Abstract
Present study discusses the experimental demonstration of optical fiber surface plasmon resonance (SPR) sensor for the determination of fluoride contamination in drinking water. Kretschmann–Raether configuration is employed on an optical fiber with sodium fluoride (NaF) contaminated distilled (DI) water that acts as a sensing analyte. A nanofilm of silver (Ag) about 50 nm in thickness is deposited over cladding etched portion of the optical fiber core. NaF salt concentration is varied from 0.005 to 0.035 g/mL in DI water and refractive index (RI) measurements of each concentration show linear increment with increase in NaF concentration. Present study is based on the principle that with a tiny variation in RI of sensing analyte, a change in resonance wavelength is detected as per SPR condition. For the designed fiber optic probe, sensitivity is calculated with variable RI and comes out to be 6489.03 nm/RIU (Refractive index unit). Results demonstrate that the developed SPR-based fiber optic sensor can be valuable for the detection of fluoride impurities in drinking water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In preceding few years, a lot many sensors have been designed and developed for the health monitoring system such as detection of different kinds of adulterants in food products and in drinking water [1]. Currently, fluoride impurities in drinking water have become a serious concern which occurs naturally or may be intensely added in water. Fluoride occurs naturally of about 0.3 g/Kg of earth’s crust. It causes many hazardous effects to human health such as dental fluorosis and its higher level also affects organs, bones and other primary parts of human body. Arsenic, cadmium, lead, chlorine and zinc are other contaminants which exists in drinking water or wastewater of industries affecting the environment also [2, 3]. Nowadays, it is becoming difficult for a common man to access pure drinking water particularly in developing countries. Study shows that excessive amount of fluoride contaminants causes skeleton, dental fluorosis and crippling skeleton fluorosis [4]. A survey exposed that around 25 nations have been affected by fluoride contaminated drinking water comprising many continents such as Asia, Africa and Australia. Over 200 million peoples have been suffering by fluoride disease, among which China and India are the most affected countries due to drinking fluoride contaminated water [5,6,7]. In India, children below 14 years of age are most affected by fluorosis (around 6 million) and the currently allowed drinking water standard limit for fluoride is 4.0 mg/L. Hence, in recent decade, detection of fluoride impurities in drinking water has drawn significant attention. A lot of optical and chemical techniques/sensors have been realized and designed so far for the detection of fluoride impurities in water such as surface enhanced Raman scattering [8], fluorescent sensors [9], colorimetric method [10], ion chromatography [11] and many more [12]. All the techniques established so far oblige suitable laboratory instrument and are a bit costly. Therefore, there is a necessity for the development of adequate optical fiber sensors for the detection of fluoride impurities in drinking water. There are several techniques and approaches for designing fiber optic refractive index (RI) sensors such as SPR sensors [13], lossy mode resonance (LMR) sensors [14], fiber Bragg grating sensors [15] and many more [16, 17]. However, optical sensors based on SPR technique show better sensing capabilities as we vary the RI of the sensing analyte and thus, often used as RI sensors [18, 19]. SPR condition is attained due to coupling between evanescent wave and surface plasmon (SP) wave supported by a nano-sized layer of metal film [20]. There are many SPR sensors that have been developed to detect several types of adulterants and contaminants in food items [21,22,23]. Also, SPR sensors have been proposed for the determination of trace metals concentration in water such as zinc (Zn), iron (Fe) and chloride (Cl) [24, 25]. Due to their light weight, compact design, label-free sensing and solicitation in remote sensing, SPR-based fiber optic sensor comes out to be an important tool in many chemical, physical and bio-sensing applications [26,27,28,29,30]. Here, we have made an effort to develop a sensor for the detection of sodium fluoride (NaF) impurities in distilled (DI) water. A familiar Kretschmann–Raether configuration [31] on a multimode optical fiber is used for the excitation of SP wave. Samples were prepared for different concentration of NaF in DI water and then placed around the sensing layer, acting as a sensing analyte.
