Abstract
Temperature influence on development and larval survivability of Culicoides peregrinus Kieffer (Diptera: Ceratopogonidae), a vector of bluetongue virus (BTV), was assessed at five rearing temperatures (15 °C, 20 °C, 26 °C, 30 °C and 35 °C), a photoperiod of 13L:11D, and relative humidity of 75%. The higher and lower threshold temperatures and developmental success of life stages during rearing were ascertained and corroborated with the seasonal abundance. Rearing temperatures significantly influenced the number of viable eggs laying by females and larval survivability. Most oviposited eggs and larval survivability (> 80%) were observed at 26 °C and the least (8%) was recorded at 35 °C. Development of immatures ceased at 15 °C. The duration from egg to adult stage was shorter (10–12 days) at 35 °C compared to that recorded at 26 °C (18–23 days). This study will be useful in the establishment of a laboratory colony of this vector species thereby, contributing to our understanding of the vectorial capacity, competence and continual supply of viable eggs throughout the year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growth and development of Culicoides insects tend to accelerate with favourable temperature range but are adversely affected when the temperature range becomes unfavourable (Veronesi et al. 2009). Biting midges Culicoides (Diptera: Ceratopogonidae) are one of the smallest haematophagous flies vectoring more than 50 arboviruses such as bluetongue virus (BTV), epizootic hemorrhagic disease virus (EHDV) and African horse sickness virus (AHSV) in sheep, cattle and wild ruminants (Mellor et al. 2009). Among these, BTV disease has been reported from several states of India, particularly in native and exotic breeds of sheep (Prasad et al. 2009). Vector abundance and appearance of Bluetongue disease (BTD) depend on climatic and seasonal variations (Prasad et al. 2009). In the southern states of India, peak abundance of Culicoides has been recorded with concomitant BTD outbreaks in monsoon period (Sreenivasulu et al. 2004). The BT vectors, C. peregrinus, C. oxystoma Kieffer, and C. fulvus Sen and Das Gupta are abundant in the state of West Bengal (Harsha et al. 2020). These vectors peak in monsoon season but show low abundance in post-monsoon (Harsha et al. 2020). The knowledge about standardization of rearing conditions, larval food, captive mating, and artificial blood feeding is important for laboratory studies on vector competence (Nayduch et al. 2014; Harsha and Mazumdar 2015). Globally, 23 species have been attempted for rearing under laboratory conditions (Narladkar et al. 2006; Barceló and Miranda 2018; Erram and Burkett-Cadena 2019) but only two colonies viz., C. sonorensis Wirth and Jones and C. nubeculosus (Meigen) are successfully maintained (Nayduch et al. 2014). Laboratory rearing of C. peregrinus with respect to the most suitable combination of food and substrate was done by Harsha and Mazumdar (2015). The temperature below which no measurable development occurs is called threshold of development (Ludwig 1928; Campbell et al. 1974). However, temperature thresholds of developmental period of this tropical vector species in India remain to be worked out. Such a study would make possible to develop suitable models of development period, estimate emergence time, and potential distribution of this vector's potential virulent populations. This knowledge can be used in practical application in epidemiology (Briere et al. 1999; Régnière et al. 2012). The influence of temperature on life stages was previously studied on C. arakawae (Arakawa), C. imicola Kieffer, and C. brevitarsis Kieffer (Kitaoka 1982; Allingham 1991; Veronesi et al. 2009). At low temperature (5 °C) the embryonic developmental period of C. variipennis sonorensis Wirth and Jones is delayed (Hunt and Tabachnick 1995). In this investigation, an experiment was set up to corroborate the development duration from freshly laid eggs to emergence of adults from pupal cases impacted by higher and lower rearing temperatures, and impact on the male: female ratio of emerged adults. Another experiment was performed to investigate the effect of refrigeration of C. peregrinus eggs and subsequent hatching in the laboratory. The viability of the stored eggs at low temperature was also studied. The objective of this study is to provide a standard laboratory rearing condition for optimum development and reproduction of this vector species of farm animals and its continuous colonization in laboratory.
