Abstract
Binary and ternary blends comprised of polypropylene (PP), polystyrene (PS) and polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene (SEBS) were prepared. The effect of phase composition of minor components on the morphology, mechanical, viscoelastic, crystallization, and thermal degradation properties was studied. Binary blends exhibited inferior properties, typical of immiscible and incompatible multi-phase systems and showed matrix-droplet phase morphologies. Ternary blends, especially those with greater concentration of SEBS minor phase, exhibited interesting properties. Scanning electron micrographs of SEBS compatibilized PP/PS blends, did not show any PS particle pulling out of the PP matrix, indicating good compatibility of SEBS with PP/PS blends. Dynamic mechanical analysis also supported the heterogeneous phase structure of the blends. Thermogravimetry and differential scanning calorimetry showed that addition of PS and SEBS decreased the thermal stability of PP marginally, but shows slight variations in melting and crystallization behavior.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer blending is a simple approach for developing advanced polymeric materials with superior properties suitable for end use applications [1]. Polypropylene (PP) is a widely used polymer with good thermo-mechanical properties. Studies revealed that polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene (SEBS) can be used as an elastomer to improve the toughness and elongation at break of PP, but at the expense of stiffness [2,3,4,5,6,7]. Presence of polystyrene (PS), which can interact favorably with SEBS, can improve the modulus of the blends. However, PP/PS blends are immiscible and incompatible. It is unequivocally established that immiscible blends possess inferior properties relative to their components due to the unfavorable interfacial interactions.
Mechanical properties of immiscible blends can be improved by compatibilization [8,9,10,11,12,13]. PP/PS blends can be effectively compatibilized by the addition of block or graft copolymers or in situ generation of compatibilizer [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Li et al. [17] have studied the phase morphology of PP/PS blends compatibilized with SBS copolymer and found that the copolymer buried in dispersed domains migrates to the interface, forming a comparatively thicker transition layer and strengthens the interfacial adhesion.
Over the past several years, ternary blends have been a topic of great interest since it is possible to develop polymeric materials with specific end use applications by the judicious selection of components and tuning the phase morphology [19,20,21,22,23,24,25]. Demarquette et al. [19] investigated the evolution of the morphology of PP/PS/polymethylmethacrylate (PMMA) ternary blends with and without compatibilizer and observed a core–shell morphology with PS as shell and PMMA as core, in every case. Wang et al. [20] have shown that a multi-phase copolymer could act as an efficient compatibilizer in a ternary blends comprising PP, PS and polyamide 6 (PA6). Favis and co-workers [21, 22] have reported a series of studies on the morphology of ternary blends and demonstrated the partial and complete wetting morphologies in ternary blends.
SEBS has been widely used as an elastomer/compatibilizer to improve the toughness of different polymers [26,27,28]. In the present study, we used SEBS for dual purposes: to improve the compatibility between PP and PS phases and enhance the ductility of the blends, by acting as impact modifier. The effects of SEBS on the morphology, mechanical, viscoelastic, crystallization, and thermal degradation studies of PP and PP/PS blends have been systemically studied. The changes in phase morphology with respect to the concentration of SEBS are correlated with thermo-mechanical properties.
Experimental
Materials and preparation of blends
Polypropylene (PP), grade 1110 MAS, with density 0.9 g/cm3 was procured from Indian Oil Corporation. Polystyrene (PS), grade POLYSTYROL 147F GR21, with density 1.05 g/cm3 was procured from Styrolution India Pvt ltd. Polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene (SEBS), with average Mw ~ 118,000 was procured from Sigma Aldrich. Thermo Haake Polylab QC system equipped with roller rotors was used for the preparation of PP/PS blends. The blending was done at 180o C with a rotor speed of 50 rpm for 8 min. For making PP/SEBS binary blends, PP was melt-mixed for two minutes, followed by the addition of the SEBS. Similarly for ternary blends, initially PP and PS were melt-mixed for two minutes, which is followed by the addition of the SEBS and the mixing was continued for another six minutes. The resulting blends were hot pressed into sheets and cut into pieces and injection molded in a DSM explore, Micro 12 cc injection molding machine, at 190o C for preparing test specimens for tensile and impact testing as per relevant ISO standards.