Theory
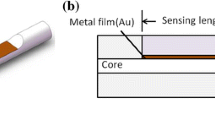
The collective oscillation of free electrons at an interface having opposite sign of their dielectric permittivity are well known as SPs. Electric field associated with SPs decays exponentially in metal as well as in dielectric medium. Since wave vector of direct light is smaller than wave vector of SPs, thus it cannot excite them. However, when a p-polarized light is incident on a prism of high RI, an evanescent wave is produced at prism-metal interface and causes excitation of SPs at metal–dielectric interface. This configuration for excitation of SPs is known as Kretschmann–Raether configuration [31]. For designing a better SPR sensor, this configuration can also be realized on multimoded optical fiber in which spectral interrogation instead of angular interrogation scheme is generally used. A thin metal film of thickness 10–100 nm is deposited over cladding detached portion of the fiber core and this layer acts as a sensing layer. After this, the sensing medium is placed in direct contact with the sensing layer.
Figure 1 displays the schematic diagram of experimental setup for the detection of fluoride contamination in water. Light from a polychromatic source is made to enter through one end of optical fiber at an angle ‘\(\theta\),’ and the other end of the fiber is attached to a spectrometer to attain SPR response. Due to greater RI of fiber core, total internal reflection (TIR) takes place and light propagate inside the fiber core. As a result of which evanescent wave having exponentially decaying nature, is produced at core-metal interface. When propagation vector of both the waves, i.e., evanescent wave and SP wave becomes equal, a resonance takes place which results in a sharp minimum in the output power of transmitted light at a wavelength called resonance wavelength. This resonance wavelength is much sensitive with a little alteration in RI of the sensing analyte. The minimum in intensity is due to the complete transfer of energy from evanescent wave to SPW. The wave vector matching condition of SPR is given by the equation as [32]
In above equation, left- and right-hand side stands for the propagation constant of the evanescent wave and SP wave, respectively, and \(\varepsilon_{{{\text{core}}}}\),\(\varepsilon_{m}\) and \(\varepsilon_{s}\) stands for dielectric permittivity of optical fiber core, Ag layer and sensing medium, respectively.
Experimental
Materials and sample preparation
Sodium fluoride (NaF) salt and Ag wire were from Alpha Easer; multimoded plastic clad silica (PCS) fiber of diameter 600 µm purchased from Science Palace Ajmer; distilled water and acetone were bought from Sarthak Sales, Jaipur. To perform the experiments, prepared NaF samples were injected in glass flow cell mounted on a 3-D translation stage acquired from Science Palace Ajmer. The halogen light source, spectrometer and detector were bought from Avantes company (USA). We prepared five samples of different concentration of NaF salt in distilled water viz. 0.005 g/mL, 0.015 g/mL, 0.025 g/mL, 0.030 g/mL, 0.035 g/mL and 0 g/mL is the reference for non-contaminated water. To measure the RI of these prepared samples, Abbes Refractometer was used.
Fabrication of fiber optic probe
For the preparation of fiber optic probe, a multimoded PCS fiber of numerical aperture 0.4 and core diameter 600 µm was taken into account. Then, a small part of the cladding of length 1.0 cm from central portion and 0.5 cm from both the ends of optical fiber was detached using a sharp blade. The end faces were made sharp and circular so that light can be guided properly through the optical fiber core. The cladding removed middle portion of the fiber was first cleaned with acetone and distilled water. Subsequently, we deposited a 50 nm thin Ag layer on cladding removed central portion of the fiber by employing thermal evaporation-based vacuum coating unit (Hind Hivac India). For the creation of high vacuum in chamber, the process was done by using two pumps viz. rotatory pump and diffusion pump. Firstly, rotatory pump was made ON in roughing position and left ON for half an hour till pressure had become better than \({10}^{-3}\) mbar. After this, diffusion pump come into action in backing position and left ON for one or two hours until a pressure better than \({5} {\text{mbar}} \times 10^{ - 6}\) was achieved. Then, the state of high vacuum was achieved and LT current knob was moved slowly until Ag metal evaporated. The rate and thickness of coating were monitored from digital thickness monitor (DTM). Since core of the optical fiber is cylindrical, therefore, optical fiber was rotated for the uniform film deposition inside the vacuum chamber.