Materials and Methods
Study Area and Collection of Field Adult
Live adults of C. peregrinus were trapped in a cage framed with aluminium wire (< 1 cm d) and covered with muslin cloth (20 cm h × 15 cm l × 15 cm w) by operating LED UV light traps from 17.30 h to 7.00 h of the next morning in a cattle shed near Gangpur, West Bengal, India (23°22’N, 87°90’E). These adults were soon transported to the laboratory to avoid direct exposure to sunlight and were initially kept for a while (15–20 min) at room temperature for acclimatization. Live engorged females were individually collected in small glass vials (5 cm l × 1 cm d) and identified under a binocular microscope (Olympus Magnus MS24).
Oviposition and Rearing at Different Temperatures
Gravid females of C. peregrinus were carefully transferred from small glass vials to large glass vials (6.5 cm l × 2 cm d) and their bottom was lined with oviposition beds provided with a light source as phototactic movement. The beds consisted of a layer of absorbent cotton (about 2 cm) kept wet by adding a few drops of distilled water and filter paper placed over it. The open ends of the vials were covered with muslin cloth. These vials were put inside the Environmental Test Chamber (model CHM-10S; REMI Elektrotechnik Ltd, Vasai, India). Seventy-five replicates (15 oviposition beds in glass vials x five different temperatures = 75:15 ± 1 °C, 20 ± 1 °C, 26 ± 1 °C, 30 ± 1 °C, 35 ± 1 °C) and at RH 75% and a photoperiod of 13L:11D hrs. The vials were checked for oviposition and the number of eggs were counted under a binocular microscope. Females that completed egg laying were preserved in 70% ethanol for mounting following Wirth and Marston (1968). Once in a day embryonated eggs were collected from the filter papers and transferred to rearing plates which were prepared following the method used by Harsha and Mazumdar (2015). For rearing experiments 30 replicates (6 rearing plates x five different temperature = 30) were used. Each successive day rearing media was replaced with a fresh one until pupation occurred. Pupae were removed from each plate with a fine paint brush (00) and carefully placed in individual glass vials (6.5 cm l × 2 cm d) layered with moistened cotton at the bottom and returned to the holding temperature.
Refrigeration of Eggs
The eggs that were laid on filter papers were counted under a binocular microscope (Magnus MS24). By using fine paint brush (00), eggs were transferred to paired plastic dishes (6 cm diameter) lined in the bottom with a clean cotton bed consisting of two layers of filter paper and wetted with deionized water. Each pair of petri dishes were sealed with paraffin film (PARAFILM “M'' Laboratory Film), and placed inside zipper bags individually. These were then kept inside a laboratory refrigerator maintained at 4 ± 1 °C. The refrigerator was for general laboratory use and was opened one to three times daily for < 1 min per opening.
In experiment I, 1196 number of oviposited eggs were used and viability of the eggs was observed by counting the hatched eggs. These eggs were selected from different clutches so that no two eggs were from the same female. Only embryonated eggs from filter paper were selected as it was observed that white or light brown eggs didn't hatch. For this purpose, eggs were removed randomly from the dish with the help of a fine-tipped brush. As a precautionary measure, dishes were kept outside the refrigerator for less than 5 min. Observed eggs were placed on a 1 cm2 piece of moist filter paper placed onto cotton bedding in an individual cell of a 24-cell costar plate incubated at 26 ± 1 °C within Environmental Test Chamber. The plates were checked for hatching every 12 h intervals till all the hatched eggs were recorded for each cell. Experiment II was carried out by following similar process but observation time was every 3rd day. The 3-day interval was chosen because this is the mean duration of egg-hatching. The oviposited eggs (n = 983) were observed till all the embryonated eggs hatched.
Statistical Analysis
The significance of differences in the oviposition, larval developmental, pupation, and adult emergence of C. peregrinus at different temperatures were analysed by using one-way ANOVA followed by Tukey’s HSD test using XLSTAT software, release 10 (Addinsoft 2010) (Zar 1999). Tukey's test was used to compare treatments at a significance level of 5%. Correction factor (CF) is also incorporated in estimation of standard error as the sample size is more than 5% of the population size. Here standard error of developmental stages is adjusted with the CF value (Williams 2011). The rate of development (1/day) of each stage was obtained using the reciprocal of the development period at each temperature. The relationships between developmental rate and temperature were determined using the best fits of non-linear regressions. Regression statistics was used to obtain the lower threshold for development (LTD), which corresponds to the intersection with the abscissa. “devRate” package (Rebaudo et al. 2018) of RStudio software was used to the empirical data through non-linear least square estimates. Standard error (SE) of the lower developmental threshold (LTD) was estimated using the formula
where s2 is the residual mean square of y, \(\overline{y }\) is the sample mean, and N is the sample size (Campbell et al. 1974).
