Abstract
Phenology of the wolf spider, Pardosa flavisterna, was studied. This species is an abundant arthropod predator in the agroecosystems of Kashmir valley and is restricted to some parts of India and Pakistan only. The life cycle pattern was deduced via sampling done by manual searching, pitfall trapping in 24 different agricultural fields, and individuals reared under laboratory conditions. Males and females underwent seven moultings to mature and showed a mean carapace width (± SEM) of 2.594 mm (± 0.026) n = 17, and 2.470 mm (± 0.040) n = 14, respectively, at maturity. Investigation of the monthly catches and outcome of the measurement of their carapace width indicated an annual-biannual life cycle of P. flavisterna having stenochronous occurrence, reproducing in spring and summer. Females produce two egg sacs. Individuals of the first brood overwinter as subadults, while the second brood overwinters as juveniles. The first brood matured in the upcoming spring, while the second brood matured the next spring after growing throughout the year. The mean clutch size (± SEM) is 68.24 (± 4.57), n = 122, range = 41–103 eggs. Weather parameters had an impact on the number of catches, with temperature showing a positive correlation, and rainfall and humidity showing a negative relationship with the number of individuals caught. A minimum of two size classes were recorded throughout the year as a result of this life cycle pattern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In agroecosystems, spiders are found in high densities and are effective pest control agents (Sunderland and Samu 2000; Maloney et al. 2003; Michalko et al. 2019). Of these, ground-dwelling wolf spiders (family Lycosidae) are the most common (Alford 2008). The genus Pardosa Koch (1847), one of the largest and most studied genera in the family Lycosidae comprising 536 species, occurs throughout the world (World Spider Catalogue 2020). The enormous prevalence of this genus practically in all environments, including agroecosystems, has prompted a substantial investigation (Buddle et al. 2004; Schmidt et al. 2005; Clough et al. 2005; Oberg 2007; Seyfulina 2010; Dippenaar-Schoeman et al. 2013; Bizuet-Flores et al. 2015; Yang et al. 2018; Song et al. 2020). The study on the life cycle of this group has significantly progressed over the years (Bonte and Maelfait 2001; Buddle 2002; Bowden and Buddle 2012; Høye et al. 2020; Rádai 2021). However, the phenology of Pardosa species found in Asia required elucidation as very little has been done at the time of this study (Fangwen 2001; Wang et al. 2017).
Pardosa flavisterna is a predominant spider species in the agroecosystems of Kashmir valley. It was first described by Caporiacco (1935) as a part of a collection of 105 new Arachnida species from Karakoram Range in India and Pakistan. According to that study, P. flavisterna was reported to be abundant in Baltistan and Kashmir at an elevation of 1700–3300 m. Marusik and Ballarian (2011) reported its distribution from the Baltistan (Pakistan) to northern Himachal Pradesh (India) between elevations of 1200–2600 m and redescribed the species based on new specimens obtained from India and Pakistan. Within the frame work of the present study, P. flavisterna was reared from egg to maturity. The carapace width analysis of each instar was done to analyse their growth and development under laboratory conditions. A year-round systematic field observations of the wolf spider were undertaken across all seasons to study phenology, effect of weather parameters on population dynamics, and oviposition and juvenile development under field conditions.
Materials and Methods
Phenology and Population Dynamics
Collections of live specimens of P. flavisterna were made using pitfall traps and visual searching at 24 agricultural field locations in the Kashmir valley (Fig. 1). Field collection sites exhibited mixed vegetation including fruit trees (apple, pear, plum, cherry, apricot, and peach), vegetables, pulses, fodder and grasses. The vegetation throughout the collection sites was nearly homogenous. At each location, 3 transects were laid 40 m away from each other in a parallel manner. Across each transect, 4 traps were placed approximately 20 m apart (4 treatments × 3 replicates = 12 traps in each field). Each trap having a diameter of 7 cm and a height of 8 cm was used. The pitfall traps were placed in a flask that was set in the ground after digging a hole. Upper edge of the outer flask was so placed that it flush with the ground level. The pitfall traps were filled with 30% ethylene glycol (antifreeze) and a few drops of soap water and operated at two weeks intervals from April 2019–March 2020 except for the winter months (December and January) during which they were operated at four weeks interval. Spiders were also collected in vials at similar intervals by the visual searching under leaf litter and at the surface for wandering spiders along transects. After emptying the traps and vials, the individuals were separated according to the size class. The data-set consisting of juveniles, subadults, adult females, and adult males were counted based on distinctions in genitalia (epigyne for adult females and pedipalp for adult males). Specimens of this study were preserved in 70% ethanol. The data, so collected, enabled us to study the phenological pattern, population dynamics, and the effect of weather parameters on the same. The weather data were sourced from the Division of Agronomy, Sher-e-Kashmir University of Agriculture, Science and Technology, Kashmir. Carapace width (CW) of all the field catches as a measure of body size and abdominal width measurements of the adult females were recorded each month as an index of body condition as used by Jakob et al. (1996). During the reproductive period egg sacs were collected from the fields to determine the clutch size. This was accomplished by opening each egg sac and counting the number of eggs within.
