Abstract
Increasing resistance against synthetic insecticides, mosquito control is becoming serious problem. For an alternative measure novel botanical sources can be used as good insecticides with less toxic hazards to the environment. The present study estimated larvicidal activities of Andrographis echioides against the vector of lymphatic filariasis Culex quinquefasciatus under laboratory conditions. Crude extracts of A. echioides mature leaves were examined for mosquito larvicidal activity against all the larval instars of Cx. quinquefasciatus. Solvent extraction was carried out using three different solvents viz. petroleum ether, ethyl acetate and acetone. Dose dependent larvicidal bioassays were performed using the solvent extractives. LC50 and LC90 values were consummated through log-probit analysis. Regression and ANOVA analyses were done for statistical justification. The effects of crude and solvent extractives were tested on a non-target water fauna. Crude extract of A. echioides leaf exhibited 100% mortality in 1.00% concentration against 1st instars larvae after 72 h of exposure. Among three used solvent extracts ethyl acetate showed efficient larvicidal effect against target mosquito. In ethyl acetate extractive 100.00% mortality was noticed at 150 ppm concentration against 1st instars larvae after 48 h of exposure with LC50 and LC90 values of 32.96 ppm and 106.96 ppm respectively. Tested non target organism was completely safe both from crude and ethyl acetate extractives. This experiment was a pioneer attempt to establish A. echioides as a precious resource for production of mosquito larvicide against Cx. quinquefasciatus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytochemicals help in defense against insects, fungi and herbivorous mammals (Hassan Adeyemi 2010). These plant derived chemicals are established as insecticidal (Hossain et al. 2011) agents.
Diseases like chikungunya, dengue, filariasis, malaria, Japanese encephalitis and yellow fever etc. are some of the deadliest mosquito born diseases that cause millions of death worldwide (ICMR Bulletin 2013). Approximately over 3500 species of mosquito under 41 genera were distributed worldwide (Harbach 2011).
Culex quinquefasciatus, also well-known as southern house mosquito, belongs to the family culicidae is the chief vector of lymphatic filariasis (Hati et al. 1989). Filariasis, a parasitic disease caused by three nematode worms Wuchereria bancrofti, Brugia timori and Brugia malayi. It also transmits West Nil Virus in few areas around the world. Almost more than 1.2 billion people facing a threat across the globe due to lymphatic filariasis (Bockarie et al. 2015). In India approximately 21 million people with symptomatic filariasis and 27 million microfilaria carriers were estimated (Reddy et al. 2000). It usually breeds in polluted surface waters and water filled artificial containers, septic tanks etc. with high organic contains. It is an anthropophagic species and live close proximate to human. It is nocturnal bitter, peak biting hours are midnight to 3 a.m. (Chandra 1995). Their preferred biting site is legs, particularly below the knee (Chandra and Hati 1993).
Control of mosquitoes become very difficult due to the development of resistance against chemically synthesized insecticides. Besides, these insecticides have many harmful effects on the environment (Russell et al. 2009). To overcome these problems botanicals may be used as suitable alternative. A lot of researchers reported various plant sources from which novel insecticide may be derived (Singha et al. 2011a; Bhattacharya et al. 2014a; Pal et al. 2016; Singh et al. 2015).
Andrographis echioides (Acanthaceae) commonly known as False Water willow is an annual herb with hairy stems growing up to 45 cm tall, branched from the base. Leaves are oblong, long up to 7.5 cm, 2.4 cm in wide and hairy on both sides. Flowers are borne in spike-like racemes, long up to 2 cm. The stalk carrying the raceme is thickly hairy. Flowers are erect and sepal tube is 2 mm long. Stamen filaments are flattened. False Water willow is found in India and Sri Lanka. Flowering time is March–June and October–December. It has various medicinal activities like diuretic, antimicrobial, anthelmintic, hepatoprotective, antioxidant, anti-inflammatory, mosquito larvicidal and antiulcer activity (Mathivanan and Suseem 2015).
The purpose of the present experiment was to assess the lethal effect of crude and solvent extracts of A. echioides foliages against the Cx. quinquefasciatus larvae. This is the first ever report of this plant as a source of larvicidal agent under laboratory conditions against Cx. quinquefasciatus.