Characterization
Scanning electron microscopy (SEM)
JEOL JCM 5000 Neoscope SEM was used to examine the blend morphology. The experiments were done by an acceleration voltage of 10 kV. The fractured surfaces were coated with thin layers of gold before analysis.
Dynamic mechanical analysis (DMA)
DMA Q-800 TA instruments were used to study the viscoelastic behavior of PP/PS blends. The analysis was done at a frequency of 1 Hz, with specimen size of 60 × 10 × 4 mm3, using a dual cantilever clamp, in the temperature range 35 to 130o C at a heating rate of 3o C/min.
Thermo gravimetric analysis (TGA)
Perkin Elmer, Diamond TG/DTA instrument was used to measure the thermal stability of the blends. Approximately 5–7 mg was used for measurements, from room temperature to 600o C at a heating rate of 20o C/min.
Differential scanning calorimetry (DSC)
Mettler Toledo DSC 822e differential scanning calorimetry was used to characterize the melting and crystallization properties of polymer blends in nitrogen atmosphere with flow rate 20 mL/min. The parameters such as melting temperature (T m ), crystallisation temperature (T c ), total enthalpy of crystallisation (ΔH c ), normalised enthalpy of fusion (ΔH f ) and percentage crystallinity (X c ) were determined from the DSC profile. Approximately 6 mg of the samples were used for measurements. The heating was done from -50 to 200 °C, followed by cooling to room temperature, heating and cooling was done at 10 °C/min.
The percentage crystalline content (X c ) was determined using Eq. 1:
where ΔH f is the normalised enthalpy of fusion and W poly is the weight fraction of a PP in the blend. The term ΔH max is a reference value and is 207.1 J/g for PP [29].
Measurement of mechanical properties
Universal testing machine (Tinius Olsen) model H 50 KT was used to measure the tensile properties of the samples, according to ISO 527. Dumbbell shaped specimens with sample dimensions of 75 × 5 × 2 mm3 were used for testing. The span length used was 55 mm. The measurements were done at a cross head speed of 50 mm/min. Impact testing was carried out according to ISO 180 using a Resil impactor junior. The sample dimensions were 80 × 10 × 4 mm3.
Results and discussion
Phase morphology
SEM micrographs of PP/SEBS binary blends are shown in Fig. 1. The SEM micrograph of neat PP shows some micro cracks (Fig. 1a). For PP/SEBS blends phase separated and coarse morphology was observed (Fig. 1b–e). It is important to mention that the surface of the PP/SEBS blends are rougher than neat PP, rougher surface is typical for tough materials [30, 31]. SEM micrographs of PP/PS blends modified with SEBS are shown in Figs. 2 and 3. The SEM micrographs reveal that the PP/PS blends are microphase separated, with large PS domains non-uniformly distributed in the PP matrix. The size of dispersed PS particles increases with the increase inconcentration of PS phase in the blend due to coalescence during the melt mixing [15]. Therefore, the PS domains in 80/20 blends are larger than 90/10 blends for the uncompatibilized blends. It can be seen that most of the PS domains in the uncompatibilized blends are removed out of the PP rich phase, which shows the poor compatibility of the PS phase with the PP matrix [32]. The extent of phase separation in PP/PS blends was decreased by the addition of SEBS. The SEBS increases the compatibility or in other words prevents the coalescence process or increases the interfacial interaction between the component polymers, and hence smaller PS droplets are dispersed in the PP matrix at higher SEBS content. These morphologies reveal that SEBS is selectively capable of interpenetrating between the homopolymer phases to provide good interconnection [33, 34]. From the micrographs, it is observed that with the addition of SEBS to PP/PS blends (1) the number of PS domains decreases (2) the number of PS domains that removed out of the PP phase decreases and (3) the size of dispersed PS domains decreases. In other words, the results indicate that some part of the SEBS phase is dispersed at the interface, between the polymer components [33, 35, 36]. These changes in morphology should reflect in the physical properties of the material, since the properties of polymer blends depends on the morphology and interfacial adhesion between the phases. The morphological parameters obtained from the SEM micrographs are given in Table 1. From the Table, the modified blends exhibits smaller PS domains compared with neat blends. It is important to add that the dispersed particle size decreases from 12 μm to 800 nm by the addition of 20 wt% of SEBS to 80/20 blends.