Laboratory Rearing
The pattern of growth was analyzed by rearing females with the egg sacs collected between 5th April and 3rd July 2019. During this period the females with egg sacs were collected from fields. Each female was placed in a cylindrical glass jar (10 cm x 6 cm) with a 6 cm diameter hole covered with cheese cloth for ventilation. The individuals of P. flavisterna were reared from egg to maturity. The freshly emerged young ones were separated and placed in the individual transparent cylindrical glass jars (5 cm height × 2.5 cm diameter) and were fed with flower thrips and their siblings up to second instar and later each instar was fed with live Drosophila and houseflies (Musca domestica) daily. Immature insects were given to instars II and III, following which they were fed mature insects till instar VIII. The prey species were alternated each day. Each spider was fed one prey item based on their feeding regime (immature/mature) per day. The prey was anaesthetized using ethyl acetate (Lorus et al. 2010) for sorting and transferring into the jars which were lined in the bottom with wet cotton covered by a filter paper for maintaining necessary moisture and humidity level. A piece of long polystyrene was placed inside each rearing jar length wise which acted as attachment site during moulting. Rearing was done in an incubator (temperature of 25 ± 5 °C; and a light: dark photoperiod of 16:8 h). The rearing jars were changed once a week and the spiders were transferred to new rearing jars using a strip of paper toweling as a substrate rolled in the form of a funnel. Any prey that had not been consumed within 24 h was removed from the jar with the help of entomological brushes and forceps. The number of developmental instars till adulthood were recorded. Body size was estimated using CW(mm). This character varies less amongst individuals of the same instar than body volume or weight (Hagstrum, 1971; Jakob et al. 1996). CW of individuals of each instar was measured under a stereo-zoom microscope (Magnus MS13/MS24; Olympus Opto Systems India Pvt. Ltd.) using an ocular micrometer. All measurements were recorded in millimeters.
Statistical Analysis
Mean, range and standard error of means of the data were determined. ANOVA was carried out and a post hoc test Fisher's Least Significant Difference (LSD) was used for comparison of the means of CW of instar stages within the same sex. Independent t-test was used to compare the mean CW of each instar between males and females at 95% confidence level. The Pearson Correlation Coefficient was used to determine the relationship between the number of catches and weather parameters at a probability level of p < 0.05. All the statistical procedures were carried out using MS Excel Software (version 2019 16.0. 6742.2048) and SPSS Software (version 1.0.0.1406).
Result
Phenology
Field data from pitfall traps and visual searching showed that adult males and females appeared in March 2019. However, males appeared a week before the females. The breeding season occurred during this time after which females were seen carrying egg sacs from April to July 2019. The first female carrying egg sac was sighted on 5th April 2019. Males disappeared from the population soon after the breeding season as they died shortly after mating. The last cocoon-carrying female was seen in the first week of August 2019. Juveniles were seen soon after the eggs started hatching in May 2019.
At the time of commencement of the winter season (November), both the adult males and females were absent from the population. Juveniles and subadults made up the population which occurred until the first week of December 2019, after which they overwintered. During late February, juveniles and subadults resumed growth, the subadults matured into adults and the population consisted of adults and juveniles. The peak in male activity was seen in May 2019, as the highest number of males was caught in pitfall traps during this period. The last male was sighted on 7th July 2019 whereas females were noticed up to the month of September 2019. No individual of P. flavisterna was collected after the second week of December to the middle of February 2020 (Fig. 2).