Results
Oviposition and Survivability of Life Stages
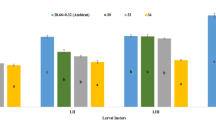
The number of eggs produced by females showed significant differences at different temperatures (F = 47.04, df = 4,45; p < 0.0001) (Fig. 1). Test temperatures revealed significant differences in the success rate of egg development at all temperatures except for 15 °C (F = 61.53, df = 4,25; p < 0.0001). Although egg development was observed at 15 °C but further development did not occur at this temperature. The highest hatching (97%) was observed at 26 °C (F = 97.14, df = 4,25; p < 0.0001) (Table 1). The 89% of the larvae completed development at 26 °C whereas at 35 °C, 78% larvae (4th instar) survived (F = 92.77, df = 4,25; p < 0.0001). At 20 °C, and 30 °C, the larval development success was 89% and 86%, respectively (Table 1). Pupa development success was 81% in 26 °C whereas at 35 °C the development success was 39% (F = 58.80, df = 4,25; p < 0.0001).
Duration of Life Stages (E > L > P > A)
Total development duration i.e., duration from egg to adult emergence from pupal case showed significant differences (F = 94.02, df = 4,25; p < 0.0001) at different rearing temperatures. The observed variations were 25–39 days (20°C), 21–27 days (26°C), 10–15 days (30°C), 10–12 days (35°C) respectively (Table 2). The egg hatching period was prolonged at low temperature i.e., 4 days at 15 °C and 20 °C and shortened to 2 days at 35 °C. Development duration of larvae to pupae took 17 to 21 days (F = 74.41, df = 4,25; p < 0.0001). The estimated average lower threshold temperature of development duration, on average, of an egg to 1st instar larva, a 4th Instar larva to a pupa and a pupa to an adult were 7.82 ± 0.06°C, 10.07 ± 0.18°C and 17.52 ± 0.43°C, respectively. Non-linear regression curves showed best pattern of development rates at different temperatures (Fig. 2). Development rates of different life stages occurred at slower rates at lower temperature, these were faster at higher temperature, and showed asymptote at 30°C and 35°C (Fig. 2a, b, c).
Refrigeration of Eggs
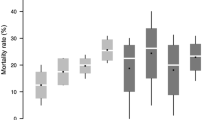
From experiment I (n = 1196) and experiment II (n = 983) embryonated eggs of C. peregrinus were kept under refrigeration to record the duration of egg development. Eggs were observed every 12 h interval and every third day, respectively, with the eggs sourced from the two experiments. In both the experiments, egg hatching was marked by the appearance of a slit initiating from the anterior end to the middle half of the dorsal surface of the eggs. First instar larvae were observed under binocular microscope. Hatching duration of eggs in experiment I was significantly shorter than in eggs from experiment II that took longer duration to hatch. There was a substantial decrease in the hatching percentage of refrigerated eggs with prolonged duration. In experiment I, hatching percentage gradually decreased with increase in the duration of storage. After 12 days, hatching percentage decreased rapidly. Similarly, in experiment II hatching percentage decreased rapidly on 12th day and onward (Table 3). No hatching was observed after day 18.
Discussion
Temperature is considered as an important factor that controls insect development and survivability (Messenger 1959). Different populations of the same species have different temperature ranges of development and different values of threshold development (Campbell et al. 1974). There are a handful of models on temperature effect on the development of ectotherms. The selection of mathematical models that describe the relationship between temperatures and the developmental rate is essential. The linear regression equation is easy to implement but it is known to be invalid at higher temperatures. The Campbell model (Campbell et al. 1974) was applied to three species of the family Ceratopogonidae to fit the development rates of C. mississippiensis Hoffman and C. variipennis (Coquillett) (Jarosík et al. 2011). In this study, the non-linear regression model was applied to calculate the lower threshold development of C. peregrinus. The result (Table 1) indicates that the developmental success of C. peregrinus was influenced by different rearing temperatures. Temperature influenced development of eggs in females and larvae, and resulted in decreased number of egg production and less number of larvae developing at low and high temperatures. These observations properly fit into the preference performance hypothesis (Allgood and Yee 2017) where gravid females are able to detect the ambient temperature to ascertain maximum larval survivability. The development success and duration of different life stages such as larval, pupal stage, and adult emergence were greatly influenced with rearing temperature (Tables 1, 2).