Materials and Methods
Collection of Plant Materials
Mature, fresh green foliages of A. echioides (Acanthaceae) were harvested randomly during March–April 2015 from the plants around the outskirts of Burdwan (23°44′41.46″N, 86°49′52.95″E), West Bengal, India. The plant was identified perfectly and a voucher specimen (Voucher No. GCZMD-11) was submitted at the Mosquito, Microbiology and Nanotechnology Research Units, Department of Zoology, The University of Burdwan.
Rearing of Larvae and Colony Set Up
By the help of standard scooping and dipping method (Robert et al. 2002) larvae of Cx. quinquefasciatus were collected from the adjacent drains of Burdwan University (23°16′N, 87°54′E). Larvae were kept in a plastic tray filled with dechlorinated tap water with proper sanitation to set up a larval colony. Larvae were fed with a composite diet of Brewer yeast, dog biscuits and algae in a ratio of 3:1:1 respectively (Kamaraj et al. 2009). Larvae became pupae within the tray and then those were shifted to an insectary (45 × 45 × 40 cm) for adult immergence. By using the mosquito identification key provided by Christophers (1933), Barraud (1934) and Chandra (2000) adult mosquitoes were identified. A multivitamin syrup and 10% sucrose solution were supplied to the adult mosquitoes for nutrition with a cotton wick in a container. Adult females were supplied a blood meal from a non-motile shaved rat on the 5th day of rearing. Petri dishes filled with 100 ml of tap water and wrinkled with filter paper were kept inside the cage for oviposition. Eggs were allowed to hatch under laboratory conditions. The colony was maintained at 80–85% relative humidity (RH) and 27 ± 2 °C temperature under the photo regime of 13:11 light-and-dark cycles. 1st generation larvae were used during bioassays.
Preparation of Crude Extract
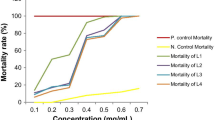
At first the collected fresh and mature foliages were rinsed well in tap water followed by distilled water and soaked on a paper towel. Mature leaves were crushed with electrical grinder and the extract was filtered using Whatman No. 1 filter paper and the filtrate was used as a stock solution (100% concentration) for further bioassay experiments. The concentrations (0.6%, 0.7%, 0.8%, 0.9% and 1.0%) were prepared with addition of distilled water with the stock solution.
Preparation of Solvent Extract
The leaves of A. echioides were rinsed well in distilled water and socked by using paper towel. Leaves were dried for few days in shed at room temperature. The dried leaves were cut in tiny pieces and put (200 g) into the thimble of Soxhlet apparatus. Separately 2000 ml of each solvent was loaded on still pot one after another. 72 h of extraction period for each solvent (8 h maximum in a day) was fixed. Three different solvents petroleum ether, ethyl acetate and acetone were passed through the thimble one after another. Extracts were accumulated from the still pot and kept on separate beakers. The extractives were intensified through rotary evaporator. The concentrated extractives were preserved at 4 °C in a refrigerator for further work.
Dose–Response Larvicidal Bioassay
The larvicidal bioassays were done at the Mosquito, Microbiology and Nanotechnology Research Units, Parasitology Laboratory, The University of Burdwan, according to the standard protocol of WHO (2005). Each larval instar of Cx. quinquefasciatus was tested against earlier prepared concentrations of crude and solvent extracts. Twenty five larvae of different instars (1st, 2nd, 3rd and 4th) were shifted into sterilized glass Petri dishes filled with 100 ml of distilled water. Crude extracts were applied in different Petri dishes in following grades viz. 0.6–1.0%. Likewise, graded concentrations of solvent extracts (from 50 to 150 ppm) were prepared and tested for their larvicidal potentiality against each instar stage. Each experiment was done in triplicate (n = 75) with a set (2 replicates) of controls. Petri dishes were placed at room temperature (30 ± 2° C) and 88 ± 2% relative humidity for 72 h of total observation. The percent mortality was recorded after 24 h, 48 h and 72 h of exposure. The larvae were assumed dead when they failed to move after probing with needle in the siphon or cervical area of it or when they were unable to reach the water surface (Pal et al. 2017).