Oviposition and Juvenile Development
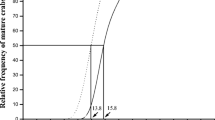
The CW frequency distribution results revealed that individuals of at least three size classes occurred during each month: CW 0.5–0.6 mm, 1.2–1.6 mm and 2.3–2.7 mm in June; 0.6–0.8 mm, 1.2–1.7 mm and 2.3–2.6 mm in July; and 0.5–0.6 mm, 0.8–1.3 mm, 1.8–2.2 mm and 2.2–2.3 mm in August. Observations of synchronized hatching of new juveniles having CW of 0.5–0.6 mm in June and August are shown in Fig. 4 where Brood 1 and Brood 2 indicate two broods (egg sacs) produced in the respective months. The mean clutch size (± SEM) was 68.24 (± 4.57), n = 122, range = 41–103. Carapace width of individuals in November showed that only two size classes were present with juveniles ranging in size from 0.8–0.9 mm and subadults from 2–2.3 mm (Fig. 4).
The mean abdominal width of females during reproductive period were found to be highest in the months of April–May (mean: 3.39 mm, n = 138) and June–July (mean: 3.17 mm, n = 116). The highest number of females during the reproductive period was recorded during this time. This indicated peak in the reproductive activity. An overlap of the two peaks in mean abdominal width and activity pattern (number of females) was also recorded (Fig. 3).
Population Dynamics
Figure 2 shows the month-wise incidence as a proportion of juveniles, subadults, adult males, and adult females in field population. Of the total 1919 individuals collected during the study period, 35.43% were juveniles, 19.54% subadults, 20.16% adult males, and 24.85% adult females. Almost 36% of the females from April to July were seen carrying egg sacs. The maximum numbers of males were found in April, May, and June 2019 while no male was found after the first week of July 2019. The adult females showed the same trend in number as adult males during the breeding period but females were seen to persist even after the males have disappeared. The juveniles were found throughout the year, but the subadults were present in the population from September 2019 to early March 2020 only.
The number of catches remained high for the major part of the year and the populations had an increase in the number of individuals at the beginning of the spring. However, there was a noticeable drop by the end of autumn after which the population declined to zero in January and it continued up to the middle of February 2020. All life stages of the species displayed marked peaks of abundance during different months of the year. The correlation analysis between the numbers of individuals against different weather parameters showed a significant effect of temperature on the individuals of P. flavisterna (r (10) = 0.645, P < 0.05). However, the number of catches showed a negative correlation with precipitation (r (10) = −0.047, P < 0.05) and humidity (r (10) = −0.491, P < 0.05).
Development of Instars Under Laboratory Conditions
In the laboratory, 26 female and 23 male juveniles were reared to maturity in order to determine their adult lifespan after which they were released. Out of these, 19 females and 17 males reached adulthood and the rest suffered mortality during different instar stages. The overall percent mortality observed during rearing of males and females were 26.08% and 26.26%, respectively. The CW of 33–49 spiders (males and females) was measured for each instar. The average time required by P. flavisterna to reach from egg to adult stage was 242.13 days under laboratory conditions. The mean CW per instar was calculated and comparisons of all the pairs of means within each sex and between the sexes were made to reveal the size distribution.
Within the sexes: In females, the mean CW of instar I–VIII range from 0.685–2.594 mm. The significant difference was observed in the mean CW values of all the instars of females (ANOVA: F7, 168 = 639.76, P < 0.05). The mean CW of males from instar I–VIII range from 0.670–2.488 mm. A significant difference was found across all the instars (ANOVA: F7, 143 = 886.70, P < 0.05) (Table 1).
Between the Sexes: The mean CW of the first three instars of males and females did not differ significantly when compared across all eight instars. (t = 3.092, P = 0.139; t = 1.490, P = 0.143; t =−0.278, P = 0.791). Also, there was no significant difference between the mean CW of females and males for Instar V and VII (t = 0.621, P = 0.538; t =−0.597, P = 0.555). However, significant differences occurred in the mean CW for instar IV, VI and VIII of males and females (t = 2.040, P = 0.048; t = -2.073, P = 0.045; t = 0.597, P = 0.008) (Table 1).