Temperature tolerance related studies were also recorded in C. variipennis and C. brevitarsis (Akey et al. 1978; Allingham 1991). In the rearing of C. variipennis it was observed that the duration for larval development at 25 °C, 27 °C and 30 °C was in the range of 11–24 days whereas early development was completed within 8–18 days at higher temperature (35 °C). At low temperature (20 °C) larval development was delayed (23–48 days) (Akey et al. 1978). Similarly, the fourth instar larval survivability of C. brevitarsis was > 80% between 26 °C and 30 °C (Allingham 1991). Kitaoka (1982) studied the effects of rearing temperature on the duration of the larval period of C. arakawae and C. maculatus (Shiraki). The increased percent of pupation was seen in both the species at 22.5 °C. Duration from egg to adult in both the species was prolonged and higher mortality was reported at 20 °C in C. arakawae but the duration was slightly extended in C. maculatus at this temperature. At 15–16 °C larval development ceased for both species and no pupae were observed at 35 °C in C. arakawae and at above 30 °C in C. maculatus. So, it may be assumed that there is a correlation between rearing temperature with larval survivability and also that suitable temperature ranges may vary with species. Davis et al. (1983) also showed rearing temperature tolerance of C. mississippiensis for maximum larval survivability (20 °C) and larval time duration showed correlation with rearing temperature. Our findings corroborate the ambient temperature range of C. peregrinus with the above-mentioned species C. brevitarsis and C. arakawae. In this empirical study, the development of C. peregrinus at five constant temperatures revealed increases in threshold development values with advantages in developmental stages. The estimated threshold development value of egg to 1st instar larvae of C. peregrinus (7.8 °C) was comparatively low in comparison with C. variipennis (9.8 °C), but the value was quite high (17.52 °C) for pupa to adult developmental stage than that noted in case of C. variipennis (10.50 °C) (Mullens and Rutz 1983). The result of this study contradicts with Jarosík et al. (2011) and lower threshold temperature of one development stage may not be used for all development of a species as the lower threshold temperature of the studied species varies with the different development stages. This knowledge regarding optimum rearing temperature value may be utilized to build laboratory colonies of this vector species, a step forward in establishing mass rearing.
Such experiments performed with vector species of C. peregrinus could also give insight into the possible thermal preference range of populations to environmental conditions and thereby increasing the understanding of vector distribution and population changes over time. So, the thermal tolerance in the field conditions may vary greatly depending on the environmental conditions, and geographic location which determines the abundance data. Our study documented the viability of eggs up to 22 days at low temperature in refrigerated conditions (4 °C). Thus, it was quite clear from the two sets of observations that the embryonated eggs of C. peregrinus can be preserved under refrigeration for 15 days with up to 56% of viable eggs (Table 3). A similar experiment was done by Hunt and Tabachnick (1995) and they showed that the cold storage (5 °C) effect on eggs of C. variipennis sonorensis. The egg hatching rate became 50% and the mortality rate increased with the increase of storage time up to 28 days. However, egg storage techniques are sometimes very advantageous to maintain laboratory colonies. McDermott et al. (2016) reported cold tolerance of eggs and life stages of C. sonorensis. They reported that eggs were the most cold-tolerant among all life stages and eggs tolerate < -20 °C temperature without facing complete mortality up to 1 h exposure. Future studies should be aimed for the probable causes of cold tolerance. However, different species of Culicoides showed differences in their ability to tolerate temperature. As Barceló and Miranda (2020) showed that C. obsoletus (Meigen) and C. circumscriptus Kieffer, C. paolae Boorman responded to the temperature differently. Culicoides obsoletus show optimum development at 18 °C, whereas the optimal rearing temperature for C. circumscriptus and C. paolae was under warmer conditions of 25–30 °C. Therefore, the different tolerance levels of different Culicoides species arouse the interest in predicting environmental change effects on these species.
Conclusion
This study showed the relationship between the range of rearing temperature with oviposition and development success of life stages. Under laboratory conditions, the lower threshold temperature for the rearing of this vector C. peregrinus was determined. Future studies with low-temperature intervals and higher observed temperatures of 40 ± 5 °C should explore the temperature effect on the development of the species. Larval survivability decreased considerably at higher (35 °C) and lower (15 °C) observed temperatures and most of the larvae survived at 26 °C. Field abundance data of C. peregrinus corroborated with experimental observation. As C. peregrinus showed a broad range of temperature tolerance, this species can expand their geographical distribution and veterinary concern. The feasibility of rearing C. peregrinus throughout the year, and the viability of eggs in refrigerated condition was also evaluated. The higher and lower threshold temperature, optimum temperature and lethal temperature of this vector species in field and laboratory conditions require further validations.