Effect on Non-target Organism
The organisms who share the same aquatic habitat with mosquito larvae are the most vulnerable group. Impact of the plant extractives on non-target group was tested on Diplonychys annulatum nymph. They were exposed to the LC50 value of 3rd instar larvae for 72 h of crude and solvent extracts. The mortality or other abnormalities like sluggishness and reduced swimming activity were observed up to 72 h of exposure.
Statistical Analyses
The observed percent mortality (%M) was calculated by using Abbott’s formula (Abbott 1925). Determination of LC50 and LC90 values of solvent extract was carried out through Log-probit and regression analyses (Y = mortality; X = concentration). For further statistical justifications three way ANOVA analyses was done by using different instars, hours and different concentrations as three random variables to validate the significance between the above parameters and larval mortality.
Result
A. echioides was found to have good mosquito larvicidal property against Cx. quinquefasciatus in laboratory observations. In crude extract of A. echioides leaf, 100% mortality was found at 1.00% concentration against 1st instars larvae after 72 h of exposure (Table 1). Among the three used solvent extracts, ethyl acetate showed most efficient larvicidal effect against the target mosquito. In ethyl acetate extractive 100.00% mortality was obtained at 150 ppm concentration against 1st instar larvae after 48 h of exposure (Table 2). With the increase in time of exposure mortality rate increased in each extract and in each instar. Results of log probit and regression analyses of larval mortality by ethyl acetate extractive are presented in Table 3. The results of log probit analysis (95% confidence level) states that LC50 and LC90 values gradually decreased with the increase of exposure period having the lowest value at 72 h of exposure in each instar. LC50 and LC90 value (95% confidence level) of ethyl acetate against 1st instars larvae at 72 h of exposure were 25.45 ppm and 70.25 ppm respectively which were significantly lower than all other instars. Regression analysis proved that mortality rate (Y) was positively correlated with time of exposure (X). The regression coefficient (R2) value was close to one in each case. Using completely randomized three way ANOVA regarding hour (H), concentration (C) and instars (I) as three parameters proved statistical significance of the larvicidal effect of ethyl acetate extractive of leaf of A. echioides against Cx. quinquefasciatus (Table 4). Tested non target organism showed no abnormality or mortality when it was treated with crude and solvent extractives of plant.
Discussion
The present study reveals that leaf extract of A. echioides has effective larvicidal property against Cx. quinquefasciatus larvae. Both crude and solvent extracts showed mosquito larvicidal potentiality against all the larval instars of Cx. quinquefasciatus. In crude extract 96.00% mortality was obtained in 1.00% concentration against 3rd instar larvae after 72 h of exposure. Usually polar fractions of plant materials are proficiently extracted using polar solvents and non polar solvents take out non polar molecules. Petroleum ether is the most non polar solvent (polarity index of 0.1) that extracts essential oil, while ethyl acetate being moderately polar (polarity index of 4.4) mainly extracts steroids, alkaloids etc. (Ghosh et al. 2012). In the present experiment we got best larvicidal potentiality in ethyl acetate extractive which probably contains steroids and alkaloids or both as secondary phytochemical. In comparison to crude extract, in ethyl acetate solvent extractive cent percent mortality of 3rd instar larvae was obtained after 72 h at a very low dose rate i.e. only 150 ppm. Tested non target organism was completely secured from the effect of crude and solvent extractives of the plant understudy.