Experimental procedure
Firstly, the fabricated fiber optic probe was fixed in the glass flowcell which comprises of two rubber corks fitted at both the ends. The prepared samples of different NaF concentration were injected in flowcell one by one from low to high concentration. A halogen source of light was used to fed light from one end of the fiber at an angle ‘θ,’ and from another end, the light was spectrally interrogated using spectrometer. The only light rays for which angle of incidence lies between critical angle to π/2 propagate inside the fiber core and hence interact with the sensing analytes. Spectrometer was interfaced to the personal laptop in which transmission spectra were recorded after installation of latest Avasoft software in it.
Results and discussion
Our main focus in the present work is to find SPR sensing capabilities for the detection of NaF impurities in distilled water. The NaF concentration was varied from 0.005 to 0.035 g/mL in DI water, and their corresponding refractive index (RI) values were estimated with the help of Abbe’s refractometer. To see the change in RI with the addition of increased NaF concentration, we plotted a graph showing the RI variation with the NaF concentration (Fig. 2). From results, we see that RI increases linearly with increase in the concentration of NaF in distilled water and its RI range lies between 1.333 and 1.336.
To check the response of fabricated fiber probe toward change in NaF concentration, transmission spectra were measured for each NaF concentration by plotting the normalized transmitted power as a function of wavelength. Initially, the sample having NaF concentration 0 g/ml, i.e., pure water, was inserted in flowcell as a reference of non-contaminated water and the corresponding transmission spectrum was measured. It was observed that SPR dip lies within in the visible range of optical spectrum and the resonance wavelength was detected as 549.926 nm. Subsequently, rest of the prepared NaF samples were poured in flowcell one by one from low to high concentration and their corresponding spectrum were measured as depicted in Fig. 3. It was observed that if we increase the NaF concentration in DI water, a red shift in resonance wavelength was detected. Corresponding to different concentrations 0.005 g/mL, 0.015 g/mL, 0.025 g/mL, 0.030 g/mL, 0.035 g/mL, the resonance wavelengths were detected at 555.262 nm, 568.588 nm, 572.915 nm, 577.905 nm and 580.898 nm, respectively. The reason for this behavior could be explained from the SPR condition specified in Eq. 1 that a little variation in the RI of the NaF samples leads to the modification in wave vector of SPW and hence the resonance condition is met at some higher wavelength value.
Hence, the SPR curves shown in Fig. 3 confirm the SPR excitation condition as explained in Eq. 1 further; Table 1 shows the experimentally observed resonance wavelength values for each NaF concentration. It should be noted that the depth of resonance dip measures the resonance strength of the corresponding SPR curves. It means that for curve with smaller depth of resonance dip, the resonance strength decreases and hence resulting in the lower penetration of evanescent wave inside the sensing medium. Correspondingly, larger depth of resonance dip results in higher penetration of evanescent wave resulting in the stronger SPR excitation which adversely affect the sensitivity of the SPR sensor. This depth of resonance dip can be further increased by using the different type of optical fiber structures such as tapered fibers and D-shaped fibers. For evaluating the shift in resonance wavelength w.r.t. each NaF concentration, a graph was plotted between resonance wavelength and RI of sensing analyte as shown in Fig. 4. The obtained curve was linearly fitted and slope of this graph gives the sensitivity of designed SPR sensor for the detection of fluoride contamination in water.
Sensitivity may be demarcated as the alteration in resonance wavelength with respect to the variation in refractive index of the NaF samples [32]. For the designed SPR sensor, the experimental sensitivity was observed to be 6489.03 nm/RIU which also give us a good estimate for the detection of fluoride impurities in drinking water. In addition, the performance of the designed SPR sensor is evaluated in terms of figure of merit (FOM) which is defined as the ratio of sensitivity to the full width at half maximum (FWHM). The FOM value for the designed SPR sensor was measured as 36.05 RIU−1.
