeds 300 °C or annealing time exceeds 1.5 h, respectively, the bonding strength of Al/Mg/Al plate decreases. The scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) analyses suggest that the appearance of thin diffusion layers between Al and Mg interfaces is helpful to improve the bonding strength, while the presence of thick diffusion layer would reduce the bonding strength greatly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnesium and its alloys are widely used metal materials. Magnesium alloys have high specific strength, specific stiffness, excellent damping properties and electromagnetic shielding performance, which can withstand greater shock and vibration loads compared with other metallic structural materials [1]. Consequently, they are extensively used in transportation, electronic industrial products, aerospace and other fields [2]. However, magnesium has a hexagonal crystal structure with few slip systems and poor plastic deformation capacity, making it difficult to manufacture into sheets, strips or other types [3]. In addition, magnesium is reactive with air by forming magnesium oxide, which leads to a low corrosion resistance [4]. A lot of researches were conducted on this important topic. For example, the bond of Mg and Al was realized by laser welding [5], friction stir welding [6] or the vacuum diffusion bonding technology [7]. Liu et al. [7] investigated the microstructure and phase constituents near the interface of Mg/Al joint, demonstrating that an obvious diffusion zone composed of intermetallic compounds of MgAl, Mg3Al2 and Mg2Al3 was formed near the Mg/Al interface. In their work, the bonding process was carried out at 480 °C with the vacuum degree of 6.5 × 10−4 Pa. In order to solve the problems mentioned above, the aluminum plate was proposed to coat on the surface of magnesium alloy. Firstly, it can prevent the magnesium from reacting with the oxygen in the air, which could increase the resistance to corrosion of magnesium alloys; at the same time, compression stress produced on the surface of magnesium alloys can reduce the deformation of small cracks of magnesium alloys, improving the processing and deformation capacity of magnesium alloys [8].
In this work, 5083 Al alloy/AZ31 Mg alloy/5083 Al alloy trilaminated plates were fabricated by one-pass hot rolling with low density, relatively dense oxide film, excellent corrosion resistance, outstanding plasticity and strong corrosion resistance of aluminum alloy. Finally, both advantages of magnesium and aluminum alloy were achieved [9]. The bonding strength of the laminated plates was investigated and measured by four-point bending method. At the same time, the microstructure of the laminated plates was analyzed. Besides reduction ratio, rolling temperature and annealing treatment were also very important parameters affecting the bonding strength [10]. Moreover, the effects of reduction ratio and rolling temperature on microstructure and bonding strength of the constituent layers after hot rolling were also studied.
2 Experimental
2.1 Materials and processing
AZ31 magnesium alloy and 5083 aluminum alloy ingots were utilized in this work. The chemical compositions of AZ31 magnesium and 5083 aluminum alloy are shown in Table 1. The Mg specimen was 50 mm in length, 40 mm in width and 2 mm in thickness. The Al specimen was 120 mm in length, 90 mm in width and 0.5 mm in thickness. All of these plates were ground with 200 mesh SiC sandpaper. One Mg plate placed in the middle with two Al sheets placed on both sides, stacking into an assemblage. Hot rolling was carried out with four reduction ratios: 30 %, 35 %, 40 % and 45 % (single pass), at four temperatures: 350, 375, 400 and 425 °C. The roller was 130 mm in diameter with rotational speed of 10 r·min−1. After surface preparation, the plates were manipulated carefully to avoid renewed contamination. Less than 50 s was allowed as the interval between surface preparation and package in an attempt to avoid the formation of a thick and continuous oxide layer on the bonding surface of the laminated plates [11]. Then, the two metal strips to be joined were positioned with the two prepared intimate surface against each other. The schematic illustration of hot rolling bonding (HTB) is presented in Fig. 1.
2.2 Microstructure and bonding strength tests
Microstructures of the bonding interface were examined by scanning electron microscopy (SEM, TESCAN-MIRA-3) and backscattered electron microscopy (BSE, TESCAN-MIRA-3). Phase and element compositions were determined by X-ray diffraction (XRD, Y-2000X) with Cu Kα radiation and energy-dispersive spectrometer (EDS).
