Abstract
The pressure nitric acid leaching of alkali-pretreated low-grade limonitic laterite, as well as removing impurity Al(III) and preparing intermediate product of nickel/cobalt sulphide from leaching liquor were investigated. After pretreatment, iron exists in the form of amorphous iron oxides, while nickel is adsorbed on the surface of iron oxides in the form of nickel oxide. The preferable pressure leaching conditions are determined as follows: leaching temperature of 458 K, leaching duration of 60 min, initial acidity of nitric acid of 1.90 mol·L−1 and liquid to solid ratio of 3:1 (volume to mass ratio). Under these conditions, the leaching efficiencies of Ni, Co and Al are 95 %, 88 % and 55 %, respectively, and that of Fe is less than 1 %. The loss rates of Ni and Co are 1.8 % and 1.5 %, respectively, during the step of removing impurity Al(III). The sulphide precipitation process produces the interim production of nickel/cobalt sulphides, recovering greater than 99 % of Ni and Co in the purified solution. The iron-rich (>60 %) pressure leaching residue with low Cr, S can be further reclaimed as the raw materials for iron making.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Among the world’s land-based nickel resources, laterite ores account for 60 %–70 % and the others are mainly sulphide nickel ores. But about 70 % of current worldwide production of nickel is derived from sulphide nickel ores, and the laterite ores are not used sufficiently [1–6]. There is an increasing interest in the economically utilizing of low-grade laterites as the continuous depletion of sulphide nickel ores and high-grade nickel laterite ores [2, 3, 7–10].
The processes applied commercially to recover nickel and cobalt from laterite include pyrometallurgical and hydrometallurgical methods [11, 12]. Compared with pyrometallurgical processes suited to treat saprolitic laterite, hydrometallurgical processes are more applicable to limonitic laterite ores and mainly include reduction-roasting ammonia–ammonium carbonate leaching (RRAL) and high pressure sulphuric acid leaching (HPAL) [13, 14]. At present, HPAL process for treating limonitic laterite becomes the hotspot of research and industrial application [15–18].
When the HPAL process was used to treat Cr-containing limonitic laterite ores from Indonesia, the leaching efficiency of Ni was less than 80 % neither in sulphuric acid nor in nitric acid medium. The phase composition analysis indicates that the un-leaching Ni embeds in the chromite, silicate mineral or other indissolvable minerals in the laterite ores. Moreover, the residues of the HPAL containing higher Cr and S are difficult to utilize in iron making. In view of the above problems, the pretreatment of complex laterite was investigated with a desire to achieve high leaching efficiency and great economic benefits.
In the studies of Guo et al. [19], the Cr-containing limonitic laterite ores from Indonesia were pretreated by alkali roasting using sodium carbonate (Na2CO3), and then leached with water to remove Cr and Al. Based on these studies, the pressure nitric acid leaching process of the pretreated limonitic laterite ore was investigated in detail. Contrast to sulphuric acid leaching, this process has low leaching temperature, and no scaling of calcium sulphate could not only extract the nickel and cobalt efficiently but also produce the iron-riched residue. The residue containing low Cr and S can be utilized for the iron making. For the iron-riched limonitic laterite ores, the sufficient utilization of iron will bring considerable economic benefits.
2 Experimental
2.1 Materials and reagents
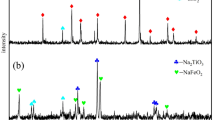
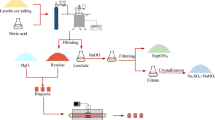
The limonitic laterite ores used in the present work were collected from Indonesia and then pretreated by alkali roasting and water leaching before pressure nitric acid leaching [19]. The leaching liquor was purified by removing Al(III) and then was used to produce the interim production of nickel/cobalt sulphides by sulphide precipitating process. Figure 1 shows the brief flow sheet of this process. Figures 2 and 3 show the XRD patterns of the limonitic laterite ores and the pretreated ores, respectively. Figure 2 indicates that the major mineral phase is goethite in the laterite ores, and magnetite, hematite, aluminium–chromite and serpentine are the minor ones. It can be found from Fig. 3 that the intensity of characteristic peaks of goethite is obviously weakened and the peak of hematite cannot be observed, but a new phase of bunsenite is discerned. During alkali-pretreated process, the main nickel and cobalt-bearing minerals are mostly transformed to amorphous phase that can be easier leached by nitric acid to recover Ni and Co. As shown in Fig. 4, the fine granular nickel oxides embed in the amorphous iron oxides. The elemental composition of pretreated laterite ore is presented in Table 1.