Discussion
Phenology
The phenology of a spider can fit in one of the following proposed patterns: 1) stenochronous in which adults are present at a certain period of the year, 2) eurychronous in which adults are present throughout the year (with or without a specific reproductive period), and 3) the spiders which show maturation in winter (Tretzel 1954). Our findings show that the population of P. flavisterna follow a stenochronous pattern of reproduction in spring and summer with a distinct phase of adult spider activity at a specific time of year, which corresponds to similar phenology described by Paquin and Duperré (2001).
The members of the family Lycosidae are found in almost all terrestrial ecosystems. So, their life cycles show adaptation to variations in the climatic conditions of different places. Thus, the phenology of these spiders differs significantly, annual (Oraze et al. 1989; Eubanks and Miller 1992), annual-biannual, and to biannual (Miller and Miller 1987; Pickavance 2001). It appears from the data that P. flavisterna in the area of this study adhere to a phenological pattern characteristic of Pardosa species living in temperate zones which involve subadults undergoing overwintering so that mating occurs in the early summer. Males die soon after mating and females carry egg sacs during the summer months (Buddle, 2000). Adult of P. flavisterna are present from March–August and breeding occurs from April– June. The highest number of males were found in the month of May. The greater catch of males during this period could possibly be attributed to males searching for mates. Adult males disappear from the population by early July but the females persist up to September.
Oviposition and Juvenile Development
The records of CW measurements indicate that synchronized hatchings occur in the month of June and in August (Fig. 4). There would have been a higher level of variation in the CW of juveniles, had the hatching period been continuous during the summer. The late season catch of females carrying egg sacs in August 2019 resulted in the greater variation of CW leading to the appearance of a new size class having CW of 0.5–0.6 mm that most likely correspond to the production of second egg sac. The higher catch of females in pitfall traps during April–May and July months suggest enhanced adult female foraging behaviour to feed themselves more actively prior to the formation of egg cases. This observation is consistent with the findings of Moring & Stewart (1992) and Kreiter & Wise (2001). The fact that the number of females showed peaks at the same time as the abdominal width supports the production of two egg sacs (Fig. 3). It appeared from the results that two egg sacs were produced and the individuals from first egg sac overwintered in its juvenile stage and those from the second egg sac overwintered in subadult stage. In temperate zones the bimodal age distribution occurs in some of the Pardosa species that show extended life cycle beyond one year and are having an annual-biannual life cycle. The late hatchlings do not mature with the early hatched ones. These need to overwinter a second time to mature, leading to differences in maturation time and developmental rates (Alderweireldt and Maelfait 1988).
Population Dynamics
Weather parameters are important factors affecting the population of P. flavisterna. According to the results of correlation analysis, the temperature showed a positive correlation with the number of catches of P. flavisterna. Positive correlation between temperature and the abundance of the species was also found by Villanueva-Bonilla et al. (2018) for two lynx spiders. This is also true for prey availability for the spiders (Kishimoto-Yamada and Itioka 2015). Similar results were found for spiders in the Brazilian savanna (Mineo et al. 2010). Precipitation and humidity had a negative effect on the incidence of this spider as is vindicated by the study of Lensing et al. (2005) which showed that spider density decreased with the increase in precipitation.
Development Under Laboratory Conditions
In the laboratory rearing of P. flavisterna, significant difference was seen in the mean CW of all the instar stages of males and females (Table 1). The results are in agreement with the study by Punzo and Farmer (2006) where an overall significant difference was observed in CW of different instars in wolf spider P. sierra. The comparison between the mean CW of females and males revealed that the first three instars and instars V and VII of the two sexes did not show significant difference whereas those of instars IV, VI and VIII between females and males showed significant difference within a narrow range. Thus, a reliable basis of identifying the sex of an instar based on CW is not evident from this study.
Some of the males matured in the VI and VII instars, while majority of them matured in VIII instar stage. The reason for the early maturation of some spiders than their female counterparts is that males have smaller body size due to which they undergo fewer moults as was also reported by Jones & Parker (2000) and Viera et al. (2006) where a proportion of male spiders reach adulthood after VI instar stage. If the males mature earlier, they could access virgin females more readily or guard penultimate females earlier, improving their reproductive success (Viera and Costa 2003).