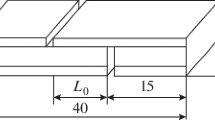
The bonding strength tests were performed using the SANS-CMT5105 tensile testing machine with an initial strain rate of 1 × 10−3 s−1 at room temperature. The sample was bonded together with cylindrical stainless steel after cutting into Φ10 mm [12]. The bonding model is shown in Fig. 2. The test model is shown in Fig. 3, which was used to measure the bonding strength of the laminated plates after being bonded as shown in Fig. 2. As can be seen from the four-point bending method depicted in Fig. 3, the middle section was pure bending, both sides were horizontal force bending, and the cross section was only moments and without shear in the pure bending section. This four-point bending method requires two loading force and the distance between the end point and the action point is a, which is equal to 0.25L (length) of the sample. The maximum bending moment (M) of the beam style is F × 0.25L, where F is the loading force, among which:
where, according to classical laminate theory (CLT) [13], σ is the maximum normal stress, characterization of the bonding strength, I is the moment of inertia, Y is the deflection, W z is the bending resistance section modulus, L is the length of the sample bar and d is the diameter of the round-shaped specimen which was cut by linear cutting machine. The final formula of the bonding strength was calculated as
3 Results and discussion
3.1 Bonding strength and effects of rolling parameters
During hot rolling, the laminated plates were subjected to a plastic deformation, which would cause grains to elongate along the rolling direction [14]. When the deformation temperature reaches the recrystallization temperature, taken as 0.4–0.6T m (T m is the melting point of the composite material), the plastic deformation exceeds a critical strain (or peak strain), and the grains between Mg and Al side would reunite and grow, which promotes the appearance of intermediate phase [15]. In this study, the rolling temperature (350–425 °C) is higher than that of the average theoretical recrystallization temperature of AZ31 magnesium alloy (260–390 °C, T m of 650 °C) and 5083 aluminum alloy (242–363 °C, T m from 570 to 640 °C) [16]. The effects of rolling parameters on bonding strength are shown in Fig. 4.
Figure 4 shows that the bonding strength increases with the reduction ratio increasing (<40 %) due to that the increase of rolling pressure leads to an increase in expanded surface area and crack number [17, 18]. However, the bonding strength decreases when the reduction ratio exceeds 40 %, which may be caused by the metallographic structure and the diffusion layer. For the sample rolled at 400 °C, the bonding strength reaches 20 MPa with a reduction ratio of 40 %, while it decreases to 12.5 MPa with a reduction ratio of 45 % due to the cracking of binding interface layer as shown in Fig. 5c. It is commonly reported that the bonding strength of laminated plates increases with the reduction ratio increasing due to an increase in contact mean pressure; however, the bonding strength decreases evidently if the reduction ratio surpasses a certain value [9]. As shown in Fig. 4, when the rolling temperature is 375 °C, the bonding strength decreases with the reduction ratio of 30 % and 35 % compared with the rolling temperature of 350 °C. The reason is that the diffusion of the atom between Mg and Al sides becomes active and forms the intermediate phase in the interface layer [19, 20]. It is clear that the strength of the majority materials decreases with the grain size increasing. However, the bonding strength increases when the rolling temperature reaches 400 °C, because the atom in the boundary obtains high activation energy and migrates to the surface to form thin diffusion layer, which promotes the combination of Al/Mg/Al laminated plates. Nonetheless, the bonding strength decreases when the temperature is 425 °C due to the conformation of interdiffusion layer as shown in Fig. 5d. The morphologies under different rolling parameters are shown in Fig. 5.
3.2 Microstructure and composition
During hot rolling, when the deformation temperature reaches the recrystallization temperature or higher, new phase would generate due to diffusion, which causes the conformation of mesosphere [21].