Mechanical properties
Table 2 displays the mechanical properties of binary and ternary blends. PP has an impact strength of 55 kJ/m2, by blending with SEBS, this value gradually increases and a maximum of 113 kJ/m2 was observed for 20 wt% SEBS modified PP. The impact strength increases by more than 100%, this increase in impact strength was due to the interaction of SEBS with PP, EB mid blocks in SEBS are compatible with noncrystalline PP fraction, which facilities segmental diffusion between the matrix and rubber particles on the interfaces and therefore excessive energy was needed to initiate cracks for the PP/SEBS blends [26]. This means that the material transform from tough to super tough material. According to Karger-Kocsis et al. the elastomers and rubbers may act as nucleating agents, therefore the spherulite size reduces and will the improve impact strength. Decrease in spherulite size increases in interfacial thickness, better spherulitic chain mobility and reduction of spherulitic defects [30]. In another study Bassani et al. [37] revealed that the increase in impact strength is due to the increase in the stress concentration region within the polymer matrix, this increases the energy absorbing capacity of the system. Several other mechanisms are proposed this include cavitation of elastomer, debounding of rubber particles, crack deflection by rubber particles, tearing of rubber particles etc. [38]. From the SEM micrographs, PP/SEBS blends posses excellent adhesion between the component phases, it is known that when the adhesion is optimum, stress transfer across the interface is continuous and in the absence of adhesion, there is discontinuity in stress transfer and therefore excellent improvement in toughness for the PP/SEBS blends [37]. Moreover, the SEM micrographs revealed a rougher surface for the PP/SEBS blends, the rough surface indicate energy dissipation and absorption leading to a tough response during an impact test [31].
From Table 2, the tensile strength gradually decreases by the addition of SEBS. This result suggests a decrease in load-bearing-cross-section of PP with SEBS due to low strength of SEBS elastomer. The elongation at break increases linearly with SEBS elastomer, a maximum of 300% increase in elongation was observed with 15 wt% SEBS. The substitution of PP matrix by ductile SEBS caused elongation of PP to increase. However, the modulus of PP/SEBS blends decreased with SEBS due to the substitution of PP matrix by soft SEBS elastomer. That means the blends become less stiff. These results are in agreement with Bassani et al. [37] and Balkan et al. [39].
It is obvious from Table 2 that PP/PS blends are highly immiscible and incompatible due to the lack of favourable interactions at the interface, which reflected in deterioration of mechanical properties. The elongation and impact strength are worst affected, which limits the use of this material for any end-use applications as it is too brittle, even if the modulus registered a 20% enhancement by the addition of 20 wt% of PS. This observation is supported by the phase morphology of binary blends as seen in the SEM micrographs (Figs. 2, 3). The PP/PS blends showed a two-phase matrix-droplet morphology, with large domains non-uniformly distributed in the PP matrix.
On the other hand, for 90/10 and 80/20 blends modified with 20 wt% SEBS, impact strength increases by 240 and 180% respectively, suggesting that in these blends, SEBS acts as an efficient compatibilizer. The smaller but finer morphology of the compatibilized blends results in PS phase with larger surface area and hence the stress is disturbed to larger surface. This reduces the maximum load applied at any point of the system and hence systems can withstand more loads. In an immiscible system the matrix takes the majority of the applied load. Therefore in PP/PS system, PP phase takes the majority of the force of the impact. The phase separation in PP/PS blends does not allow the impact energy to travel easily between the component phases. However, the presence of SEBS strengthens the interface between the PP and PS; hence impact energy can travel easily between the component phases. Thus SEBS acts an agent that allows better dispersion of impact energy or in other words SEBS allows a better stress transfer across the phase boundary [40].