Conclusion
This study has revealed that P. flavisterna is a stenochronous species, reproducing in spring and summer in the study area. It took eight instar stages to reach maturity under laboratory conditions. The female produced two egg sacs, where the progeny of the first egg sac develops faster and mature in the upcoming spring while those of the second egg sac are slow developers and mature in the next spring, consequently making it an annual-biannual species. The exact mechanism that leads to two distinct life cycle patterns by the broods requires further study.
References
Alderweireldt, M., and J.P. Maelfait. 1988. Life cycle, habitat choice and distribution of Pardosa amentata (Clerck, 1757) in Belgium (Araneae, Lycosidae). Bulletin De La Société Scientifique De Bretagne 59 (1): 7–16.
Alford, D.V., ed. 2008. Biocontrol of oilseed rape pests. United States: John Wiley & Sons. New Jersey.
Bizuet-Flores, M.Y., M.L. Jiménez-Jiménez, A. Zavala-Hurtado, and P. Corcuera. 2015. Diversity patterns of ground dwelling spiders (Arachnida: Araneae) in five prevailing plant communities of the Cuatro Ciénegas Basin, Coahuila. Mexico. Revista Mexicana De Biodiversidad 86 (1): 153163. https://doi.org/10.7550/rmb.45444.
Bonte, D., and J. P. Maelfait. 2001. Life history, habitat use and dispersal of a dune wolf spider (Pardosa monticola (Clerck, 1757) Lycosidae, Araneae) in the Flemish coastal dunes (Belgium). Belgian Journal of Zoology 131(2): 145 - 158. http://hdl.handle.net/1854/LU-363607
Bowden, J.J., and C.M. Buddle. 2012. Life history of tundra-dwelling wolf spiders (Araneae: Lycosidae) from the Yukon Territory. Canada. Canadian Journal of Zoology 90 (6): 714–721. https://doi.org/10.1139/Z2012-038.
Buddle, C.M., S. Higgins, and A.L. Rypstra. 2004. Ground-dwelling spider assemblages inhabiting riparian forests and hedgerows in an agricultural landscape. The American Midland Naturalist 151 (1): 15–26. https://doi.org/10.1674/0003-0031(2004)151[0015:GSAIRF]2.0.CO;2.
Buddle, C.M. 2002. Interactions among young stages of the wolf spiders Pardosa moesta and P.mackenziana (Araneae: Lycosidae). Oikos 96 (1): 130–136. https://doi.org/10.1034/j.1600-0706.2002.960114.x.
Buddle, C.M. 2000. Life history of Pardosa moesta and Pardosa mackenziana (Araneae, Lycosidae) in central Alberta Canada. The Journal of Arachnology 28 (3): 319–328. https://doi.org/10.1636/0161-8202(2000)028[0319:LHOPMA]2.0.CO;2.
Caporiacco, L.D. 1935. Aracnidi dell’Himalaia e del Karakoram raccolti dalla Missione Italiana al Karakoram (1929–VII). Memorie Della Società Entomologica Italiana 13: 113–250.
Clough, Y., A. Kruess, D. Kleijn, and T. Tscharntke. 2005. Spider diversity in cereal fields: Comparing factors at local, landscape and regional scales. Journal of Biogeography 32 (11): 2007–2014. https://doi.org/10.1111/j.1365-2699.2005.01367.x.
Dippenaar-Schoeman, A.S., A.M. Van den Berg, R. Lyle, and C.R. Haddad. 2013. Current knowledge of spiders in South African agroecosystems (Arachnida, Araneae). Transactions of the Royal Society of South Africa 68 (1): 57–74. https://doi.org/10.1080/0035919X.2012.755136.
Eubanks, M. D., and G. L. Miller. 1992. Life cycle and habitat preference of the facultatively arboreal wolf spider, Gladicosa pulchra (Araneae, Lycosidae). Journal of Arachnology 1: 157-164. https://www.jstor.org/stable/3705875
Fangwen, T. 2001. Study on biological characteristics and predation function of Pardosa astrigera, the predator of locust. Plant Protection Technology and Extension 7. n.p.
Hagstrum, D.W. 1971. Carapace width as a tool for evaluating the rate of development of spiders in the laboratory and the field. Annals of the Entomological Society of America 64 (4): 757–760. https://doi.org/10.1093/aesa/64.4.757.