In this study, the analytical grade reagents, including nitric acid, sulphuric acid, calcium carbonate, sodium sulphide, etc., were used, and all aqueous solutions were prepared using distilled water.
2.2 Pressure nitric acid leaching
The leaching experiments were performed in 2-vertical titanium autoclave equipped with magnetic stirrer with a digital controller unit, an internal water-cooling titanium spiral tube and an electrical heating mantle which controlled the temperature of the reaction medium with an accuracy of ±1 °C.
The reactor was charged with slurry which was prepared by mixing leaching solvent of nitric acid and pretreated ore and then agitating continuously with a constant speed and heating up to a required temperature. After the leaching reaction was sustained for a predetermined time, the autoclave was cooled to the temperature of 353 K and then the gas was exhausted till the pressure was zero. The leaching slurry was withdrawn from reactor, and was vacuum filtered. The filter cake was dried in oven at 353 K for 8 h for sample preparation. The residue and leached liquor samples were chemically analysed for determining the elements of Ni, Co, Fe and Al.
2.3 Sulphide precipitation of nickel and cobalt
The removal of Al(III) from leaching liquor was carried out in the glass reactor before sulphide precipitation. Leaching liquor was adjusted to the appropriate pH value of 4.0 by adding 30 wt% CaCO3 slurry for 60 min to precipitate the major portion of Al(III) as aluminium hydroxides. The purified solution and precipitation were separated by filtration and then analyzed, respectively, to determine the impurity removal efficiency and loss of nickel and cobalt. To the purified solution, sodium sulphide was added to precipitate the Ni and Co as nickel and cobalt sulphides which were filtrated from solution and then analyzed.
2.4 Analysis methods
The metal concentrations in solution were determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES, ICPE-9000), and the pH value was measured with a pH/mV-metre. The phase composition and micro-morphology of solid samples were investigated by X-ray diffraction (XRD, Ultima IV), reflection macroscope and scanning electron microscopy (SEM, HITACHI S-3500N).
3 Results and discussion
3.1 Pressure nitric acid leaching
The metal oxides like NiO, CoO and Fe2O3 in pretreated laterite ore are soluble under autoclave conditions and react with acid according to the following stoichiometric reactions [1, 15]:
Ferric cations hydrolyse rapidly after the dissolution of iron oxide, forming directly hematite according to Reaction (4) [15]:
The effects of leaching temperature, leaching time, initial acid concentration and the liquor to solid ratio (volume to mass ratio) on the Ni and Co leaching efficiency were investigated to obtain the optimal reaction conditions.
3.1.1 Effect of leaching temperature
The experiments were performed in the temperature range of 433–478 K under the conditions: initial nitric acid concentration of 1.60 mol·L−1, liquid to solid ratio of 3:1 and agitation speed of 500 r·min−1 for 60 min, to study the effects of temperature on the leaching efficiencies of Ni, Co, Fe and Al. The results are shown in Fig. 5. It can be observed that the Ni and Co leaching efficiencies increase rapidly with the increase of the temperature from 433 to 458 K, but rise slowly with the temperature above 458 K. The leaching efficiency of Fe intends to decrease during the whole temperature range because the ferric hydrolytic reaction, as shown in Reaction (4), processes increasingly readily with the temperature elevating. The leaching efficiency of Al shows a pronounced decline with the temperature of below 443 K, while maintains about 50 %–55 % when the temperature exceeds 443 K. Therefore, the temperature is chosen as 458 K.