Conclusion
A fiber optic SPR sensor was fabricated and utilized for the detection of fluoride contamination in water using Kretschmann–Raether configuration. NaF concentration was varied from 0.005 to 0.035 g/mL in DI water and the resonance wavelengths values shifted to higher wavelengths with increase in NaF concentration. The variation of resonance wavelength is linear with increase in NaF concentration. The obtained sensitivity and FOM of present fiber optic probe were observed to be 6489.03 nm/RIU and 36.05 RIU−1, respectively. Thus, the present study can be beneficial for the detection of fluoride impurities in drinking water with higher sensitivity values.
References
B. Glisic, D. Inaudi, Fibre Optic Methods for Structural Health Monitoring (John Wiley & Sons, Chichester, 2008)
S.D. Richardson, Disinfection by-products and other emerging contaminants in drinking water. TrAC Trends Anal. Chem 22(10), 666–684 (2003)
R.L. Calderon, The epidemiology chemical contaminants of drinking water. Food. Chem. Toxicol. 38, S13–S20 (2000)
V. Jain, S. Kumbhaj, P.K. Sen, Long-period fiber grating sensor to determine fluoride contamination in water. Res. Rev. J. Phys. 7(3), 36–43 (2018)
S. Ayoob, A.K. Gupta, Fluoride in drinking water: a review on the status and stress effects. Crit. Rev. Environ. Sci. Tech 36(6), 433–487 (2006)
Y. Zhang, S.R. Cao, Coal burning induced endemic fluorosis in China. Fluoride 29(4), 187–260 (1996)
S. Jagtap, M.K. Yenkie, N. Labhsetwar, Fluoride in drinking water and defluoridation of water. Chem. Rev. 112(4), 2454–2466 (2012)
X. Li, M. Zhang, Y. Wang, X. Wang, H. Ma, P. Li, W. Song, X. Xia Han, B. Zhao, Direct detection of fluoride ions in aquatic samples by surface-enhanced Raman scattering. Talanta 178, 9–14 (2018)
A.V. Budeev, L.K. Sadieva, I.S. Kovalev, G.V. Zyryanov, Pyrene-derived benzimidazoles as fluorescent sensors for detection of fluoride anion, in AIP Conference Proceedings 2063 1 040012 (2019)
R. Chavli, N.S. Gunda, S. Naicker, S.K. Mitra, Rapid detection of fluoride in potable water using a novel fluorogenic compound 7-O-tert-butyldiphenylsilyl-4-methylcoumarin. Anal. Chem. Res. 6, 26–31 (2015)
H. Jason, L. Christina, Determination of chloride, fluoride, and sulfate ions in various water samples using ion chromatography. J. Anal. Chem. 3, 24–28 (2012)
B. Qiu, Y. Zeng, L. Cao, R. Hu, X. Zhang, T. Yu, J. Chen, G. Yang, Y Li (2016) A colorimetric and ratiometric fluorescence sensor for sensitive detection of fluoride ions in water and toothpaste. RSC Adv. 6(54), 49158–49163 (2016)
S. Vikas, K. Gupta, R.K. Tejavath, Verma, urea detection using bio-synthesized gold nanoparticles: an SPR/LSPR based sensing approach realized on optical fiber. Opt. Quantum Electron. 52(278), 1–14 (2020)
Vikas, R.K. Verma, Sensitivity enhancement of a lossy mode resonance based tapered fiber optic sensor with an optimum taper profile. J. Phys. D Appl. Phys. 51(41), 415302 (2018)
G. Woyessa, A. Fasano, A. Stefani, C. Markos, K. Nielsen, H.K. Ramussen, O. Bang, Single mode step-index polymer optical fiber for humidity insensitive high temperature fiber Bragg grating sensors. Opt. Exp. 24(2), 1253–1260 (2016)
J. Zhou, Y. Wang, C. Liao, B. Sun, J. He, G. Yin, S. Liu, Z. Li, G. Wang, X. Zhong, J. Zhao, Intensity modulated refractive index sensor based on optical fiber Michelson interferometer. Sens. Actuat. B Chem. 208, 315–319 (2015)