Høye, T.T., J.C. Kresse, A.M. Koltz, and J.J. Bowden. 2020. Earlier springs enable High-Arctic wolf spiders to produce a second clutch. Proceedings of the Royal Society B 287 (1929): 20200982. https://doi.org/10.1098/rspb.2020.0982.
Jakob, E.M., S.D. Marshall, and G.W. Uetz. 1996. Estimating fitness: A comparison of body condition indices. Oikos. https://doi.org/10.2307/3545585.
Jones, T.C., and P.G. Parker. 2000. Costs and benefits of foraging associated with delayed dispersal in the spider Anelosimus studiosus (Araneae, Theridiidae). Journal of Arachnology 28 (1): 61–69. https://doi.org/10.1636/0161-8202(2000)028[0061:CABOFA]2.0.CO;2.
Kishimoto-Yamada, K., and T. Itioka. 2015. How much have we learned about seasonality in tropical insect abundance since Wolda (1988)? Entomological Science 18 (4): 407–419. https://doi.org/10.1111/ens.12134.
Kreiter, N.A., and D.H. Wise. 2001. Prey availability limits fecundity and influences the movement pattern of female fishing spiders. Oecologia 127 (3): 417–424. https://doi.org/10.1007/s004420000607.
Lensing, J.R., S. Todd, and D.H. Wise. 2005. The impact of altered precipitation on spatial stratification and activity-densities of springtails (Collembola) and spiders (Araneae). Ecological Entomology 30 (2): 194–200. https://doi.org/10.1111/j.0307-6946.2005.00669.x.
Loru, L., A. Sassu, X. Fois, and R.A. Pantaleoni. 2010. Ethyl acetate: A possible alternative for anaesthetizing insects. Annales De La Société Entomologique De France 46 (3–4): 422–424. https://doi.org/10.1080/00379271.2010.10697677.
Maloney, D., F. A. Drummond, and R. Alford. 2003. TB190: Spider predation in agroecosystems: Can spiders effectively control pest populations? Dissertation, University of Maine.
Marusik, Y. M., and F. Ballarin. 2011. Redescription of the Himalaian Pardosa flavisterna Caporiacco, 1935 (Aranei: Lycosidae) with notes of the Pardosa nebulosi spp. -group. Proceedings of the Zoological Institute RAS 315(1): 63 - 69.
Michalko, R., S. Pekár, and M.H. Entling. 2019. An updated perspective on spiders as generalist predators in biological control. Oecologia 189 (1): 21–36. https://doi.org/10.1007/s00442-018-4313-1.
Miller, G. L., and P. R. Miller. 1987. Life cycle and courtship behavior of the burrowing wolf spider Geolycosa turricola (Treat) (Araneae, Lycosidae). Journal of Arachnology 1: 385 - 394. https://www.jstor.org/stable/3705854
Mineo, M.F., K. Del-Claro, and A.D. Brescovit. 2010. Seasonal variation of ground spiders in a Brazilian Savanna. Zoologia (curitiba). 27: 353–362. https://doi.org/10.1590/S1984-46702010000300006.
Moring, J. B., and K. W. Stewart. 1992. Influence of sex and egg-case presence on predatory behavior of the wolf spider Pardosa valens Barnes (Araneae: Lycosidae). The Southwestern Naturalist 132–137. https://www.jstor.org/stable/3671661
Oberg, S. 2007. Diversity of spiders after spring sowing- influence of farming system and habitat type. Journal of Applied Entomology 131 (8): 524–553. https://doi.org/10.1111/j.1439-0418.2007.01173x.
Oraze, M. J., A. A. Grigarick, and K. A. Smith. 1989. Population ecology of Pardosa ramulosa (Aranaea, Lycosidae) in flooded rice fields of northern California. Journal of Arachnology 1: 163 - 170. https://www.jstor.org/stable/3705624
Paquin, P., and N. Dupérré. 2001. On the distribution and phenology of Argyrodes fictilium (Araneae, Theridiidae) at its northern limit of North America. Journal of Arachnology. 29(2), 238–243. https://www.jstor.org/stable/3706102
Pickavance, J.R. 2001. Life-cycles of four species of Pardosa (Araneae, Lycosidae) from the island of Newfoundland Canada. Journal of Arachnology 29 (3): 367–377. https://doi.org/10.1636/0161-8202(2001)029[0367:LCOFSO]2.0.CO;2.