3.1.2 Effect of initial nitric acid concentration
Figure 6 shows the effects of initial nitric acid concentration on the leaching efficiencies of Ni, Co, Fe and Al. The Fe leaching efficiency is less than 1 % and that of Al alters little, maintaining about 50 % with the acid concentration varying from 1.00 to 2.70 mol·L−1. By contrast, the Ni and Co leaching efficiencies increase significantly with the initial nitric acid increasing and the acid level of 1.90 mol·L−1 is sufficient to leach most of the Ni and Co.
3.1.3 Effect of leaching time
Leaching experiments were conducted to investigate the effect of leaching time under the conditions: temperature of 458 K, initial acid concentration of 1.90 mol·L−1, liquor to solid ratio of 3:1 and agitation speed of 500 r·min−1. Results are plotted in Fig. 7, which show that leaching time has no remarkable effect on the Fe leaching efficiency which remains less than 1 %, but evidently affects the leaching efficiency of Ni and Co in the initial 60 min. It is seen that Al leaching efficiency increases slowly with an increase in leaching time and remains about 50 % after 80 min. Thus, leaching time for higher Ni and Co recovery is determined to be 60 min.
3.1.4 Effect of liquid to solid ratio
The pretreated ore was leached in solution containing 1.90 mol·L−1 nitric acid for 60 min at 458 K with liquid to solid ratios changing from 3:2 to 5:1. As shown in Fig. 8, with the liquid to solid ratio changing from 3:2 to 3:1, the leaching efficiencies of Ni and Co increase evidently, but maintain steadily with the liquid to solid ratio increasing continually. The leaching efficiencies of Fe and Al remain constant within the whole liquid to solid ratio range. The optimal liquid to solid ratio is therefore considered as 3.
Based on the above experiments, the optimal conditions of pressure acid leaching pretreated laterite ore are determined as leaching temperature of 458 K, leaching time of 60 min, initial nitric acid of 1.90 mol·L−1 and liquid to solid ratio of 3:1. Under the optimal conditions, the leaching efficiencies of Ni, Co, Al and Fe are about 95 %, 88 %, 55 % and less than 1 %, respectively. During the process, Ni and Co are selectively leached and the leaching residue contains about 0.028 % of Ni, 0.005 % of Co and more than 60.000 % of Fe.
The XRD pattern of the leaching residue is shown in Fig. 9, which indicates that the main diffraction peaks of the leaching residue match with the standard diffraction peaks of hematite and talcum. The result illustrates that the amorphous iron phase in pretreated laterite ore transforms to hematite phase during the pressure acid leaching. The SEM image of the leaching residue is shown in Fig. 10, and the further SEM–EDS analysis indicates that the main elements contained in hematite phase are Fe (70.63 %), O (26.66 %) and Si (2.62 %).
3.1.5 Contrast of leaching residues produced by nitric/sulphuric acid leaching of pretreated ores and nitric acid direct leaching of raw ores
The alkali pretreatment destroys the mineral lattices bearing Ni of the laterite, which makes more Ni be exposed and then easily leached during the subsequent pressure acid leaching process.
The subsequent contrast experiments of pressure nitric acid leaching for raw laterite ore and pretreated laterite ore, and pressure sulphuric acid leaching for pretreated laterite ore were carried out to evaluate the effects of pretreatment and leaching media on the leaching of Ni and Co and the iron grade of acid leaching residues.
The contrast experiments of pressure acid leaching were performed under their respective optimal conditions. The main compositions of the pressure acid leaching residues are presented in Table 2. It can be seen that the residue of direct pressure nitric acid leaching for the raw laterite ore contains more Cr, Ni and Co with low grade of iron; the residue of pressure sulphuric acid leaching for the pretreated ore also with lower grade of iron contains relatively plenty of S which is detrimental to the utilization of the residue in iron making. By contrast, the residue of pressure nitric acid leaching for pretreated ore has lower content of Ni, S, Co and Cr and higher grade of iron, thus it can be utilized as iron-making material.