P. Polynkin, A. Polynkin, N. Peyghambarian, M. Mansuripur, Evanescent field-based optical fiber sensing device for measuring the refractive index of liquids in microfluidic channels. Opt. let. 30(11), 1273–1275 (2005)
T. Hu, Y. Zhao, A.-N. Song, Fiber optic SPR sensor for refractive index and temperature measurement based on MMF-FBG-MMF structure. Sens. Actuators B Chem. 237, 521–525 (2016)
A.K. Mishra, S.K. Mishra, B.D. Gupta, SPR based fiber optic sensor for refractive index sensing with enhanced detection accuracy and figure of merit in visible region. Optics Comm. 344, 86–91 (2015)
R.C. Jorgenson, S.S. Yee, A fiber-optic chemical sensor based on surface plasmon resonance. Sens. Actuators B Chem. 12, 213–220 (1993)
K. Narsaiah, S.N. Jha, R. Bhardwaj, R. Sharma, R. Kuma, Optical biosensors for food quality and safety assurance—a review. J. Food Sci. Technol. 49(4), 383–406 (2012)
K.V. Ragavan, S. Neethirajan, Nanoparticles as biosensors for food quality and safety assessment. Nanomater. Food Appl. 147, 202 (2019)
Vikas, M.K. Yadav, P. Kumar, R.K. Verma, Detection of adulteration in pure honey utilizing Ag-graphene oxide coated fiber optic SPR probes. Food Chem. 332, 127346 (2020)
R. Verma, B.D. Gupta, Detection of heavy metal ions in contaminated water by surface plasmon resonance based optical fibre sensor using conducting polymer and chitosan. Food Chem. 166, 568–575 (2015)
B.S. Bourah, R. Biswas, An optical fiber based surface plasmon resonance technique for sensing of lead ions: a toxic water pollutant. Opt. Fib. Technol. 46, 152–156 (2018)
C. Liedberg, I. Nylander, Sundstrom, surface plasmon resonance for gas detection and biosensing. Sens. Actuators B Chem. 4, 299–304 (1983)
S.K. Mishra, S.N. Tripathi, V. Choudhary, B.D. Gupta, SPR based fibre optic ammonia gas sensor utilizing nanocomposite film of PMMA/reduced graphene oxide prepared by in situ polymerization. Sens. Actuators B Chem. 199, 190–200 (2014)
P. Bhatia, B.D. Gupta, Fabrication and characterization of a surface plasmon resonance based fiber optic urea sensor for biomedical applications. Sens. Actuators B Chem. 161(1), 434–438 (2012)
A.K. Mishra, S.K. Mishra, R.K. Verma, An SPR-based sensor with an extremely large dynamic range of refractive index measurements in the visible region. J. Phys. D Appl. Phys. 48(43), 435502 (2015)
D.J. Gentleman, L.A. Obando, J.F. Masson, J.R. Holloway, K.S. Booksh, Calibration of fiber optic based surface plasmon resonance sensors in aqueous systems. Anal. Chim. Acta. 515(2), 291–302 (2004)
E. Kretschmann, H. Raether, Radiative decay of non-radiative surface plasmons excited by light. Z. Naturforschung A. 23(12), 2135–2136 (1968)
V. Vikas, R.K. Verma, Design considerations of a surface plasmon resonance (SPR) based tapered fiber optic bio-sensing probe with graphene-MoS2 over layers. Optik Int. J. Light Electron. Opt. 180, 330–343 (2019)
Acknowledgements
The author Mr. Vikas would like to acknowledge Department of Science and Technology (DST), INDIA, for funding the INSPIRE fellowship (Registration no- IF170543). Also, this work is partially supported by DST SERB CRG/2020/005593, India.
Funding
The funding was provided by Science and Engineering Research Board, (CRG/2020/005593), Rajneesh Verma
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vikas, Sharma, S. & Verma, R.K. Optical fiber SPR sensor for the detection of fluoride contamination in drinking water. J Opt 51, 707–712 (2022). https://doi.org/10.1007/s12596-021-00803-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12596-021-00803-x