Punzo, F., and C. Farmer. 2006. Life history and ecology of the wolf spider Pardosa sierra Banks (Araneae: Lycosidae) in southeastern Arizona. Southwest Naturalist 51(3): 310 - 319. https://www.jstor.org/stable/20424725
Rádai, Z. 2021. Cohort splitting from plastic bet-hedging: Insights from empirical and theoretical investigations in a wolf spider. Theoretical Ecology 14 (1): 9–21. https://doi.org/10.1007/s12080-020-00475-6.
Schmidt, M. H., I. Roschewitz, C. Thies, and T. Tscharntke. 2005. Differential effects of landscape and management on diversity and density of ground‐dwelling farmland spiders. Journal of Applied Ecology 42(2): 281 - 287. https://www.jstor.org/stable/3505721
Seyfulina, R.R. 2010. The spider assemblage (Arachnida, Aranei) in agroecosystems of the Kuban Plain: spp. composition, spatial distribution, and seasonal dynamics. Entomological Review 90 (4): 494–510. https://doi.org/10.1134/S001387381004010X.
Song, X., T. Yang, X. Xu and Y. Zhong. 2020. A checklist of spiders in tea plantations of China. Biodiversity data journal 8. Pensoft publishers. Sofia, Bulgaria.
Sunderland, K., and F. Samu. 2000. Effects of agricultural diversification on the abundance, distribution, and pest control potential of spiders: A review. Entomologia Experimentalis Et Applicata 95 (1): 1–3. https://doi.org/10.1046/j.1570-7458.2000.00635.x.
Tretzel, E. 1954. Reife-und Fortpflanzungszeit bei Spinnen. Zeitschrift für Morphologie und Ökologie der Tiere 42(6/7), 634–691. https://www.jstor.org/stable/43261884
Villanueva-Bonilla, G.A., S. Safuan-Naide, and J. Vasconcellos-Neto. 2018. Population dynamics and phenology of two congeneric and sympatric lynx spiders Peucetia rubrolineata Keyserling, 1877 and Peucetia flava Keyserling, 1877 (Oxyopidae). Journal of Natural History 52 (5–6): 361–376. https://doi.org/10.1080/00222933.2018.1433339.
Viera, C., and S. Ghione. 2003. Sexual behavior and ritualised fight of adult males of a social spider, Anelosimus studiosus (Araneae, Theridiidae). In Proceedings XXVIII International Ethological Conference, Florianópolis, Brasil, 218–219.
Viera, C., S. Ghione, and F.G. Costa. 2006. Regurgitation among penultimate juveniles in the subsocial spider Anelosimus cf. studiosus (Theridiidae): are males favored? Journal of Arachnology 258–260. https://www.jstor.org/stable/4489069
Wang, J., L. Zhao, Y. Shi, M. Tang, and Z. Wang. 2017. Effect of temperature on growth and development of Pardosa pseudoannulata. Chinese Journal of Biological Control 33 (5): 597–603.
World Spider Catalog. 2020 Version 21.5. Natural History Museum Bern. http://wsc.nmbe.ch.
Yang, H., Y. Peng, J. Tian, J. Wang, B. Wei, C. Xie, and Z. Wang. 2018. Rice field spiders in China: A review of the literature. Journal of Economic Entomology 111 (1): 53–64. https://doi.org/10.1093/jee/tox319.
Acknowledgements
The author is grateful to Dr. Sameera Qayoom, Agromet Field Unit, Sher-e-Kashmir University of Agricultural Sciences & Technology- Kashmir for providing the Meteorological Data.
Author information
Authors and Affiliations
Contributions
Both the authors conceived and designed the study. Shazia Riyaz Shah conducted the experiment, analyzed the data and wrote the paper. Both the authors contributed to manuscript revision and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shah, S.R., Buhroo, A.A. Phenology of the Himalayan Wolf Spider, Pardosa flavisterna Caporiacco, 1935 (Araneae: Lycosidae) in the Agroecosystems of Kashmir, India . Proc Zool Soc 75, 292–300 (2022). https://doi.org/10.1007/s12595-022-00435-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-022-00435-4