The elongation at break also increases linearly with the addition of SEBS elastomer. For the 90/10 and 80/20 blends, elongation at break increases by 600 and 250% respectively with the addition of 20 wt% SEBS. The sharp increase in elongation at break is due to the enhanced adhesion between the PP matrix and PS dispersed phase as observed from the SEM micrographs [14]. However, the tensile strength and modulus decreases for the PP/PS blends by the addition of soft SEBS elastomer [41]. Note that the modulus of 20 wt% SEBS modified PP/PS (90/10) blend is nearly two times than that of SEBS modified PP blends. This means that these blends exhibit excellent toughness and reasonably good strength and modulus, suitable for various end-use applications. The other series of ternary blends also possess good strength and modulus and reasonably good elongation and impact strength.
Thus it can be concluded that the SEBS phase plays a dual role in PP/PS blends. Even if it forms an independent phase in PP matrix which acts as a plasticizer to improve the flexibility of hard PP and PS chains, a fraction of SEBS chains rupture apart from the bulk phase and migrate under the influence of shear force. They eventually locate at the interface between the PP/PS interfaces and act as compatibilizer. When SEBS dispersed phase tends to increase the flexibility and ductility of the system, the PS dispersed phase compatibilized with SEBS tries to retain the stiffness of the blends.
Dynamic mechanical properties
Figure 4 shows the storage modulus, which gives an idea about the stiffness, a critical parameter in determining the suitability of a blend for specific applications, of binary and ternary blends. Figure 4a reveals that all the PP/PS blends possess a heterogeneous phase structure, typical of immiscible and incompatible blends, as evident from the phase morphology. Addition of 10 wt% of PS into PP increased the storage modulus, up to a temperature of about 115 °C, the glass transition temperature (T g ) of PS. Further addition of PS into PP increased the storage modulus of the blends only marginally, without changing the T g of PS. On the other hand, addition of even 5 wt% of SEBS decreased the storage modulus of PP (Fig. 4b), in the entire temperature range. Further addition resulted in a significant drop in the modulus. The modulus of PP/SEBS blends decreased with addition of SEBS due to the substitution of PP matrix by soft SEBS elastomer. The SEBS embeds themselves between the PP chains and hence increases the free volume, making it softer [42]. Ternary blends also displayed similar behavior, as the amount of SEBS in the blends increased modulus decreased and conversely, as the amount of PS in the blends increased modulus increased.
Figure 5 shows the variation of loss modulus of the blends as a function of temperature. The loss modulus represents the amount of energy released during the DMA running. The PP/PS blends showed glass transition peaks of PS at around 115 °C. The ternary blends also possess glass transition peaks, in the same region. Blending and compatibilization have no impact on the T g of PS, due to the immiscible nature of the blends. This means that the presence of SEBS in PP/PS blends does not provide any molecular level miscibility, but SEBS forms a separate phase, as evident from the phase morphology studies. Otherwise, there would have been a remarkable shift in the T g of PS, in the presence of SEBS. Thus, it is obvious that the blends exhibited a three-phase morphology in which PP forms the matrix and PS and SEBS form dispersed phases. But, in every case it is seen that as the amount of SEBS in the blend increased, loss modulus decreased. As mentioned earlier, the soft SEBS molecules may get in between the PP polymer chains and hence increases the free volume, making it softer. Thus the addition of SEBS decreases the stress present within the polymer chains and hence decreases the (loss modulus) amount of energy released during the DMA running. These results are in agreement with recent finding by Zhao et al. [43]. This decrease in stress within the polymer matrix enhances the impact strength and strain at break.
The variation of tan δ with temperature is shown in Fig. 6. The variations in tan δ represent the segmental mobility of the polymer chains. It is seen from the figure that there is no appreciable change in the T g of PS by blending and compatibilization, indicating the heterogeneous nature of the system. However, as the amount of PS in the blend increased peak height is increased. After the T g the polymer chains are free to move such that there is no stress available and hence the tan δ is minimum. As expected the response of the PP/SEBS and SEBS modified PP/PS blends are greater than the uncompatibilized blends. This is because the soft SEBS molecules increase the free volume and hence an increase in the segmental mobility for the SEBS modified PP or PP/PS blends.