References
Addinsoft, S.A.R.L. 2010. XLSTAT software, Ver 9.0. Addinsoft, Paris.
Akey, D.H., H.W. Potter, and R.H. Jones. 1978. Effects of rearing temperature and larval density longevity, size and fecundity in the biting gnat Culicoides variipennis. Annals of the Entomological Society of America 71: 411–418. https://doi.org/10.1093/aesa/71.3.411.
Allgood, D.W., and D.A. Yee. 2017. Oviposition preference and offspring performance in container breeding mosquitoes: Evaluating the effects of organic compounds and laboratory colonisation. Ecological Entomology 42: 506–516. https://doi.org/10.1111/een.12412.
Allingham, P.G. 1991. Effect of temperature on late immature stages of Culicoides brevitarsis (Diptera: Ceratopogonidae). Journal of Medical Entomology 28: 878–881. https://doi.org/10.1093/jmedent/28.6.878.
Barceló, C., and M.A. Miranda. 2018. Bionomics of livestock-associated Culicoides (biting midge) bluetongue virus vectors under laboratory conditions. Medical and Veterinary Entomology 32: 216–225. https://doi.org/10.1111/mve.12286.
Barceló, C., and M.A. Miranda. 2020. Development and lifespan of Culicoides obsoletus ss (Meigen) and other livestock-associated species reared at different temperatures under laboratory conditions. Medical and Veterinary Entomology 35: 187–201. https://doi.org/10.1111/mve.12487.
Briere, J.F., P. Pracros, A.Y. Le Roux, and J.S. Pierre. 1999. A novel rate model of temperature-dependent development for arthropods. Environmental Entomology 28 (1): 22–29.
Campbell, A., B.D. Frazer, N. Gilbert, A.P. Gutierrez, and M. Mackauer. 1974. Temperature requirements of some aphids and their parasites. Journal of Applied Ecology 11: 431–438.
Davis, E.L., D.L. Kline, J.F. Relnert, R.H. Roberts, and J.F. Butler. 1983. Development of immature Culicoides mississippiensis (Diptera: Ceratopogonidae) in the laboratory. Annals of the Entomological Society of America 76: 918–924. https://doi.org/10.1093/aesa/76.5.918.
Erram, D., and N. Burkett-Cadena. 2019. Laboratory rearing of Culicoides stellifer (Diptera: Ceratopogonidae), a suspected vector of Orbiviruses in the United States. Journal of Medical Entomology 57: 25–32. https://doi.org/10.1093/jme/tjz154.
Harsha, R., and A. Mazumdar. 2015. Laboratory rearing of Culicoides peregrinus Kieffer (Diptera: Ceratopogonidae), a potential vector of Bluetongue disease. Medical and Veterinary Entomology 29: 434–438. https://doi.org/10.1111/mve.12136.
Harsha, R., S.M. Mazumdar, and A. Mazumdar. 2020. Abundance, diversity and temporal activity of adult Culicoides spp. associated with cattle in West Bengal. India. Medical and Veterinary Entomology 34: 327–343. https://doi.org/10.1111/mve.12446.
Hunt, G.J., and W.J. Tabachnick. 1995. Cold storage effects on egg hatch in laboratory reared Culicoides variipennis sonorensis (Diptera: Ceratopogonidae). Journal of the American Mosquito Control Association 11: 335–338.
Jarosík, V., A. Honek, R.D. Magarey, and J. Skuhrovec. 2011. Developmental database for phenology models: Related insect and mite species have similar thermal requirements. Journal of Economic Entomology 104: 1870–1876.
Kitaoka, S. 1982. Effects of rearing temperature on length of larval period and size of adults in Culicoides arakawae and Culicoides maculatus (Dipteria: Ceratopogonidae). National Institute of Animal Health Quarterly 22: 159–162.
Ludwig, D. 1928. The effects of temperature on the development of an insect (Popillia japonica Newman). Physiological Zoology 1 (3): 358–389.