Mosquito control mainly depends on control of larvae and adults. Due to larval low mobility and confinement to water bodies, larval control is easier than control of adults. Many researchers reported a lot of plants which showed effective mosquito larvicidal (Singha and Chandra 2011; Singh Ray et al. 2014; Bhattacharya and Chandra 2013, 2015; Mondal et al. 2016) repellent, smoke toxicity, pupicidal and adulticidal (Singha et al. 2011b; Bhattacharya and Chandra 2014; Rawani et al. 2012) properties. Rawani et al. (2010) published that ethyl acetate extract of Solanum nigrum showed potent larvicidal property against Cx. quinquefasciatus and the LC50 value was 17.04 ppm after 24 h of exposure. Singha Ray et al. (2015) reported that ethyl acetate extractive of Capparis zeylanica leaf showed larvicidal efficiency against Cx. quinquefasciatus with lowest LC50 and LC90 values of 12.44 ppm and 33.88 ppm respectively. According to Kundu et al. (2013) ethyl acetate solvent extractive of seed coat of Cassia sophera showed potent larvicidal property against Cx. quinquefasciatus. In comparison, we found lowest LC50 and LC90 values 25.45 ppm and 70.25 ppm respectively. Bhattacharya et al. (2014b) reported that chloroform: methanol (1:1 v/v) extractives of Ravenala madagascariensis leaves showed larvicidal property against the same mosquito species. The LC50 and LC90 values were 25.41 ppm and 90.98 ppm respectively against 1st instar larvae of Cx. quinquefasciatus after 72 h of exposure that is higher dose in comparison to our study. In another work, Rawani et al. (2013) reported that chloroform: methanol (1:1 v/v) extract of Solanum nigrum berry showed effective larvicidal property against Cx. quinquefasciatus. Hexane flower extract of Nerium oleander (Raveen et al. 2014), Acetone extractives of leaf of Nicotiana plumbaginifolia (Singh et al. 2016), petroleum ether and N-butanol extract of Cassia occidentalis (Kumar et al. 2014) also showed potent larvicidal effect against Cx. quinquefasciatus.
The above study showed that the foliages of A. echioides exhibited significant mortality of Cx. quinquefasciatus larvae. Therefore, it can be concluded that further detail studies on foliages of A. echioides may fulfill the search of establishing a novel bio-insecticide.
References
Abbott, W.S. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology 18: 265–266.
Barraud, P.J. 1934. The Fauna of British India, including Ceylon and Burma. Diptera Vol -IV, 1–455. London: Taylor and Francis.
Bhattacharya, K., and G. Chandra. 2013. Bioactivity of Acyranthes aspera (Amaranthaceae) Foliage against the Japanese Encephalitis Vector Culex vishnui Group. Journal of Mosquito Research 3(13): 89–96.
Bhattacharya, K., and G. Chandra. 2014. Phagodeterrence, larvicidal and oviposition deterrence activity of Tragia involucrata L. (Euphorbiaceae) root extractives against vector of lymphatic filariasis Culex quinquefasciatus (Diptera: Culicidae). Asian Pacific Journal of Tropical Disease 4(Suppl 1): S226–S232.
Bhattacharya, K., and G. Chandra. 2015. Biocontrol efficacy of Operculina turpethum (L.) (Convolvulaceae) leaf extractives against larval form of malarial mosquito Anopheles stephensi (Liston 1901). International Journal of Pharma and Bio Sciences 6(3): 460–468.
Bhattacharya, K., S. Burman, S. Nandi, P. Roy, D. Chatterjee, and G. Chandra. 2014a. Phytochemical extractions from the leaves of Ravenala madagascariensis from Sundarban area and its effect on southern house mosquito (Culex quinquefasciatus Say 1823) larvae. Journal of Mosquito Research 4(12): 1–6.
Bhattacharya, K., I. Chandra, P. Kundu, S. Ray, D. Halder, and G. Chandra. 2014b. Larval control of Culex vishnui group through bio-active fraction of traveller’s tree, Ravenala madagascariensis Sonn. (Strelitziaceae). Journal of Mosquito Research 4(15): 1–6.
Bockarie, J.M., M.P. Erling, B.W. Graham, and E. Michael. 2015. Role of vector control in the global program to eliminate lymphatic filariasis. Annual Review of Entomology 54: 469–487.
Chandra, G. 1995. Peak period of filarial transmission. The American Journal of Tropical Medicine and Hygiene 53(4): 378–379.
Chandra, G. 2000. Mosquito, 1–102. Kolkata: Sribhumi Publication Co.
Chandra, G., and A.K. Hati. 1993. Correlation between the preferred biting site of Culex quinquefasciatus and the region of the body affected by clinical filariasis. Annals of Tropical Medicine and Parasitology 87(4): 393–397.