3.2 Removal of aluminium
As shown in Table 3, the pressure acid leaching solution contains impurity Al and Fe which are required to remove for the subsequent step of sulphide precipitation. The separation was performed in a glass reactor. The leaching solution was adjusted to the appropriate pH value of 4.0 by adding 30 wt% CaCO3 slurry for 60 min to the neutralization and precipitation of the major portion of aluminium. The pressure nitric acid leaching is an oxidation process; therefore, the iron in leaching solution is ferric cation which can also be removed in the process of neutralization and precipitation.
The experiments of removing Al(III) were carried out at 298 K, because the former experiments indicate that the effect of temperature on the Al(III) precipitating efficiency is less when the terminal pH value is selected. Figure 11 presents the effect of terminal pH values on the precipitating efficiency of Al and the loss of Ni and Co. It can be seen that the Al(III) precipitating efficiency increases significantly to about 99 % with terminal pH value increasing in the range of 3.5–3.8 and the loss of Ni and Co are about 1.8 % and 1.5 %, respectively. No remarkable Al is precipitated with the terminal pH value exceeding 3.8, but the loss of Ni and Co increases, correspondingly. Therefore, the feasible pH value ranges from 3.8 to 4.0. After this process, the Fe(III) and Al(III) in solution are less than 0.005 and 0.010 g·L−1, respectively. The SEM image of depositing aluminium residue is shown in Fig. 12. It indicates that the residue exists in the form of aggregate granules and the particle diameters are about 20 μm.
3.3 Sulphide precipitating of nickel and cobalt
Metal sulphide precipitation is a frequently used and important process in hydrometallurgical treatment of ores and effluents with some advantages, including lower solubility of metal sulphide precipitates, potential for selective metal removal, fast reaction rates, etc. In this research, sodium sulphide was used as sulphide agent for collecting Ni and Co from leaching liquor. Previous work related to the sulphide precipitation of metals was well documented [20]. It is also found that the process of sulphide precipitation is sensitive to the dose of sulphide agent added in the liquor [20, 21]; hence, the effect of mole ratio (n) of Na2S to (Ni2++ Co2+) on Ni precipitating efficiency was studied. The results are shown in Fig. 13, which indicate that the optimum mole ratio is 1.2:1.0 with the Ni and Co precipitating efficiency of greater than 99 %. The Ni and Co in the sulphide are 42.72 % and 2.07 %, respectively. The SEM image of Ni/Co sulphide powder is shown in Fig. 14. As shown in Fig. 14, the Ni/Co sulphide powder is amorphous aggregation.
4 Conclusion
The process of alkali-pretreated pressure nitric acid leaching can selectively leach Ni and Co efficiently and produce iron-riched residue which can be utilized for iron making. The leaching liquor was purified by removing Al(III) and then was used to produce the interim production of nickel/cobalt sulphides. The optimal pressure nitric acid leaching conditions are determined as leaching temperature of 458 K, leaching time of 60 min, initial concentration of nitric acid of 1.90 mol·L−1 and liquid to solid ratio of 3:1. Under these conditions, the leaching efficiencies of Ni, Co and Al are about 95 %, 88 % and 55 %, respectively, and that of Fe is less than 1 %. More than 99 % Al(III) and Fe(III) are removed from leaching solution by neutralization precipitating with less than 2 % loss of Ni and Co, respectively. Greater than 99 % of the Ni and Co in the purified solution are precipitated as Ni/Co sulphide which can be further utilized to refine Ni and Co.
References
Baghalha M, Papangelakis VG. Pressure acid leaching of laterites at 250 °C: a solution chemical model and its applications. Metall Mater Trans B. 1998;29B(10):945.