With rolling temperature of 400 °C and reduction ratio of 40 %, the rolling marks are the dislocation of the plastic deformation during the rolling process and neatly arrange in parallel as shown in Fig. 6a, b, which could improve the bonding strength [22]. However, with the rolling temperature increasing, lots of intermediate phases generated both in Al side and in Mg side, in other words, the cracking of the laminated plates start with intermediate compound layer as shown in Fig. 6c, d. These results happen in the breaking and locking of the brittle interface layers. The thicker the mesophase is, the less the compaction surface of the laminated plates is. Consequently, the bonding strength decreases consciously [23]. The results of XRD analysis are shown in Figs. 7 and 8. The constituent phases on the surface of the Al plate are α-Al and a small amount of Al 3 Mg 2 , while those of the Mg plate are mainly β-Mg and Al 12 Mg 17 in the experimental laminated plates. In addition, there is no discrepancy in the phases present for all cases [24].
3.3 Effects of annealing treatment on bonding strength
As shown in Fig. 9, the optimal annealing temperature is 300 °C. When it exceeds 300 °C, the bonding strength decreases. The reason is that the width of the diffusion of the Al/Mg/Al laminated plate increases with the annealing temperature increasing. The reason is that temperature has a significant impact on the rate of the diffusion atom. It is confirmed that the rate of the diffusion atom is slow when the temperature is low, which is unable to form intermetallic compound; when the temperature increases, the diffusion atom are qualified with thermodynamic and kinetic conditions for the formation of intermetallic compounds [25]. Consequently, the thickness of the diffusion layer increases as shown in Fig. 10. When annealed at 300 °C for 20 min, the interface of the laminated plates is divided evidently without any intermediate phase (Fig. 10a); when the annealing temperature reaches 400 °C, parts of intermediate phases generate (Fig. 10b). As a result, the combination degree of the Al and Mg matrixes degrades, which causes the debridement of the bonding strength.
The effect of annealing time on the diffusion layer was also investigated after hot rolling at 400 °C with reduction ratio of 35 %. The effects of annealing time on the bonding strength are mainly attributed to the effects of the thickness of interdiffusion layers. The morphologies of the interface of the samples are shown in Fig. 11. As shown in Fig. 11, the thickness of the interdiffusion layers increases with the annealing time increasing. Consequently, the binding property of the laminated sheets degrades obviously.
4 Conclusion
The microstructure and bonding strength of 5083 Al/AZ31 Mg/5083 Al laminated plate after one-pass hot rolling were investigated in this study. In summary, the width of the diffusion layer increases with the rolling temperatures increasing and annealing time extending, and the bonding strength of the samples decreases when the rolling temperature exceeds 400 °C due to the diffusion of the atom between the two sides of the matrixes and the growth of the intermediate phase. The maximum bonding strength of the thin plate is nearly 20 MPa with rolling temperature of 400 °C and reduction ratio of 40 %, and the bonding strength of the experimental laminated plate decreases when the reduction ratio exceeds 40 % due to the appearance of crack and flow. When the annealing temperature is 250–300 °C or annealing time is 1.5 h, a better property of the materials is achieved. When the annealing temperature exceeds 300 °C or annealing time exceeds 1.5 h, respectively, the bonding strength of Al/Mg/Al plate decreases.
References
Abbasid M, Toroghinejad MR. Effects of processing parameters on the bond strength of Cu/Cu roll-bonded strips. J Mater Process Technol. 2010;210(18):560.
An J, Liu YB, Lu Y, Sun D. Hot roll bonding of Al-Pb-bearing alloy strips and steel sheets using an aluminized interlayer. Mater Charact. 2001;47(3–4):291.
Danes H, Manesh A, Tamera AK. The effect of annealing treatment on mechanical properties of aluminum clad steel sheet. J Mater Des. 2003;24(17):617.
Li XR, Liang W, Zhao XG, Zhang Y, Fu XP, Liu FC. Bonding of Mg and Al with Mg–Al eutectic alloy and its application in aluminum coating on magnesium. J Alloys Compd. 2009;471(3):408.
Rattans B, Yukio M, Yoshiharu M. Dissimilar material laser welding between magnesium alloy AZ31B and aluminum alloy A5052-O. Sci Technol Adv Mater. 2005;6(8):199.
Somasekharan AC, Murray LE. Microstructures in friction-stir welded dissimilar magnesium alloys and magnesium alloys to 6061-T6 aluminum alloy. Mater Charact. 2004;52(1):49.