Differential Scanning Calorimetry
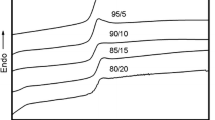
Table 3 summarizes the crystallization parameters derived from the heating and cooling DSC curves, and % crystallinity calculated from the enthalpy of fusion values. It can be seen that addition of SEBS to PP has no effect on the melting and crystallization temperature. However, the overall enthalpy of fusion decreases with SEBS addition that means the overall crystallinity of the PP/SEBS blends decreases. On the other hand, the enthalpy of crystallization slightly increases at lower concentrations of SEBS. This behavior is also true for SEBS modified PP/PS blends; therefore, the % crystallinity was calculated, the values obtained are given in Table 3. For the blends studied, the % crystallinity of PP was slightly increased by the addition of SEBS. This means that SEBS may act as a nucleating agent by providing nucleation sites for PP molecular segments [26, 44].
Thermal degradation properties of the blends
The important thermal degradation parameters such as initial degradation temperature (T i ), maximum degradation temperature (T max ) and final degradation temperature (T f ) of the blends are given in Table 4. It is important to mention that all the blends show a single step degradation characteristic, even if the blends are immiscible. This is because the concentrations of minor phases are too small to show separate degradation peaks. Addition of PS and SEBS into PP decreased the thermal stability of PP. The latter has a slightly pronounced deteriorating effect. Compatibilization has no appreciable effect on the thermal degradation properties of the blends.
Conclusion
Binary and ternary blends composed of PP, PS and SEBS were prepared. The effect of concentration of minor phases on the morphology, and thermo-mechanical properties was studied. Binary blends exhibited inferior properties and matrix-droplet phase morphology. With respect to mechanical performance, PP/PS blends were inferior to PP/SEBS blends. Ternary blends, especially those with greater concentration of SEBS, exhibited excellent toughness and reasonably good strength and modulus, suitable for several end-use applications. This behaviour could be correlated with the phase morphology, which demonstrated that the SEBS phase served a dual role of compatibilizer and plasticizer. Dynamic mechanical analysis reveals that the presence of SEBS decreases the stress present within the polymer matrix and hence enhances the toughness. DSC measurements reveal that SEBS acts as a nucleating agent for the PP polymer chains. Thermogravimetry showed that addition of PS and SEBS decreased the thermal stability of PP marginally. To conclude, the results may help us to judicially select the blend composition for specific applications.
References
Utracki Leszek A, Mukhopadhyay P, Gupta RK (2014) Chapter 1, Polymer blends introduction. In: Utracki Leszek A, Wilkie Charles A (eds) Polymer blends handbook, 2nd edn. Springer, NY
Vuluga Z, Panaitescu DM, Radovici C, Nicolae C, Iorga MD (2012) Effect of SEBS on morphology, thermal, and mechanical properties of PP/organoclay nanocomposites. Polym Bull 69:1073–1091
Azizi A, Arefazar A, Jazani OM (2013) Effects of core-shell particles on fracture micromechanisms of PP/PC/SEBS ternary blends. Polym Plast Technol Eng 52:1595–1603
Drozdov AD, Sanporean CG, Christiansen JDC (2014) Enhancement of mechanical properties of polypropylene by blending with styrene- (ethylene-butylene)-styrene tri-block copolymer. J Polym Eng 34:765–774
Sharma R, Maiti SN (2014) Effects of crystallinity of PP and flexibility of SEBS-g-MA copolymer on the mechanical properties of PP/SEBS-g-MA blends. Polym Plast Technol Eng 53:229–238
Azizi A, Arefazar A, Jazani OM (2014) Fracture micromechanisms of polypropylene/polycarbonate/poly(styrene-b-(ethylene-co-butylene)-b-styrene) (PP/PC/SEBS) ternary blends: the effects of SEBS content. J Macromol Sci B Phys 53:1103–1115