McDermott, E.G., C.E. Mayo, and B.A. Mullens. 2016. Low temperature tolerance of Culicoides sonorensis (Diptera: Ceratopogonidae) eggs, larvae, and pupae from temperate and subtropical climates. Journal of Medical Entomology 54: 264–274. https://doi.org/10.1093/jme/tjw190.
Mellor, P.S., S. Carpenter, and D.M. White. 2009. Bluetongue virus in the insect host. Bluetongue (ed. by P.S. Mellor, M. Baylis and P.P.C. Mertens), Academic Press, Elsevier, Paris.
Messenger, P.S. 1959. Bioclimatic studies with insects. Annual Review of Entomology 4: 183–206.
Mullens, B.A., and D.A. Rutz. 1983. Development of immature Culicoides variipennis (Diptera: Ceratopogonidae) at constant laboratory temperatures. Annals of the Entomological Society of America 76 (4): 747–751.
Narladkar, B.W., P.D. Deshpande, and P.R. Shivpuje. 2006. Bionomics and life cycle studies on Culicoides sp. (Diptera: Ceratopogonidae). Journal of Veterinary Parasitology 20: 7–12.
Nayduch, D., L.W. Cohnstaedt, C. Saki, D. Lawson, P. Kersey, M. Fife, and S. Carpenter. 2014. Studying Culicoides vectors of BTV in the post–genomic era: Resources, bottlenecks to progress and future directions. Virus Research 182: 43–49. https://doi.org/10.1016/j.virusres.2013.12.009.
Prasad, G., D. Sreenivasulu, K.P. Singh, P.P.C. Mertens, and S. Maan. 2009. Bluetongue in the Indian subcontinent. Bluetongue (ed. by P.S. Mellor, M. Baylis and P.P.C. Mertens), Academic Press, Elsevier, Paris.
Rebaudo, F., Q. Struelens, and O. Dangles. 2018. Modelling temperature-dependent development rate and phenology in arthropods: The devRate package for R. Methods in Ecology and Evolution 9 (4): 1144–1150.
Régnière, J., J. Powell, B. Bentz, and V. Nealis. 2012. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. Journal of Insect Physiology 58 (5): 634–647.
Sreenivasulu, D., M.V. Subba Rao, Y.N. Reddy, and G.P. Gard. 2004. Overview of bluetongue disease, viruses, vectors, surveillance and unique features: The Indian sub-continent and adjacent regions. Veterinaria Italiana 40: 73–77.
Veronesi, E., G.J. Venter, K. Labuschagne, P.S. Mellor, and S. Carpenter. 2009. Life-history parameters of Culicoides (Avaritia) imicola Kieffer in the laboratory at different rearing temperatures. Veterinary Parasitology 163: 370–373. https://doi.org/10.1016/j.vetpar.2009.04.031.
Williams, D.C. 2011. Finite sample correction factors for several simple robust estimators of normal standard deviation. Journal of Statistical Computation and Simulation 81 (11): 1697–1702.
Wirth, W.W., and N. Marston. 1968. A method for mounting small insects on microscope slides in Canada balsam. Annals of the Entomological Society of America 61: 783–784. https://doi.org/10.1093/aesa/61.3.783.
Zar, J.H. 1999. Biostatistical Analysis, 4th ed. New Delhi, India: Pearson Education.
Acknowledgements
The authors are indebted to Basudev Das, Senior Technical Assistant, University Science Instrumentation Centre (USIC), The University of Burdwan, for the fabrication and maintenance of the UV LED light trap. We would like to thank the Head, Department of Zoology, The University of Burdwan for providing laboratory facilities. Authors are grateful to Prof. Gautam Aditya, Department of Zoology, University of Calcutta for helping with statistical data analysis. Authors also thank anonymous reviewers for their valuable suggestions on the manuscript.
Author information
Authors and Affiliations
Contributions
PB, RH and AM had developed the idea for the article. PB, AS and RH had done the investigation and data analysis, PB wrote the original manuscript. AM and AS edited and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we don’t have any conflict of interest.
Copy right transfer statement
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banerjee, P., Sarkar, A., Harsha, R. et al. Influence of Rearing Temperatures on Oviposition and Survivability of Developmental Stages of Culicoides peregrinus Vector of Bluetongue Virus with a Note on Egg Viability. Proc Zool Soc 77, 232–239 (2024). https://doi.org/10.1007/s12595-024-00527-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-024-00527-3