Liu P, Li YJ, Gang HR. Jawing. A study of phase constitution near the interface of Mg/Al vacuum diffusion bonding. Mater Lett. 2005;59(6):2001.
Matsumoto H, Watanabe S, Hamada S. Fabrication of pure Al/Mg–Li alloy clad plate and its mechanical properties. J Mater Process Technol. 2005;169(5):9.
Ueda TT, Tuatara M, Kamala Y, Kikuchi S. Preparation and hydrogen storage properties of Mg–Ni–Mg2Ni laminate composites. J Alloys Compd. 2005;386(1–2):253.
Takeichi N, Tanaka K, Tanaka HH, Ueda TT, Kamiya Y, Tsukahara M, Miyamura H, Kikuchi S. Hydrogen storage properties of Mg/Cu and Mg/Pd laminate composites and metallographic structure. J Alloys Compd. 2007;446–447(39):543.
Froes FH, Eliezer D, Aghion E. The science, technology, and applications of magnesium. JOM. 1998;9(1):30.
Kojima Y. Platform science and technology for advanced magnesium alloys. Mater Sci Forum. 2000;350–351(26):73.
Polmear I. Magnesium alloys and applications. J Mater Sci Technol. 1994;10(478):1.
Liu FC, Liang W, Li XR. Improvement of corrosion resistance of pure magnesium via vacuum pack treatment. J Alloys Compd. 2008;461(1–2):399.
Luo CZ, Liang W, Li XR, Zhao XG. Study on interface characteristics of Al/Mg/Al composite plates fabricated by two-pass hot rolling. Mater Sci Forum. 2013;747–748(8):346.
Li YJ, Liu P, Wang J, Ma HJ. XRD and SEM analysis near the diffusion boding interface of Mg/Al dissimilar materials. Vacuum. 2008;82(1):15.
Zhao LM, Zhang ZD. Effect of Zn alloy interlayer on interface microstructure and strength of diffusion-bonded Mg-Al joints. Scr Mater. 2008;58(4):283.
Matsumoto H, Watanabe S, Hamada S. Fabrication of pure Al/Mg–Li alloy clad plate and its mechanical properties. J Mater Process Technol. 2005;169(1):9.
Movahedi M, Madaah Hosseini HR, Kokabi AH. The influence of roll bonding parameters on the bond strength of Al-3003/Zn soldering sheets. Mater Sci Eng A. 2008;487(23):417.
Lu C, Tieu K, Wexler D. Significant enhancement of bond strength in the accumulative roll bonding process using nano-sized SiO2 particles. J Mater Process Technol. 2009;209(10):4830.
Zhang XP, Yang TH, Castagne S, Gu CF, Wang JT. Proposal of bond criterion for hot roll bonding and its application. J Mater Des. 2011;32(4):2239.
Liu LM, Zhao LM, Xu RZ. Effect of interlayer composition on the microstructure and strength of diffusion bonded Mg/Al joint. J Mater Des. 2009;30(9):4548.
Mahendranal G, Bal Subramanian V, Senthilvelan T. Developing diffusion bonding windows for joining AZ31B magnesium–AA2024 aluminum alloys. J Mater Des. 2009;30(41):1240.
Li XR, Liang W, Zhao XG, Zhang Y, Fu XP, Liu FC. Bonding of Mg and Al with Mg–Al eutectic alloy and its application in aluminum coating on magnesium. J Alloys Compd. 2009;471(65):408.
Zhang XP, Yang TH, Liu JQ, Luo XF, Wang JT. Mechanical properties of an Al/Mg/Al trilaminated composite fabricated by hot rolling. J Mater Sci. 2010;45(9):3457.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Nos. 51301118 and 51175363), the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20050112001) and the Natural Science Foundation of Shanxi Province (No. 2006011051).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, AL., Liu, XH., Dong, L. et al. Binding property of Al/Mg/Al thin plates fabricated by one-pass hot rolling with different reduction ratios, temperatures and annealing treatments. Rare Met. 37, 136–142 (2018). https://doi.org/10.1007/s12598-015-0519-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0519-0