Sharma R, Maiti SN (2015) Effects of crystallinity of polypropylene (PP) on the mechanical properties of PP/styrene-ethylene-butylene-styrene-g-maleic anhydride (SEBS-g-MA)/teak wood flour (TWF) composites. Polym Bull 72:627–643
Lyu S (2003) Block copolymers suppressing droplet coalescence through stopping film rupture. Macromolecules 36:10052–10055
Sundararaj U (2006) Phase morphology development in polymer blends: processing and experimental aspects. In: Harrats C, Thomas S, Groeninckx G (eds) Micro- and nanostructured multiphase polymer blend systems: phase morphology and interfaces. Taylor & Francis Group, Boca Raton, pp 133–164
Huang H (2011) Macro-micro and nanostructured morphologies of multiphase polymer systems, Chapter 6. In: Boudenne A, Ibos L, Candau Y, Thomas S (eds) Handbook of multiphase polymer systems, vol 1. Wiley, New York, pp 151–249
Jose S, Thomas S, Biju PK, Kargr-Kocsis J (2013) Mechanical and dynamic mechanical properties of polyolefin blends: effect of blend ratio and copolymer monomer fraction on the compatibilisation efficiency of random copolymers. J Polym Res 20:303–316
Parameswarnpillai J, Joseph G, Chellappan RV, Zachakariah AK, Hameed N (2015) The effect of polypropylene-graft-maleic anhydride on the morphology and dynamic mechanical properties of polypropylene/polystyrene blends. J Polym Res 22:1–11
Parameswaranpillai J, Joseph G, Jose S, Hameed N (2015) Phase morphology, thermomechanical, and crystallization behavior of uncompatibilized and PP-g-MAH compatibilized polypropylene/polystyrene blends. J Appl Polym Sci 132:42100
Dı´az MF, Barbosa SE, Capiati NJ (2005) Improvement of mechanical properties for PP/PS blends by in situ compatibilization. Polymer 46:6096–6101
Waldman WR, De Paoli MA (2008) Photodegradation of polypropylene/polystyrene blends: styrene–butadiene–styrene compatibilisation effect. Polym Degrad Stabil 93:273–280
Al-Saleh MH, Sundararaj U (2008) Nanostructured carbon black filled polypropylene/polystyrene blends containing styrene–butadiene–styrene copolymer: influence of morphology on electrical resistivity. Eur Polym J 44:1931–1939
Li Y, Hu S, Sheng J (2007) Evolution of phase dimensions and interfacial morphology of polypropylene/polystyrene compatibilized blends during mixing. Eur Polym J 43:561–572
Lin BH, Du MC, Zhu YJ, Liang YW (2014) Non-isothermal crystallization behavior and kinetics of compatibilized β nucleated polypropylene/polystyrene blends. Adv Mater Res 893:291–294
de Freitas CA, Valera TS, de Souza AMC, Demarquette NR (2007) Morphology of compatibilized ternary blends. Macromol Symp 247:260–270
Wang D, Li Y, Xie XM, Guo BH (2011) Compatibilization and morphology development of immiscible ternary polymer blends. Polymer 52:191–200
Le Corroller P, Favis BD (2011) Effect of viscosity in ternary polymer blends displaying partial wetting phenomena. Polymer 52:3827–3834
Ravati S, Favis BD (2013) Tunable morphologies for ternary blends with poly(butylene succinate): partial and complete wetting phenomena. Polymer 54:3271–3281
Rastin H, Jafari SH, Saeb MR, Khonakdar HA, Wagenknecht U, Heinrich G (2014) On the reliability of existing theoretical models in anticipating type of morphology and domain size in HDPE/PA-6/EVOH ternary blends. Eur Polym J 53:1–12
Kolahchi AR, Ajji A, Carreau PJ (2014) Surface morphology and properties of ternary polymer blends: effect of the migration of minor components. J Phys Chem B 118:6316–6323
Dou R, Wang W, Zhou Y, Li L, Gong L, Yin B, Yang M (2013) Effect of core-shell morphology evolution on the rheology, crystallization, and mechanical properties of PA6/EPDM-g-MA/HDPE ternary blend. J Appl Polym Sci 129:253–262
Abreu FOMS, Forte MMC, Liberman SA (2005) SBS and SEBS block copolymers as impact modifiers for polypropylene compounds. J Appl Polym Sci 95:254–263