Christophers, S.R. 1933. The Fauna of British India, including Ceylon and Burma. Diptera Vol -V, 360. London p: Taylor and Francis.
Ghosh, A., N. Chowdhury, and G. Chandra. 2012. Plant extracts as potential mosquito larvicides. Indian Journal of Medical Research 135(5): 581–598.
Harbach, R.E. 2011. Mosquito taxonomic inventory. http://mosquito-taxonomic-inventory.info/. Accessed 12 Mar 2011.
Hassan Adeyemi, M.M. 2010. The potential of secondary metabolites in plant material as deterrents against insect pests: A review. African Journal of Pure and Applied Chemistry 4(11): 243–246.
Hati, A.K., G. Chandra, A. Bhattacharyya, D. Biswas, K.K. Dwibedi, and H.N. Chatterjee. 1989. Annual transmission potential of bancroftian filariasis in an urban and a rural area of West Bengal, India. The American Journal of Tropical Medicine and Hygiene 40(4): 365–367.
Hossain, E., A. Rawani, G. Chandra, S.C. Mandal, and J.K. Gupta. 2011. Larvicidal activity of Dregea volvubilis and Bombax malabaricum leaf extracts against the filarial vector Culex quinquefasciatus. Asian Pacific Journal of Tropical Medicine 4(6): 436–441. https://doi.org/10.1016/S1995-7645(11)60121-1.
ICMR Bulletin. 2013. Prospects of using herbal products in the control of mosquito vectors. Indian Council of Medical Research Bulletin 12: 406–408.
Kamaraj, C., A. Bagavan, A.A. Rahuman, A.A. Zahir, G. Elango, and G. Pandiyan. 2009. Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitology Research 104: 1163–1171.
Kumar, D., R. Chawla, P. Dhamodaram, and N. Balakrishnan. 2014. Larvicidal activity of Cassia occidentalis (Linn.) against the larvae of bancroftian filariasis vector mosquito Culex quinquefasciatus. Journal of Parasitology Research. https://doi.org/10.1155/2014/236838.
Kundu, M., A. Rawani, and G. Chandra. 2013. Evaluation of mosquito larvicidal activities of seed coat extract of Cassia sophera L. Journal of Mosquito Research 3(11): 76–81.
Mathivanan, D., and S.R. Suseem. 2015. Phytochemical and pharmacological review of Andrographis echiodies. Journal of Chemical and Pharmaceutical Research 7(7): 1167–1171.
Mondal, R.P., A. Singh, A. Ghosh, and G. Chandra. 2016. Studies on larvicidal activity of some plant extracts against filarial vector Culex quinquefasciatus. Journal of Mosquito Research 6(7): 1–6.
Pal, J.K., A. Singh, A. Rawani, and G. Chandra. 2016. Larvicidal activity of Tinospora crispa (Menispermaceae) fruit extract against filarial vector Culex quinquefasciatus. Journal of Mosquito Research 6(35): 1–8.
Pal, J.K., A. Singh, and G. Chandra. 2017. Larvicidal efficacy of Cleistanthus patulus Muell. Arg. (Euphorbiaceae) leaf extract against filarial vector Culex quinquefasciatus (Say 1823). Journal of Mosquito Research 7(12): 96–103.
Raveen, R.K., T. Kamakshi, M. Deepa, S. Arivoli, and S. Tennyson. 2014. Larvicidal activity of Nerium oleander L. (Apocynaceae) flower extracts against Culex quinquefasciatus Say (Diptera: Culicidae). International Journal of Mosquito Research 1(1): 38–42.
Rawani, A., A. Ghosh, and G. Chandra. 2010. Mosquito larvicidal activities of Solanum nigrum L. leaf extract against Culex quinquefasciatus Say. Parasitology Research 107(5): 1235–1240.
Rawani, A., A. Ghosh, S. Laskar, and G. Chandra. 2012. Aliphatic amide from seeds of Carica papaya as mosquito larvicide, pupicide, adulticide, repellent and smoke toxicant. Journal of Mosquito Research 2(2): 8–18.
Rawani, A., N. Chowdhury, A. Ghosh, S. Laskar, and G. Chandra. 2013. Mosquito larvicidal activity of Solanum nigrum berry extracts. Indian Journal of Medical Research 137: 972–976.