McDonald RG, Whittington BI. Atmospheric acid leaching of nickel laterites review. Part I. Sulphuric acid technologies. Hydrometallurgy. 2008;91(1):35.
McDonald RG, Whittington BI. Atmospheric acid leaching of nickel laterites review. Part II. Chloride and bio-technologies. Hydrometallurgy. 2008;91(1):56.
Lu HB. Thermodynamic research on production of ferronickel alloy by electric furnace reduction from lateritic nickel ore. Chin J Rare Met. 2012;36(5):785.
Loveday BK. The use of oxygen in high pressure acid leaching of nickel laterites. Miner Eng. 2008;21(7):533.
Qiu S, Che XK, Zheng Q, Duan J. Experimental study on laterite-nickel ore with sulfating roasting-water immersion methods. Chin J Rare Met. 2010;34(3):406.
Lee HY, Kim SG, Oh JK. Electrochemical leaching of nickel from low-grade laterites. Hydrometallurgy. 2005;77(3):263.
Simate GS, Ndlovu S, Walubita LF. The fungal and chemolithotrophic leaching of nickel laterites––challenges and opportunities. Hydrometallurgy. 2010;103(2):150.
Stamboliadis E, Alevizos G, Zafiratos J. Leaching residue of nickeliferous laterites as a source of iron concentrate. Miner Eng. 2004;17(2):245.
Li JH, Li XH, Hu QY, Wang ZX, Zhou YY, Zheng JC, Liu WR, Li LJ. Effect of pre-roasting on leaching of laterite. Hydrometallurgy. 2009;99(1):84.
Guo XY, Li D, Park KH, Tian QH, Wu Z. Leaching behavior of metals from a limonitic nickel laterite using a sulfation-roasting-leaching process. Hydrometallurgy. 2009;99(3):144.
Mudd GM. Global trends and environmental issues in nickel mining: sulfides versus laterites. Ore Geol Rev. 2010;38(1):9.
Zhai XJ, Fu Y, Zhang X, Ma LZ, Xie F. Intensification of sulphation and pressure acid leaching of nickel laterite by microwave radiation. Hydrometallurgy. 2009;99(3):189.
Sudol S. The thunder from down under: everything you wanted to know about laterites but were afraid to ask. Can Min J. 2005;126(5):8.
Georgiou D, Papangelakis VG. Sulphuric acid pressure leaching of a limonitic laterite: chemistry and kinetics. Hydrometallurgy. 1998;49(1):23.
Rubisov DH, Papangelakis VG. Sulphuric acid pressure leaching of laterites-a comprehensive model of a continuous autoclave. Hydrometallurgy. 2000;58(2):89.
Rubisov DH, Krowinkel VG, Papangelakis VG. Sulphuric acid pressure leaching of laterites–universal kinetics of nickel dissolution for limonites and limonitic/saprolitic blends. Hydrometallurgy. 2000;58(1):1.
Whittington BI, McDonald RG, Johnson JA, Muir DM. Pressure acid leaching of Bulong nickel laterite ore. Part I. Effect of water quality. Hydrometallurgy. 2003;70(1):31.
Guo Q, Qu JK, Qi T, Wei GY, Han BB. Activation pretreatment of limonitic laterite ores by alkali-roasting method using sodium carbonate. Miner Eng. 2011;24(8):825.
Lewis AE. Review of metal sulphide precipitation. Hydrometallurgy. 2010;104(2):222.
Lewis AE, Swartbooi A. Factors affecting metal removal in mixed sulfide precipitation. Chem Eng Technol. 2006;29(2):277.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 51274044, 51304023, and U1302274).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, YL., Wang, CY., Yang, YQ. et al. Pressure nitric acid leaching of alkali-pretreated low-grade limonitic laterite. Rare Met. 34, 64–70 (2015). https://doi.org/10.1007/s12598-014-0432-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0432-y