Parameswaranpillai J, Joseph G, Shinu KP, Sreejesh PR, Jose S, Salim NV, Hameed N (2015) The role of SEBS in tailoring the interface between the polymer matrix and exfoliated graphene nanoplatelets in hybrid composites. Mater Chem Phys 163:182–189
Parameswaranpillai J, Joseph G, Shinu KP, Jose S, Salim NV, Hameed N (2015) Development of hybrid composites for automotive applications: effect of addition of SEBS on the morphology, mechanical, viscoelastic, crystallization and thermal degradation properties of PP/PS–xGnP composites. RSC Adv 33:25634–25641
Van der Wal A, Mulder JJ, Gaymans RJ (1998) Fracture of polypropylene: the effect of crystallinity. Polymer 39:5477–5481
Karger-Kocsis J, Kalló A, Szafner A, Bodor G, Zs Sényei (1979) Morphological study on the effect of elastomeric impact modifiers in polypropylene systems. Polymer 20:37–43
Tortorella N, Beatty CL (2008) Morphology and mechanical properties of impact modified polypropylene blends. Polym Eng Sci 48:2098–2110
Macau´bas PHP, Demarquette NR (2001) Morphologies and interfacial tensions of immiscible polypropylene/polystyrene blends modified with triblock copolymers. Polymer 42:2543–2554
Ismail HH, Nasir M (2002) Morphological studies of uncompatibilized and compatibilized polystyrene/polypropylene blend. Polym Test 21:263–267
Wildes G, Keskkula H, Paul DR (1999) Coalescence in PC/SAN blends: effect of reactive compatibilization and matrix phase viscosity. Polymer 40:5609–5621
Welander M, Rigdahl M (1989) Use of an emulsifying block copolymer to improve time-dependent mechanical properties of polyethylene-polystyrene blends. Polymer 30:207–212
Manglio G, Palumbo R (1984) Polymer blends, processing, morphology and properties. Plenum press, New York, p 41
Bassani A, Pessan LA, Hage E (2001) Toughening of polypropylene with styrene/ethylene-butylene/styrene tri-block copolymer: effects of mixing condition and elastomer content. J Appl Polym Sci 82:2185–2193
Mcgrath GC, Fracture and toughening in fiber reinforced polymer composites, in rubber toughened engineering plastics, Chapman & hall, 1994, 61
Balkan O, Demirer H, Kayali ES (2011) Effects of deformation rates on mechanical properties of PP/SEBS blends. J Achiev Mater Manuf Eng 47:26–33
Brostow W, Grguric TH, Olea-Mejia O, Pietkiewicz D, Rek V (2008) Polypropylene + polystyrene blends with a compatibilizer. Part 2. Tribological and mechanical properties. e-Polymers, no. 034
Samsudin SA, Hassan A, Mokhtar M, Jamaluddin SMS (2005) Effect of SEBS on the mechanical properties and miscibility of polystyrene rich polystyrene/polypropylene blends. Prog Rubber Plast Recycl Technol 21:261–276
Ahmad Z, Kumar KD, Saroop M, Preschilla N, Biswas A, Bellare JB, Bhowmick AK (2010) Highly transparent thermoplastic elastomer from isotactic polypropylene and styrene/ethylene-butylene/styrene triblock copolymer: structure-property correlations. Polym Eng Sci 50:331–341
Zhao X, Huang Y, Kong M, Yang Q, Li G (2014) Retarded stress and morphology relaxation of deformed polymer blends in the presence of a triblock copolymer. RSC Adv 4:59302–59309
Liao CZ, Tjong SC (2011) Effects of carbon nanofibers on the fracture, mechanical, and thermal properties of PP/SEBS-g-MA blends. Polym Eng Sci 51:948–958
Acknowledgements
J P acknowledges the Department of Science and Technology, Government of India, for financial support under an Innovation in Science Pursuit for Inspired Research (INSPIRE) Faculty Award (contract Grant Number IFA-CH-16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parameswaranpillai, J., Jose, S., Siengchin, S. et al. Phase morphology, mechanical, dynamic mechanical, crystallization, and thermal degradation properties of PP and PP/PS blends modified with SEBS elastomer. Int J Plast Technol 21, 79–95 (2017). https://doi.org/10.1007/s12588-017-9172-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12588-017-9172-9