Reddy, G.S., N. Venkatesvarlou, P.K. Das, P. Vanamail, S.K. Vijayan, and S.P. Pani. 2000. Tolerability and efficacy of single dose diethylcarbamazine (DEC) or ivenmectin the clearance of Wuchereria bancrofti microfilaraemia at Pondicherry, south India. Tropical Medicine & International Health 5: 779–785.
Robert, V., G.L. Goff, F. Ariey, and J.B. Duchemin. 2002. A possible alternative method for collecting mosquito larvae in rice fields. Malaria Journal 1: 4.
Russell, T.L., B.H. Kay, and G.A. Skilleter. 2009. Environmental effects of mosquito insecticides on saltmarsh invertebrate fauna. Aquatic Biology 6: 77–90.
Singh Ray, A., K. Bhattacharya, A. Singh, and G. Chandra. 2014. Larvicidal activity of Nelumbo nucifera Gaertn. (Nymphaeaceae) against Anopheles stephensi (Liston 1901) and its effect on non-target organisms. Journal of Mosquito Research 4(10): 1–7.
Singh, A., K. Bhattacharya, and G. Chandra. 2015. Efficacy of Nicotiana plumbaginifolia (solanaceae) leaf extracts as larvicide against malarial vector Anopheles stephensi Liston 1901. International Journal of Pharma and Bio Sciences 6(1): 860–868.
Singh, A., K. Bhattacharya, A. Singh Ray, and G. Chandra. 2016. Larvicidal efficacy of mature leaf extract of Nicotiana plumbaginifolia viv. (Solanaceae) against southern house mosquito. International Journal of Pharma and Bio Sciences 7(2): 162–167.
Singha, S., and G. Chandra. 2011. Mosquito larvicidal activity of some common spices and vegetable waste on Culex quinquefasciatus and Anopheles stephensi. Asian Pacific Journal of Tropical Biomedicine 4(4): 288–293. https://doi.org/10.1016/S1995-7645(11)60088-6.
Singha Ray, A., K. Bhattacharya, and G. Chandra. 2015. Target specific larvicidal effect of Capparis zeylanica L. (Capparaceae) foliages against lymphatic filarial vector culex quinquefasciatus say (1823). International Journal of Pharma and Bio Sciences 6(3): 139–148.
Singha, S., U. Adhikari, and G. Chandra. 2011a. Smoke repellency and mosquito larvicidal potentiality of Mesua ferra L. leaf extract against filarial vector Culex quinquefasciatus Say. Asian Pacific Journal of Tropical Biomedicine 1(1): S119–S123.
Singha, S., S. Banerjee, and G. Chandra. 2011b. Synergistic effect of Croton caudatus (fruits) and Tiliacora acuminate (flowers) extracts against filarial vector Culex quinquefasciatus. Asian Pacific Journal of Tropical Biomedicine 1(2): S159–S164.
World Health Organization. 2005. Guidelines for laboratory and field testing of mosquito larvicides, 13. Geneva WHO/CDS/WHOPES/GCDPP/: WHO.
Acknowledgements
Authors are indebted to UGC for financial support for providing CAS (RTI/1309/184) and Rajiv Gandhi National Fellowship (RGNF-2013-14-ST-WES-52307).
Author information
Authors and Affiliations
Contributions
The screening was done and the plant having mosquitocidal activity was selected by Moumita Das. Rearing of mosquito larvae and colony set up was done by Moumita Das. Extract preparation and laboratory bioassay was carried out by Moumita Das and Aniket Singh. Manuscript preparation was done by Kuntal Bhattacharya. Plant identification was done by Professor Ambarish Mukherjee. Professor Goutam Chandra designed the study and supervised the whole work. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Das, M., Singh, A., Bhattacharya, K. et al. Mosquito Larvicidal Efficacy of Andrographis echioides (Acanthaceae) Foliages Against Vector of Lymphatic Filariasis Culex quinquefasciatus Say (1823). Proc Zool Soc 72, 283–289 (2019). https://doi.org/10.1007/s12595-018-0273-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-018-0273-z